Abstract
A 10:1 pooled test strategy on-site at an airport of China was pursued, resulting in increased test throughput, limited use of reagents, and increased testing efficiency without loss of sensitivity. This testing approach has the potential to reduce the need for contact tracing when the results are delivered first time.
Keywords: coronavirus disease 2019, infection, screening, on-site, pooled test
The spread of global coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has led to the infection of over 4 million persons globally. Shelter in place, as one of the most effective public control measures, has been implemented by many countries as a countermeasure against the estimated high reproductive number of the SARS-CoV-2 [1]. In China, where the outbreak started in December of 2019, life is returning to normal, but continued vigilance is required because of the high rate of asymptomatic infection with COVID-19 [2, 3]. New approaches to preventing transmission, especially by travelers from previously endemic areas, should be considered.
The main challenge of traveler screening is the projected volume of testing required, along with the time required to obtain actionable results from conventional reverse transcription polymerase chain reaction (RT-PCR) technologies. Specimen pooling strategies were used successfully in the initial stages of testing for human immunodeficiency virus (HIV) in the blood supply and were deployed successfully in early evaluations of HIV antibody prevalence in developing countries [4]. Recently, pooling of respiratory specimens has been investigated as an approach to increasing testing capacity for the comprehensive screening of SARS CoV-2 [5–11].
Hainan is the second largest island in China, and its 3 airports represent a major connection hub to mainland China. To date, the total COVID-19 cases reported in Hainan were 170 with 131 (77.1%) imported, such that imported cases represent a relatively high proportion of overall case load. To prevent importation of COVID-19 cases transmitted from mainland China to Hainan Island, the local government initiated testing of incoming domestic passengers from presumptive “high-risk” areas such as Wuhan in the Sanya Airport.
Starting on 8 April 2020, both SARS-CoV-2 nucleic acid and antibody (both immunoglobulin M [IgM] and immunoglobulin G [IgG]) testing were performed on all disembarking passengers at Sanya Airport. Three commercial RT-PCR assays (Daan, Guangzhou; BGI, Wuhan; and Fosun, Shanghai) [12] were deployed on nasopharyngeal swab (NPS) specimens throughout the entire screening period. The Xpert Xpress SARS-CoV-2 assay (Sunnyvale, CA) was implemented on 3 May 2020 using NPS specimens pooled in a 10:1 ratio. NPS was collected by using the Virus Collection and Preservation System (Jiangsu Kangjian Medical Apparatus, Taizhou, China). In brief, 1 flocked swab was used to collect NPS via 2 nostrils and added into 1-mL preservation medium. The mixture was then vortexed and aliquoted. The first aliquot was used for 10:1 pooled testing on site in a real-time manner, wherein 10 individual samples (50 µL each) were pooled and vortexed, and 300 µL of pooled specimen were added into the Xpert cartridge and run on GeneXpert as previously described [13]. The second aliquot was transported to and tested in the Sanya Centers for Disease Control and Prevention by using the Daan SARS-CoV-2 assay according to the manufacturer’s instructions.
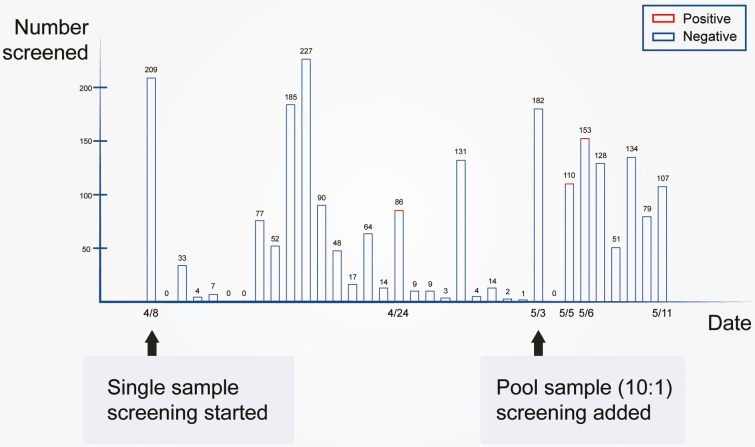
Between 8 April and 11 May 2020, a total of 2230 NPS specimens collected from arriving passengers were tested for SARS-CoV-2 RNA. A total of 944 specimens were tested by using the Xpert assay on 10:1 pooled-specimens (Figure 1), using a total of 117 (95 + 10 + 10 + 2) test cartridges. This specifically saved 827 (87.6%) testing cartridges, approximately 3 hours staff hands-on time and $49 620 in costs. After deconvolution of positive pools in real-time, 2 SARS-CoV-2 RNA-positive passengers were detected in this period. Because the 2 cases were detected before entering the general population, closed-loop management involving immediate quarantine was carried out, resulting in no local contacts. In contrast, before the Xpert assay was implemented, 1286 NPS specimens were collected, and 1 asymptomatic SARS-CoV-2 carrier was detected by the Daan assay on 24 April, but the 36-hour delay in getting results from the central lab resulted in the need for contact tracing of 68 close contacts and 196 others with some level of contact (Figure 1).
Figure 1.
Time distribution of 2230 passengers screened for SARS-CoV-2 in NPS specimens between 8 April and 11 May 2020 in Sanya Airport, Hainan, China. Abbreviations: NPS, nasopharyngeal swab; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Case 1 detected by Xpert pooled testing was a 27-year old female Wuhan resident who took a 1-stop flight from Wuhan to Sanya via Liuzhou; she was in the transfer terminal for 1 hour. She arrived at Sanya Airport at 12:19pm on 5 May 2020 together with 28 other passengers. She tested positive for IgG antibody against SARS-CoV-2 (initially detected by up-conversion luminescence of Rejing, Beijing, and confirmed by chemilluminescence of Boaosaisi, Tianjin). Her NPS was positive for SARS-CoV-2 RNA by Xpert in a 10:1 specimen pool (Ct value, 43.8), and her single specimen yielded a value consistent with this (Ct value, 40.6). The other NP swab aliquot tested positive by the Daan assay at Sanya CDC laboratory 1 day later on 6 May. She had lived for the past 6 months with her mother who was infected with COVID-19 on 26 January 26 and was discharged on 29 February with intermittent nucleic acid positivity until 25 April. The passenger denied any SARS-CoV-2-related symptoms and signs, and she tested negative for SARS-CoV-2 nucleic acids and antibodies on 30 April as part of trip preparation. She and all 28 passengers were put in isolation at a nearby hotel.
The other case detected by Xpert assay was a 30-year old male Wuhan resident who took a nonstop flight from Wuhan to Hainan as a tourist. He arrived at Sanya Airport at 12:30pm on 6 May 2020. He also tested positive for IgG antibody against SARS-CoV-2 (method as in case 1). His NPS was positive for SARS-CoV-2 RNA in an Xpert 10:1 specimen pool (Ct value, 43.9), and his individual specimen testing yielded a Ct value of 41.6. His second NPS aliquot tested negative by the Daan assay at Sanya CDC laboratory on 7 May but was found to be positive by 2 other commercial assays (BGI, Shenzhen, and Fosun, Shanghai) on 8 May. He had no any SARS-CoV-2-related symptoms and signs and was put in isolation at a nearby hotel.
Successful implementation of specimen pooling and real-time screening strategies for asymptomatic COVID-19 infection will depend on test methods comprising high sensitivity; the Xpert Xpress SARS-CoV-2 test displays better sensitivities than several other assays that can be run on-demand [14–16]. The data generated in this study suggested the sensitivity of the Xpert assay done on 10:1 pooled-specimens is equal to or better than the commercial Daan assay done on single specimens. Second, the Xpert assay can be operated at the point of screening and generates test results within 1 hour; because the vast majority (93/95) of pools tested was negative, most passengers could be released immediately. Retesting of the 2 positive pools took an additional cycle of testing but could be performed immediately on the 16-module instrument. Performance of the pooled testing strategy was surprising good, which may relate in part to collection of NPS specimens using 1 flocked swab from both nostrils and adding it to 1-mL preservation medium that provided RNA stability and higher concentration than conventional transport media [11].
In conclusion, we found that a 10:1 pooled test strategy could be successfully implemented on-site for SARS-CoV-2 detection. The advantages included increased test throughput, limited use of reagents, increased overall testing efficiency without significant loss of sensitivity, and the potential to limit the need for contact tracing if results can be delivered before reentry in the general population.
Although promising, our study is limited in that it was performed at a single airport with limited positive findings. This pooling sample strategy may not be appropriate in settings where high frequencies of symptomatic patients and asymptomatic carriers exist.
Notes
Acknowledgments. The authors thank Yujun Fu, Shuai Yu, Dian Cao, Caihong Wang, Jiaqi Li, Youhui Li, Yihan Wang, Ge Gao, Eugene Peng, Jane Ren, and David Wen for their excellent assistance.
Financial support. This work was supported by the Science and Technology Development and Strategy grant from the Chinese Academy of Engineering (19-HN-XZ-10), the Medical Innovation grant from the Sanya Government (2020YW01), and Investigator Initiated Study grants (Cepheid-IIS-2020–0007 and Cepheid-IIS-2020–0011) to H. L.
Potential conflicts of interest. D. H. P. and Y. W. T. are employees of Cepheid, the commercial manufacturer of the Xpert® Xpress SARS-CoV-2 test. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Li Q, Guan X, Wu P, et al. . Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bai Y, Yao L, Wei T, et al. . Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020; 323:1406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu Z, Song C, Xu C, et al. . Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci 2020; 63:706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tamashiro H, Maskill W, Emmanuel J, Fauquex A, Sato P, Heymann D. Reducing the cost of HIV antibody testing. Lancet 1993; 342:87–90. [DOI] [PubMed] [Google Scholar]
- 5. Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am J Clin Pathol 2020; 153:715–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hogan CA, Sahoo MK, Pinsky BA. Sample pooling as a strategy to detect community transmission of SARS-CoV-2. JAMA 2020; 323:1967–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yelin I, Aharony N, Shaer Tamar E, et al. . Evaluation of COVID-19 RT-qPCR test in multi-sample pools. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torres I, Albert E, Navarro D. Pooling of nasopharyngeal swab specimens for SARS-CoV-2 detection by RT-PCR. J Med Virol 2020; 5:25971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ben-Ami R, Klochendler A, Seidel M, et al. . Large-scale implementation of pooled RNA extraction and RT-PCR for SARS-CoV-2 detection. Clin Microbiol Infect 2020; 22:30349–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wacharapluesadee S, Kaewpom T, Ampoot W, et al. . Evaluating the efficiency of specimen pooling for PCR-based detection of COVID-19. J Med Virol 2020; 13:26005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt M, Hoehl S, Berger A, et al. . Novel multiple swab method enables high efficiency in SARS-CoV-2 screenings without loss of sensitivity for screening of a complete population. Transfusion 2020; 6:15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loeffelholz MJ, Tang YW. Laboratory diagnosis of emerging human coronavirus infections: the state of the art. Emerg Microbes Infect 2020; 9:747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loeffelholz MJ, Alland D, Butler-Wu SM, et al. . Multicenter evaluation of the Cepheid Xpert Xpress SARS-CoV-2 test. J Clin Microbiol 2020; 4:00926–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lieberman JA, Pepper G, Naccache SN, Huang ML, Jerome KR, Greninger AL. Comparison of commercially available and laboratory developed assays for in vitro detection of SARS-CoV-2 in clinical laboratories. J Clin Microbiol 2020; 29:00821–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moran A, Beavis KG, Matushek SM, et al. . The detection of SARS-CoV-2 using the Cepheid Xpert Xpress SARS-CoV-2 and Roche cobas SARS-CoV-2 assays. J Clin Microbiol 2020; 17:00772–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhen W, Smith E, Manji R, Schron D, Berry GJ. Clinical evaluation of three sample-to-answer platforms for the detection of SARS-CoV-2. J Clin Microbiol 2020; 24:00783–20. [DOI] [PMC free article] [PubMed] [Google Scholar]