Abstract
Background
HER2 overexpression has been investigated as a potential biomarker and therapeutic target in biliary tract cancer (BTC), but a prognostic role of such alteration has not been demonstrated yet.
Materials and Methods
We retrospectively evaluated HER2 protein expression by immunohistochemistry (IHC) in 100 patients with radically resected BTC. HER2 gene amplification was assessed by fluorescence in situ hybridization (FISH) in 2+ and 3+ cases at IHC. High HER2 protein expression was defined as either IHC 3+ or 2+ associated with FISH positivity. The primary objective of the study was to evaluate the prognostic role of HER2 overexpression in terms of disease‐free survival (DFS) and overall survival (OS). Secondary endpoints were the prevalence of HER2 overexpression and the possible correlation with other clinicopathological features.
Results
HER2 overexpression was identified in 11 patients and was not related to other clinicopathological factors. DFS was significantly shorter in HER2‐positive compared with HER2‐negative patients (10.6 vs. 20.9 months, log‐rank p = .017). HER2 confirmed its prognostic value for DFS at multivariate analysis (hazard ratio 2.512; 95% confidence interval, 1.232–5.125; p = .011) together with nodal stage (p < .001), resection margin (p = .027), and tumor site (p = .030). There was no difference in OS between HER2‐positive and ‐negative patients (p = .068).
Conclusion
HER2 overexpression represents an independent prognostic factor for disease recurrence in patients with BTC treated with potentially curative surgery.
Implications for Practice
HER2 overexpression may play an independent role in promoting an aggressive behavior in resectable biliary tract cancer. This evidence could be helpful in improving prognostic stratification after resection and, primarily, should endorse the rationale to investigate HER2 as a therapeutic target in biliary tract cancer.
Keywords: Biliary tract cancer, HER2, Prognosis, Cholangiocarcinoma, Gallbladder cancer
Short abstract
Studies suggest that the HER2 pathway could play a role in the development and growth of biliary tract cancer. This study investigated the incidence and effect of HER2 expression on the risk of recurrence in a large series of resected biliary tract cancer cases at a single Institution.
Introduction
Biliary tract cancer (BTC) represents an infrequent and heterogeneous group of neoplasms characterized by poor prognosis 1. The only curative option consists of surgical resection, even though the disease often presents as locally advanced or metastatic, thus making chemotherapy the only treatment possibility in the majority of patients.
Despite the advances in the knowledge about carcinogenesis and molecular alterations characterizing different subtypes of BTC 2, 3, reliable molecular markers of prognosis are lacking. Both in clinical trials and in routine practice, BTC undergoing radical surgery are stratified on the basis of site of origin and several clinicopathological factors (such as nodal involvement, surgical margins, and tumor grade), which demonstrated an impact on prognosis in different series of resected patients 4, 5, 6. Among resected patients, the role of adjuvant treatment has been recently established in randomized studies. A few years ago, a meta‐analysis of 20 trials supported the use of adjuvant treatment, especially in the case of nodal involvement or positive margins after resection 7. Nonetheless, limitations in the design, size, and treatment regimens used for the included studies undermined the results of the metanalysis. Recently, two prospective trials evaluated the role of adjuvant chemotherapy compared with observation in patients with resected BTC: whereas the combination of gemcitabine and oxaliplatin failed to demonstrate a benefit in relapse‐free survival (RFS) 8, capecitabine monotherapy showed to improve both RFS and overall survival (OS) at the price of acceptable toxicity 4.
However, the usefulness of the identified molecular alteration in guiding treatment decisions after resection is unclear.
Among the most investigated biomarkers, the human epidermal growth factor receptor 2 (HER2) is a member of the ErbB family and is involved in tumor proliferation by downstream signaling activation 9. Its overexpression, measured by immunohistochemistry (IHC) and/or fluorescence in situ hybridization (FISH), represents a predictive biomarker for HER2‐targeted agents in different tumor types, such as breast and gastric cancer 10, 11.
Preclinical studies suggest that the HER2 pathway could have a role in the development and growth of BTC: in transgenic mice, overexpression of ErbB‐2 in the basal layer of biliary tract epithelium led to the development of adenocarcinoma 12, 13. In cholangiocarcinoma cell lines, HER2 expression was associated with increased invasiveness, motility, and proliferation through AKT/p70S6K pathway 14.
Different retrospective analyses have been conducted in BTC, reporting a rate of HER2 overexpression or amplification depending on tumor site (more than 19% in extrahepatic and about 5% in intrahepatic cholangiocarcinoma) 15 and etiology (10.4% in Fluke‐positive and 2.7% in Fluke‐negative cholangiocarcinoma) 16. However, a prognostic role of such alteration has not been demonstrated yet. In this study, we investigated the incidence and explore the impact of HER2 expression on the risk of recurrence in a large series of BTC cases resected at a single Institution.
Materials and Methods
Patients
We retrospectively collected a cohort of 100 consecutive patients with radically resected BTC followed at the Pisa University hospital between 2006 and 2015. Inclusion criteria were age > 18 years; diagnosis of intrahepatic or extrahepatic cholangiocarcinoma, gallbladder carcinoma, or ampullary carcinoma; resectable disease at presentation, treated by surgery with curative intent; availability of paraffin‐embedded tumor sample; and access to clinical information, pathological features, and survival data. Data have been collected and analyzed assuring patient anonymization. The analyses included in this study were performed in accordance with the Declaration of Helsinki and were approved by the Ethics Committee of the Pisa University Hospital. Written informed consent from the patients for research use of data was obtained before the investigation.
HER2 protein expression was evaluated by IHC in all samples. HER2 gene amplification was assessed by FISH only in cases judged 2+ and 3+ at IHC. High expression of HER2 protein was defined as either IHC 3+ or IHC 2+ associated with FISH positivity.
HER2 IHC Analysis
Three‐micrometer‐thick tumor sections were stained with HER2/neu (4B5) Rabbit Monoclonal Primary Antibody Pathway (Ventana Medical Systems, Inc., Oro Valley, AZ). Staining was done on an automated IHC/ISH (chromogenic in situ hybridization) slide staining system (BenchMark XT, Ventana Medical Systems, Inc.) using the ultraView DAB Detection Kit (Ventana Medical Systems, Inc.).
HER2 scoring was conducted according to criteria used for gastric cancer 17. The key feature of the scoring criteria was the inclusion of complete or incomplete membrane staining as HER2‐positive if ≥10% of cells from surgical samples were stained. As previously described, HER2 IHC was reported as a score ranging from 0 to 3+. In particular, IHC was defined as follows: IHC 3+ staining is any membranous staining visible at low magnification (×2.5–5). Lateral or U‐shaped membranous staining is typically seen at cell‐cell junctions. Immunohistochemistry 2+ membranous staining is visible at ×10–20 magnification. Immunohistochemistry 1+ staining is visible only with ×40 magnification and should be considered immunohistochemistry negative. No staining is defined as IHC 0.
HER2 FISH Analysis
Immunohistochemistry 2+ and 3+ cases were retested using FISH. The FISH analysis was performed on 4–6‐μm‐thick paraffin sections of tumor tissues using Paraffin Pretreatment Reagent Kit II and PathVysion HER2 DNA Probe Kit II (Abbott Molecular, Abbott Park, IL). Probe, composed of Vysis CEP17 SpectrumGreen and Vysis LSI HER2/neu SpectrumOrange; Abbott Molecular), was used to detect gene amplification involving the HER2 gene as per the manufacturer's instructions. FISH results were expressed as the ratio between the number of copies of the HER2 gene and the number of copies of chromosome 17 within the nucleus counted in at least 20 cancer cells. The definition of FISH positivity was HER2:chromosome 17 ratio of ≥2.0 18.
Statistical Analysis
The primary objective of the study was to evaluate the prognostic role of HER2 overexpression in patients with resected BTC in terms of disease‐free survival (DFS) and OS. Secondary endpoints were the prevalence of HER2 overexpression in resected BTC and the possible correlation with other clinic‐pathological features.
DFS was measured from the date of surgery to the date of disease recurrence or death, whichever occurred first. Patients who were free of recurrence after resection were censored at the date of the last radiological assessment. OS was measured from the date of surgery to the date of death or the last follow‐up visit. DFS and OS were estimated using the Kaplan‐Meier product‐limit method. Median values with corresponding 95% confidence intervals (95% CIs) were reported.
The following factors were included in the analyses for DFS and OS: HER2 status (negative vs. positive), age (≤65 vs. >65 years), gender, Eastern Cooperative Oncology Group (ECOG) performance status (PS) (0–1 vs. >1), tumor location (gallbladder vs. intrahepatic vs. extrahepatic vs. ampullary), T stage (T1 vs. T2 vs. T3–4), N stage (N0 vs. N1/Nx), resection margin (R0 vs. R1/R2), tumor grading (G2 vs. G3), lymphadenectomy at the time of primary tumor resection (yes vs. no), adjuvant chemotherapy (yes vs. no), and adjuvant radiotherapy (yes vs. no).
Correlation between HER2 status and other clinicopathological features was performed by chi‐square test.
Kaplan‐Meier method was used to estimate survival curves that were compared by the use of the log‐rank test. We performed a univariate assessment of the effect of HER2 and the other variables on DFS and OS. Hazard ratios and 95% CIs were calculated with the Cox proportional hazard regression model. A two‐sided p value of ≤.05 was considered significant at univariate analysis. A multivariate analysis was carried out using stepwise Cox proportional hazards regression modeling, stratifying for the stage and setting statistical significance at p ≤ .05 for a two‐sided test. Statistical analyses were carried out using the statistical software package SPSS 19.0 (SPSS, Chicago, IL).
Results
Patient Characteristics
One hundred patients who met the eligibility criteria were included in the study. Baseline characteristics are reported in Table 1.
Table 1.
Patients' characteristics (n = 100)
| Characteristic | All patients (n = 100), n | HER2− (n = 89), n | HER2+ (n = 11), n | p value |
|---|---|---|---|---|
| Sex | .536 | |||
| Male | 55 | 50 | 5 | |
| Female | 45 | 39 | 6 | |
| Age, median (range) | 68 (33–84) | 69 (35–84) | 65 (33–79) | .561 |
| ECOG PS | .854 | |||
| 0 | 55 | 51 | 4 | |
| 1 | 38 | 34 | 4 | |
| 2/3 | 7 | 4 | 3 | |
| Tumor grade | .698 | |||
| G2 | 59 | 52 | 7 | |
| G3 | 36 | 33 | 3 | |
| Gx | 5 | 4 | 1 | |
| Tumor site | .901 | |||
| Intrahepatic | 36 | 32 | 4 | |
| Extrahepatic | 28 | 24 | 4 | |
| Ampullary | 23 | 21 | 2 | |
| Gallbladder | 13 | 12 | 1 | |
| Resection margins | 1.000* | |||
| R0 | 94 | 83 | 11 | |
| R1 | 5 | 5 | 0 | |
| R2 | 1 | 1 | 0 | |
| T stage | .051 | |||
| T1 | 19 | 18 | 1 | |
| T2 | 28 | 24 | 4 | |
| T3 | 49 | 44 | 5 | |
| T4 | 3 | 3 | 0 | |
| Tx | 1 | 0 | 1 | |
| N stage | .379 | |||
| N0 | 39 | 35 | 4 | |
| N1 | 40 | 37 | 3 | |
| Nx | 21 | 17 | 4 | |
| Adjuvant chemotherapy | .201 | |||
| Yes | 50 | 47 | 3 | |
| No | 50 | 42 | 8 | |
| Type of adjuvant treatment | 1.000** | |||
| Gemcitabine | 44 | 41 | 3 | |
| Capecitabine | 4 | 4 | 0 | |
| Other regimens | 2 | 2 | 0 | |
| Adjuvant radiotherapy | .515 | |||
| Yes | 4 | 4 | 0 | |
| No | 96 | 85 | 11 | |
| Type of first‐line chemotherapy | .145a | |||
| Gemcitabine | 12 | 9 | 3 | |
| Gemcitabine + platinum | 22 | 19 | 3 | |
| Fluoropyrimidines | 2 | 2 | 0 | |
| Fluoropyrimidines + platinum | 8 | 8 | 0 | |
| Other regimens | 6 | 6 | 0 | |
| Best supportive care | 12 | 8 | 4 | |
| Not available | 6 | 5 | 1 |
Combination treatment versus monotherapy versus best supportive care.
Radical resection versus R1/R2 Resection.
Gemcitabine versus others.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; G, grade.
HER2 Analyses
High expression of HER2 protein at IHC was recorded in 33 patients (2+ in 24 and 3+ in 9 samples, respectively). In the other 67 patients, IHC score was negative (0 in 50 patients and 1+ in 17 patients; Fig. 1). FISH was performed on 28 of 33 high IHC‐positive samples (five cases were excluded because of inadequate tissue for analysis). Gene amplification was found in 2 out of 22 patients with IHC 2+ (9%) and in 5 out of 6 patients with IHC 3+ (83%). There was a statistically significant correlation between IHC expression 3+ and FISH amplification (p = .0012; supplemental online Table 1).
Figure 1.
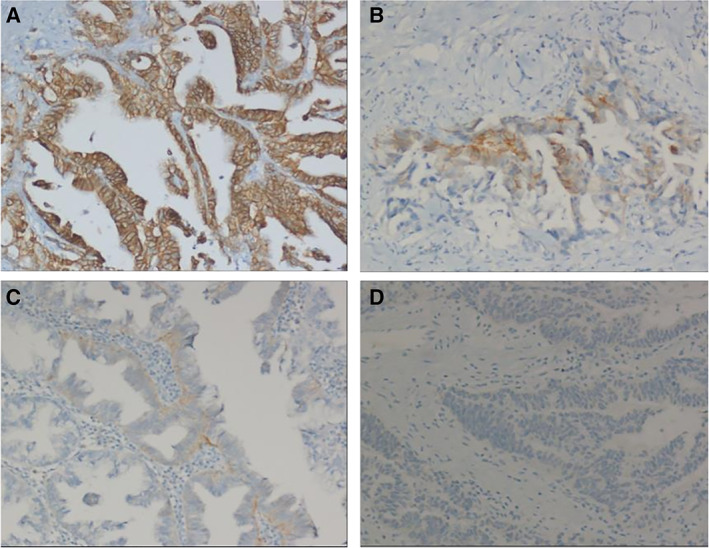
Examples of HER2 immunohistochemistry (IHC) staining. IHC evaluation of HER2 in biliary tract cancer is scored as follows: score 3+ (A), score 2+ (B), score 1+ (C), score 0 (D).
According to combined IHC and FISH results, HER2 overexpression was identified in 11 patients (nine cases with IHC 3+ and two cases with IHC 2+ and FISH amplification).
As reported in Table 1, there was no statistically significant difference in baseline characteristics according to HER2 status.
Survival Analyses
At a median follow‐up of 82.8 months, 68 patients had experienced disease progression and 64 were expired. In the global population, median DFS and OS were 20.5 months (95% CI, 16.01–24.99) and 40.9 months (95% CI, 30.87–50.92), respectively. Data about treatment received after progression are listed in Table 1.
DFS significantly differed according to HER2 status; median DFS was 20.9 months in HER2‐negative and 10.6 months in HER2‐positive patients (log‐rank p = .017; Fig. 2). At univariate analysis, three other parameters resulted significantly associated with DFS: nodal status (p = .0005), ampullary tumor site (p = .009), and resection margin (p = .037). The results of univariate analyses are reported in Table 2.
Figure 2.
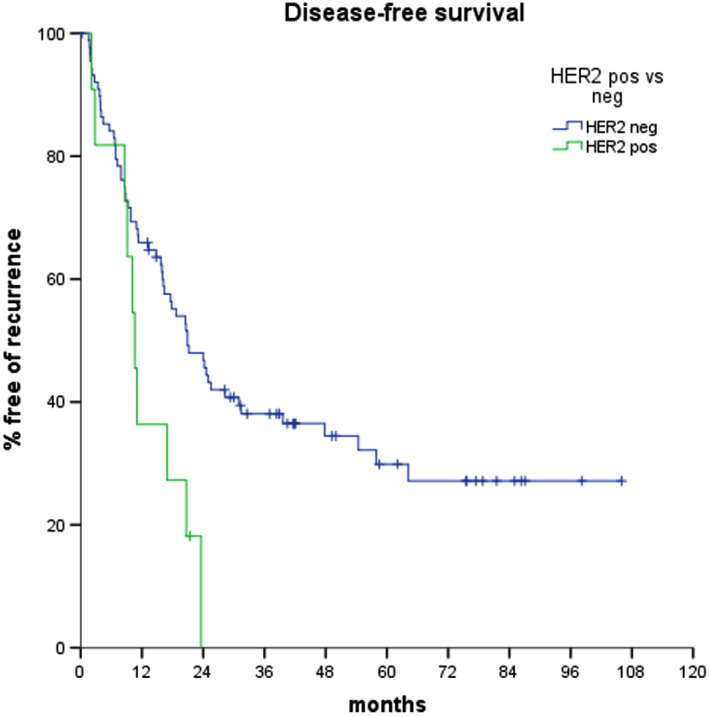
Disease‐free survival curves according to HER2 status.
Table 2.
Univariate analyses for disease‐free survival and overall survival
| Characteristic | Disease‐free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| Median, months | HR (95% CI) | p value | Median, months | HR (95% CI) | p value | |
| Age | ||||||
| >65 years | 20.9 | 1.013 (0.626–1.640) | .957 | 39.7 | 1.288(0.779–2.129) | |
| ≤65 years | 20.5 | 1 | 44.0 | .323 | ||
| Gender | ||||||
| Female | 16.4 | 1.161 (0.720–1.874) | .540 | 43.4 | 1.097 (0.857–1.405) | |
| Male | 23.6 | 1 | 29.1 | .463 | ||
| ECOG PS | ||||||
| 0/1 | 20.7 | 0.561 (0.224–1.402) | .216 | 42.7 | 0.349 (0.149–0.817) | |
| >1 | 8.6 | 1 | 18.0 | .015 | ||
| Tumor site | ||||||
| Intrahepatic | 11.2 | 1 | .063 | 42.7 | 1 | .008 |
| Ampullary | 64.2 18.7 | 0.379 (0.182–0.786) | .009 | Not reached | 0.353 (0.194–0.643) | .001 |
| Gallbladder | 13.3 | 0.837 (0.380–1.846) | .660 | 18.0 | 1.693 (1.004–2.856) | .048 |
| Extrahepatic | 18.7 | 0.931 (0.532–1.626) | .801 | 29.0 | 1.357 (0.892–2.064) | .154 |
| T stage | ||||||
| T1 | 31.4 | 0.527 (0.264–1.054) | .079 | Not reached | 0.463 (0.261–0.996) | .101 |
| T2 | 23.6 | 0.600 (0.337–1.069) | .070 | 43.4 | 0.690 (0.389–1.223) | .051 |
| T3–4 | 16.2 | 1 | .083 | 30.4 | 1 | .204 |
| Nodal stage | ||||||
| N0 | 57.9 | 0.387 (0.227–0.661) | 75.3 | 0.417 (0.241–0.747) | ||
| N1/Nx | 15.7 | 1 | .0005 | 30.4 | 1 | .002 |
| Resection margins | ||||||
| R0 | 20.6 | 1 | 42.0 | 1 | ||
| R1/R2 | 3.7 | 2.674 (1.059–6.753) | .037 | 14.1 | 3.135 (1.339–7.339) | .008 |
| Grading | ||||||
| G2 | 23.6 | 0.847 (0.662–1.084) | 43.4 | 0.879 (0.680–1.136) | ||
| G3 | 13.3 | 1 | .188 | 30.4 | 1 | .324 |
| Lymphadenectomy | ||||||
| No | 24.6 | 1.074 (0.810–1.426) | 31.0 | 1.358 (1.031–1.790) | ||
| Yes | 20.5 | 1 | .619 | 43.4 | 1 | .029 |
| Adjuvant CT | ||||||
| No | 15.7 | 1.113 (0.876–1.415) | 37.4 | 1.178 (0.920–1.510) | ||
| Yes | 20.7 | 1 | .382 | 51.2 | 1 | .194 |
| Adjuvant RT | ||||||
| No | 17.8 | 1.495 (0.739–3.023) | 39.5 | 1.415 (0.699–2.864) | ||
| Yes | 28.3 | 1 | .263 | 51.2 | 1 | .334 |
| HER2 | ||||||
| Negative | 20.9 | 1 | 42.7 | 1 | ||
| Positive | 10.6 | 2.261 (1.134–4.507) | .020 | 27.9 | 1.861 (0.945–3.665) | .073 |
Abbreviations: HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; G, grade; CT, chemotherapy; RT, radiotherapy.
A multivariate regression analysis was conducted including these four parameters, and all of them confirmed their independent prognostic value (HER2 overexpression, p = .011; nodal stage, p < .001; resection margin, p = .027; and tumor site, p = .030; Table 3).
Table 3.
Multivariate analyses for disease‐free survival and overall survival
| Disease‐free survival | Overall survival | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| HER2 | ||||
| Negative | 1 | — | — | |
| Positive | 2.512 (1.232–5.125) | .011 | ||
| Nodal stage | ||||
| N0 | 1 | 1 | ||
| N1/Nx | 2.969 (1.705–5.170) | .00012 | 3.516 (1.864–6.633) | .0001 |
| Resection margin | ||||
| R0 | 1 | 1 | ||
| R1/R2 | 2.503 (1.109–5.651) | .027 | 1.826 (0.857–3.892) | .119 |
| Tumor site | ||||
| Intrahepatic | 1 | .030 | 1 | .004 |
| Ampullary | 0.347 (0.165–0.728) | .005 | 0.235 (0.095–0.583) | .002 |
| Gallbladder | 0.882 (0.380–2.045) | .77 | 1.571 (0.716–3.451) | .260 |
| Extrahepatic | 1.017 (0.576–1.794) | .954 | 0.984 (0.504–1.922) | .963 |
| ECOG PS | ||||
| 0/1 | — | — | 1 | |
| >1 | 2.933 (1.140–7.86) | .026 | ||
| Lymphadenectomy | ||||
| No | — | — | 1 | |
| Yes | 0.876 (0.621–1.237) | .453 | ||
ECOG PS and lymphadenectomy were not tested in the multivariate model for disease‐free survival because significance was not reported at univariate analysis (as was for HER2 status in the multivariate model for overall survival).
Abbreviations: —, not performed; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio.
Median OS was 55.2 and 23.4 months in HER2‐negative and ‐positive patients, respectively (log‐rank p = .068; Fig. 3). The following covariates resulted associated with poorer OS: ECOG PS (p = .015), N stage (p = .002), and tumor site (p = .008 for intrahepatic, p = .001 for ampullary, and p = .048 for gallbladder tumors, respectively; Table 2). In particular, the median OS was not reached in ampullary tumors, 55.3 months in intrahepatic tumors, 34.7 months in extrahepatic tumors, and 18.1 months in gallbladder tumors.
Figure 3.
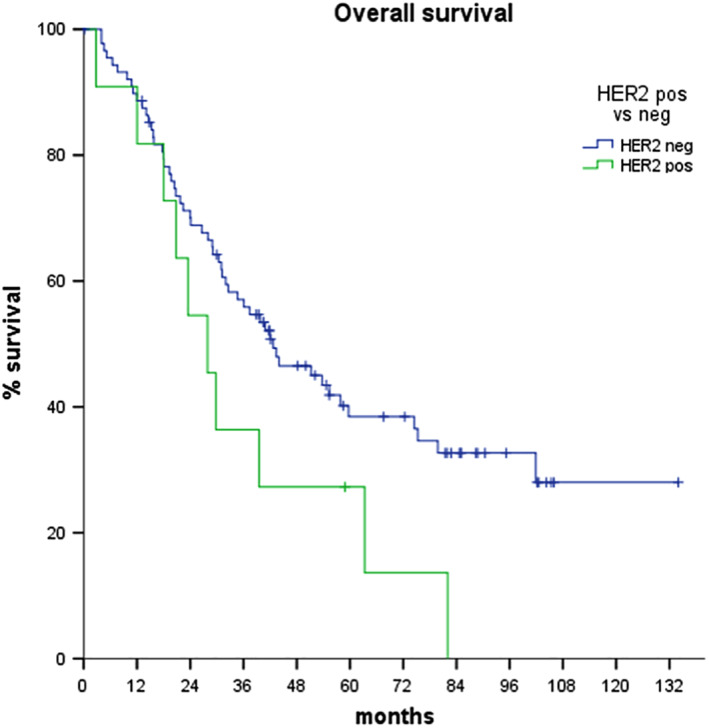
Overall survival curves according to HER2 status.
In the OS multivariate model, tumor site (p = .005) and nodal stage (p = .004) confirmed their prognostic role (Table 3).
Also excluding ampullary tumors the difference in DFS was statistically significant (median DFS of 10.1 months for HER2 positives vs. 17.8 months for HER2 negatives, p = .040), whereas the difference in OS remained not statistically significant (median OS 23.5 months vs. 36.1 for HER2 positives vs. negatives, respectively, p = .241).
Tumor Characteristics and Association with HER2 Status
HER2 status was not correlated with any of the clinicopathological features analyzed. In particular, HER2 overexpression was not associated with tumor site (14.3% of extrahepatic, 11.1% of intrahepatic, 8.7% of ampullary, and 7.7% of gallbladder tumors were HER2 positive: p = .9), tumor grade (p = .737), T stage (p = .713), or N stage (p = .712). In particular, HER2 overexpression was 8% in stage II cases, 0% in stage III cases, and 11% in stage IV cases (p = .717).
Discussion
BTC represents a group of rare cancers with a dismal prognosis both in localized and distant metastatic cases 19. Chemotherapy has shown to minimally improve outcome in this disease 20. For this reason, molecular characterization is becoming crucial for the identification of potential therapeutic targets and more accurate patient stratification beyond conventional clinicopathological features. Previous studies described the incidence of HER2 alterations in different cohorts of BTC, but the clinical implication of such alteration has not been clarified 21, 22.
Among 100 cases of resected BTC, we reported HER overexpression (defined as score 3+ by IHC or score 2+ confirmed by FISH amplification) in 11% of cases: this rate was in line with that reported by other authors 15. Moreover, in line with previous reports, in our cohort, the incidence of HER2 overexpression did not differ between tumor sites (p = .9) or with other clinical and pathologic factors 15. As described, we found a strong correlation between IHC expression and amplification (p = .0012) 23. These data confirm that a subset of BTC cases is defined by HER2 overexpression and that both IHC and FISH are reliable techniques for HER2 assessment in this setting, as already observed in other tumor types 17, 24.
Although previous studies had suggested a putative prognostic role for other members of the ErbB family in BTC such as EGFR and HER3 25, 26, data regarding the prognostic role of HER2 overexpression were not conclusive. To our knowledge, this is the first report describing that HER2 expression is significantly associated with prognosis in resected BTC. In particular, the prognostic role of HER2 overexpression for DFS emerged in univariate analysis and was confirmed in multivariate analysis. In our series, HER2‐positive patients experienced disease progression after a median of 10.6 months from surgery, whereas DFS was almost twice as high among HER2‐negative cases (median, 20.9 months). Other known prognostic determinants such as nodal status, resection margin, and tumor site were confirmed as factors influencing disease recurrence in resected patients, thus making our results more robust in terms of external validity 27. Although the number of patients in the HER2‐positive group is small, the DFS prognostic significance of HER2 is confirmed by descriptive statistics that show no difference in terms of other baseline characteristics according to the HER2 status.
Although a substantial difference in median OS between HER2‐positive and ‐negative patients was observed (23.4 and 55.2 months, respectively), this parameter did not reach statistical significance (p = .068), probably because of heterogeneity in metastatic spreading and treatment administered after relapse. Notably, our study confirmed at the multivariate analysis that PS, nodal stage, and tumor site are associated with OS 28. In particular, ampullary cancer confirmed its better prognosis compared with gallbladder and intrahepatic or extrahepatic cholangiocarcinoma 29.
Although anti‐HER2 agents failed to show activity in treating unselected patients with BTC 30, preliminary reports have shown interesting results in preclinical setting 31 and subsequently in patients with BTC harboring HER2 overexpression. A pivotal case report published by Law et al. in 2012 described the case of a patient with metastatic HER2‐positive BTC treated with paclitaxel and trastuzumab achieving a dramatic disease response 32. A subsequent retrospective analysis described eight cases of BTC treated with anti‐HER2 agents (trastuzumab, lapatinib, and pertuzumab) obtaining promising results in terms of response rate (50%) and duration of response (median 40 weeks) 33. Recently, preliminary data of two basket trials with HER2‐targeted agents (trastuzumab plus pertuzumab and trastuzumab emtansine) confirmed HER2 as a promising therapeutic target in advanced BTC 34, 35.
Although the retrospective nature of our study and the number of analyzed samples represent potential limitations, results reported in terms of DFS and OS according to HER2 status suggest that this biomarker may play a role in promoting a more aggressive behavior in a subset of BTC cases. Because of the exploratory nature of our study, our results should be confirmed in prospective validating cohorts. Intriguingly, as no correlation between HER2 status and the other clinical and pathological parameters has emerged, in future studies all patients with BTC should be screened for HER2 overexpression and could thus potentially benefit from anti‐HER2 therapy. Moving from our data as well as considering the preliminary results achieved with targeted agents in selected BTC cases, prospective studies are warranted in order to improve the results achieved with cytotoxic chemotherapy in first‐line or even in the adjuvant setting.
Conclusion
Our study shows that HER2 expression represents an independent prognostic factor in BTC cases treated with potentially curative surgery. This evidence could help in refining prognostic stratification after resection but, more importantly, should be the rationale to investigate HER2 as a therapeutic target in this challenging clinical situation.
Author Contributions
Conception/design: Caterina Vivaldi, Lorenzo Fornaro, Enrico Vasile
Provision of study material or patients: Caterina Vivaldi, Lorenzo Fornaro, Clara Ugolini, Cristina Niccoli, Gianna Musettini, Irene Pecora, Andrea Cacciato Insilla, Francesca Salani, Giulia Pasquini, Silvia Catanese, Monica Lencioni, Gianluca Masi, Daniela Campani, Gabriella Fontantini, Alfredo Falcone, Enrico Vasile
Collection and/or assembly of data: Caterina Vivaldi, Lorenzo Fornaro, Clara Ugolini, Cristina Niccoli, Gianna Musettini, Irene Pecora, Andrea Cacciato Insilla, Francesca Salani, Giulia Pasquini, Silvia Catanese, Monica Lencioni, Gianluca Masi, Daniela Campani, Gabriella Fontantini, Alfredo Falcone, Enrico Vasile
Data analysis and interpretation: Caterina Vivaldi, Lorenzo Fornaro, Clara Ugolini, Enrico Vasile
Manuscript writing: Caterina Vivaldi, Lorenzo Fornaro, Clara Ugolini, Enrico Vasile
Final approval of manuscript: Caterina Vivaldi, Lorenzo Fornaro, Clara Ugolini, Cristina Niccoli, Gianna Musettini, Irene Pecora, Andrea Cacciato Insilla, Francesca Salani, Giulia Pasquini, Silvia Catanese, Monica Lencioni, Gianluca Masi, Daniela Campani, Gabriella Fontantini, Alfredo Falcone, Enrico Vasile
Disclosures
Caterina Vivaldi: Eli Lilly & Co. (H), Bayer (other—travel expenses); Lorenzo Fornaro: Eli Lilly & Co. (H), Merck Sharp & Dohme (SAB), Gilead Sciences, Merck Sharp & Dohme (RF), Celgene (other—travel expenses); Alfredo Falcone: Bayer, Bristol, Eli Lilly & Co., Merck, Pierre‐Fabre, Roche, Servier (C/A, H), AstraZeneca, Bayer, Bristol, Eli Lilly & Co., Merck, Merck Sharp & Dohme, Novertis, Roche, Sanofi, Servier (RF—institutional).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Table 1 Correlation between IHC and FISH results
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Sohal DPS, Shrotriya S, Abazeed M et al. Molecular characteristics of biliary tract cancer. Crit Rev Oncol Hematol 2016;107:111–118. [DOI] [PubMed] [Google Scholar]
- 3. Valle JW, Lamarca A, Goyal L et al. New horizons for precision medicine in biliary tract cancers. Cancer Discov 2017;7:943–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Primrose JN, Fox R, Palmer DH et al. Adjuvant capecitabine for biliary tract cancer: The BILCAP randomized study. J Clin Oncol 2017;35:4006. [Google Scholar]
- 5. Mavros MN, Economopoulos KP, Alexiou VG et al. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: Systematic review and meta‐analysis. JAMA Surg 2014;149:565–574. [DOI] [PubMed] [Google Scholar]
- 6. Saito H, Noji T, Okamura K et al. A new prognostic scoring system using factors available preoperatively to predict survival after operative resection of perihilar cholangiocarcinoma. Surgery 2016;159:842–851. [DOI] [PubMed] [Google Scholar]
- 7. Horgan AM, Amir E, Walter T et al. Adjuvant therapy in the treatment of biliary tract cancer: A systematic review and meta‐analysis. J Cinical Oncol 2012;30:1934–1940. [DOI] [PubMed] [Google Scholar]
- 8. Edeline J, Bonnetain F, Phelip JM et al. Gemox versus surveillance following surgery of localized biliary tract cancer: Results of the PRODIGE 12‐ACCORD 18 (UNICANCER GI) phase III trial. J Clin Oncol 2017;35:225–225. [Google Scholar]
- 9. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127–137. [DOI] [PubMed] [Google Scholar]
- 10. Slamon DJ, Leyland‐Jones B, Shak S et al. Use of Chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 11. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): A phase 3, open‐label, randomised controlled trial. Lancet 2010;376:687–697. [DOI] [PubMed] [Google Scholar]
- 12. Kiguchi K, Carbajal S, Chan K et al. Constitutive expression of ErbB‐2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res 2001;61:6971–6976. [PubMed] [Google Scholar]
- 13. Pellat A, Vaquero J, Fouassier L. Role of ErbB/HER family of receptor tyrosine kinases in cholangiocyte biology. Hepatology 2017;67:762–773. [DOI] [PubMed] [Google Scholar]
- 14. Treekitkarnmongkol W, Suthiphongchai T. High expression of ErbB2 contributes to cholangiocarcinoma cell invasion and proliferation through AKT/p70S6K. World J Gastroenterol 2010;16:4047–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galdy S, Lamarca A, McNamara MG et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta‐analysis: A potential therapeutic target? Cancer Metastasis Rev 2017;36:141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jusakul A, Cutcutache I, Yong CH et al. Whole‐genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov 2017;7:1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hofmann M, Stoss O, Shi D et al. Assessment of a HER2 scoring system for gastric cancer: Results from a validation study. Histopathology 2008;52:797–805. [DOI] [PubMed] [Google Scholar]
- 18. Rüschoff J, Hanna W, Bilous M et al. HER2 testing in gastric cancer: A practical approach. Mod Pathol 2012;25:637–650. [DOI] [PubMed] [Google Scholar]
- 19. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Valle J, Weigt J, Malfertheiner P et al.; Investigators A‐02 T. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;4:395–397. [DOI] [PubMed] [Google Scholar]
- 21. Yoshikawa D, Ojima H, Iwasaki M et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer 2008;98:418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ata A, Polat A, Serinsöz E et al. Prognostıc value of increased HER2 expression in cancers of pancreas and biliary tree. Pathol Oncol Res 2015;21:831–838. [DOI] [PubMed] [Google Scholar]
- 23. Yang X, Wang W, Wang C et al. Characterization of EGFR family gene aberrations in cholangiocarcinoma. Oncol Rep 2014;32:700–708. [DOI] [PubMed] [Google Scholar]
- 24. Hanna WM, Kwok K. Chromogenic in‐situ hybridization: A viable alternative to fluorescence in‐situ hybridization in the HER2 testing algorithm. Mod Pathol 2006;19:481–487. [DOI] [PubMed] [Google Scholar]
- 25. Yoshikawa D, Ojima H, Iwasaki M et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer 2008;98:418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee HJ, Chung JY, Hewitt SM et al. HER3 overexpression is a prognostic indicator of extrahepatic cholangiocarcinoma. Virchows Arch 2012;461:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spolverato G, Yakoob MY, Kim Y et al. The impact of surgical margin status on long‐term outcome after resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol 2015;22:4020–4028. [DOI] [PubMed] [Google Scholar]
- 28. Ohtsuka M, Ito H, Kimura F et al. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg 2002;89:1525–31. [DOI] [PubMed] [Google Scholar]
- 29. De Groen P, Gores G, LaRusso N et al. Bilary tract cancers. N Engl J Med 1999;341:1368–1378. [DOI] [PubMed] [Google Scholar]
- 30. Peck J, Wei L, Zalupski M et al. HER2/neu may not be an interesting target in biliary cancers: Results of an early phase II study with lapatinib. Oncology 2012;82:175–179. [DOI] [PubMed] [Google Scholar]
- 31. Kawamoto T, Ishige K, Thomas M et al. Overexpression and gene amplification of EGFR, HER2, and HER3 in biliary tract carcinomas, and the possibility for therapy with the HER2‐targeting antibody pertuzumab. J Gastroenterol 2015;50:467–479. [DOI] [PubMed] [Google Scholar]
- 32. Law LY. Dramatic response to trastuzumab and paclitaxel in a patient with human epidermal growth factor receptor 2‐positive metastatic cholangiocarcinoma. J Clin Oncol 2012;30:e271–e273. [DOI] [PubMed] [Google Scholar]
- 33. Javle M, Churi C, Kang HC et al. HER2/neu‐directed therapy for biliary tract cancer. J Hematol Oncol 2015;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Javle MM, Hainsworth JD, Swanton C et al. Pertuzumab + trastuzumab for HER2‐positive metastatic biliary cancer: Preliminary data from MyPathway. J Clin Oncol 2017;35:402–402.27893326 [Google Scholar]
- 35. Li BT. A multi‐histology basket trial of ado‐trastuzumab emtansine in patients with HER2 amplified cancers. J Clin Oncol 2018;36:2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Table 1 Correlation between IHC and FISH results


