Abstract
Lessons Learned
Moxifloxacin plus continuation of the previous treatment of physician's choice shows promising efficacy in patients with metastatic breast cancer.
The addition of moxifloxacin shows well‐tolerated toxicities.
Background
Recent studies have confirmed bacterial infection as an important contributor in cancer. Elimination of tumor‐associated microbes may lead to a reduction in tumors and improved survival. Moxifloxacin is an orally administrated fourth‐generation quinolone with broad‐spectrum coverage against tumor‐associated bacteria.
Methods
In this study, we assessed the efficacy and safety of moxifloxacin in combination with treatment of physician's choice (TPC) in patients with metastatic breast cancer (MBC). In this single‐arm, phase II study, we recruited 30 patients with MBC who had a trend toward disease progression (stable disease [SD] with increased tumor size) during TPC before enrollment at Sun Yat‐sen University Cancer Center between January 1 and July 30, 2018. Eligible patients were given moxifloxacin once daily at a dose of 400 mg from days 1 to 7 of a 28‐day cycle, in addition to continuing to receive the therapy previously selected by their physicians. Tumor response was determined according to RECIST (version 1.1). Progression‐free survival (PFS) was calculated using the Kaplan‐Meier method.
Results
The concomitant use of moxifloxacin and previous TPC yielded a median PFS of 6.6 months (95% confidence interval [CI]: 4.0–9.1) and a 1‐year PFS of 25.9% (95% CI: 10.0%–41.9%). Objective responses were achieved in seven (23.3%, 95% CI: 7.3%–39.4%) patients. The clinical benefit rate was 46.7% (95% CI: 27.7%–65.6%). No grade 4 adverse events (AEs) and four grade 3 AEs were observed, none of which were considered to have definite relation to moxifloxacin.
Conclusion
The combination of moxifloxacin with previous TPC shows promising efficacy and well‐tolerated toxicities in patients with MBC.
Discussion
Breast cancer tissue harbors its own unique microbiota, which contributes to DNA damage, angiogenesis, drug resistance, and tumor progression. Previous research has indicated that antibiotics treatment resulted in reduction of tumor size in vitro and successfully abolished drug resistance; therefore, eliminating tumor‐associated bacteria by using broad‐spectrum antibiotics may be effective and feasible for patients with MBC. Moxifloxacin is a well‐tolerated fourth‐generation quinolone with broad‐spectrum coverage against gram‐positive bacteria, gram‐negative bacteria, atypical microorganisms, and anaerobes; however, there have been no investigations for concomitant use of moxifloxacin and TPC in patients with MBC so far.
In this phase II, single‐arm study, we aimed to evaluate the efficacy and safety of oral moxifloxacin in combination with TPC in MBC patients. The Consolidated Standards of Reporting Trials flow diagram is shown in Figure 1. According to RECIST (version 1.1), “SD with increased tumor size” was defined as “no more than 20% of relative increase or no more than 5 mm of absolute increase in the sum of diameters of target lesions.” We screened patients with MBC who had been receiving TPC and use computed tomography (CT) scans for assessment of objective tumor response evaluation. A total of 30 patients who had SD with increased tumor size in the latest response evaluation by CT scans were included in the study. Baseline characteristics are presented in Table 1. Then patients were given moxifloxacin in addition to previous TPC as the study treatment. The pre‐enrollment data are shown in Figure 2 in blue. The concomitant use of moxifloxacin and TPC yielded a median PFS of 6.6 months (95% CI: 4.0–9.1) and a 1‐year PFS of 25.9% (95% CI: 10.0%–41.9%). Moreover, half of the patients had tumor shrinkage and nearly a quarter (23.3%) had partial response (PR) after the addition of moxifloxacin (Fig. 2, yellow). The overall response rate (ORR) and clinical benefit rate (CBR) of the entire cohort were 23.3% (95% CI: 7.3%–39.4%) and 46.7% (95% CI: 27.7%–65.6%), respectively. In addition, moxifloxacin plus TPC showed well‐tolerated toxicities. No grade 4 AEs were observed. The most commonly reported AE was fatigue (36.7%).
Figure 1.
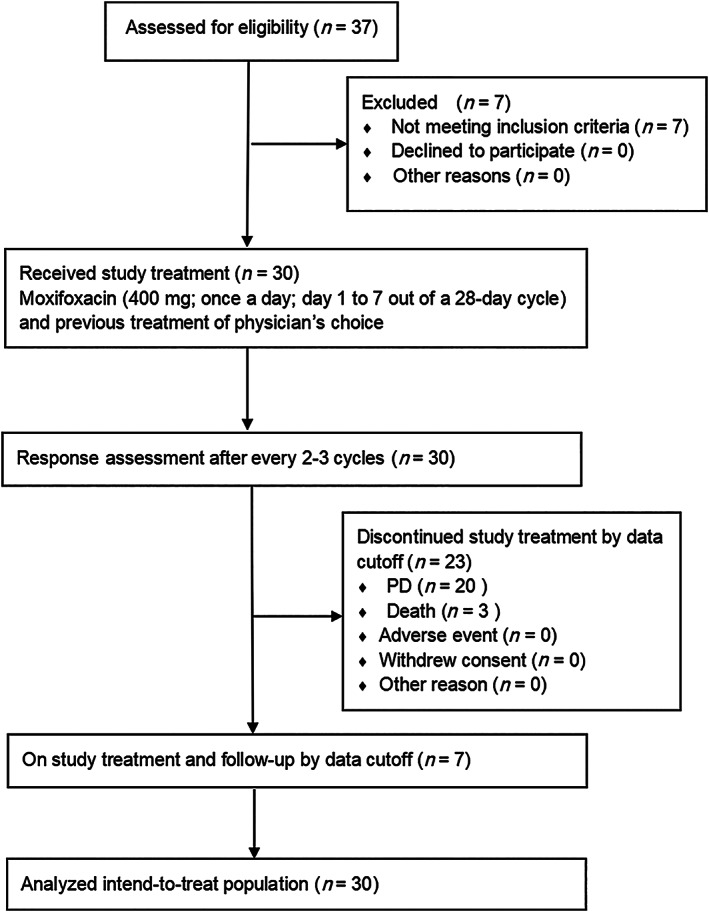
Consolidated Standards of Reporting Trials flow diagram.
Abbreviation: PD, progressive disease.
Figure 2.
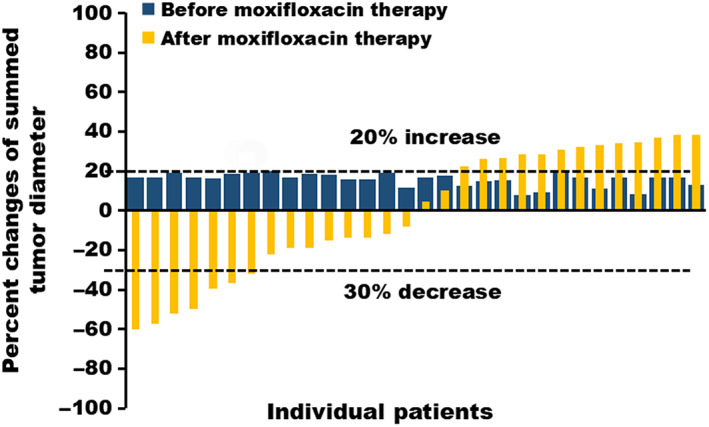
Waterfall plot of the percentage change in tumor size for each patient. The postenrollment data are shown in yellow, indicating the best percentage change from baseline in tumor size for each patient under moxifloxacin plus previous treatment of physician's choice (TPC). The pre‐enrollment data are shown in blue, indicating that patients exhibited stable disease with increased tumor size under TPC only.
In conclusion, our results suggested that moxifloxacin in combination with TPC might help postpone disease progression and prolong survival in patients with MBC. Future direction for this work should include initiating randomized clinical trials with a larger sample size to validate results from the current study.
Trial Information
| Disease | Breast cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | No designated number of regimens |
| Type of Study | Phase II, single arm |
| Primary Endpoint | Progression‐free survival |
| Secondary Endpoints | Overall response rate, clinical benefit rate |
| Additional Details of Endpoints or Study Design | |
|
All enrolled patients who received at least two cycles of moxifloxacin in combination with their ongoing treatment (intention‐to‐treat population) were included for efficacy and safety analysis. Tumor assessments were conducted with CT scans at baseline, after every 2–3 cycles of study medication until a 6‐month visit, and every 2 months thereafter until 12 months. Tumor response to treatment was determined according to RECIST (version 1.1). All CT scans of enrolled patients were evaluated independently by two experienced radiologists. Safety evaluation and survival follow‐up, including vital signs, physical examinations, and laboratory tests, were performed every month until the 6‐month visit, then every 2 months until the 12‐month visit. Continuous variables were described using mean and standard deviation or median and interquartile range when appropriate. Categorical variables were described using frequency and percentage. The primary endpoint of the present study was PFS, defined as the duration from the date of administration of moxifloxacin to the date of progressive disease (PD), death from any cause, or the last follow‐up, whichever came first. Secondary endpoints were ORR and CBR. ORR was defined as the proportion of patients with a complete response (CR) and PR. CBR was defined as the proportion of patients with CR, PR, and SD for at least 6 months. PFS was calculated using the Kaplan‐Meier method. The statistical analysis was performed using SPSS version 22.0 software (SPSS A, Inc., Chicago, IL). | |
| Investigator's Analysis | Active and should be pursued further |
Drug Information
| Generic/Working Name | Moxifloxacin |
| Trade Name | Avelox |
| Company Name | Bayer |
| Drug Type | Small molecule |
| Drug Class | Antibiotics |
| Dose | 400 mg per flat dose |
| Route | p.o. |
| Schedule of Administration | Once daily at a dose of 400 mg from days 1 to 7 of a 28‐day cycle, in addition to continuing to receive therapy previously selected by their physicians. |
Patient Characteristics
| Number of Patients, Male | 0 |
| Number of Patients, Female | 30 |
| Stage | IV |
| Age | Median (range): 53.5 (29.0–72.0) years |
| Number of Prior Systemic Therapies | Median (range): 1 (0–7) |
| Performance Status: ECOG |
0 — 0 1 — 17 2 — 13 3 — 0 Unknown — 0 |
| Cancer Types or Histologic Subtypes | Luminal A, 7; Luminal B (HER2 negative), 12; Luminal B (HER2 positive), 1; HER2 enriched, 6; Triple negative, 4 |
Primary Assessment Method
| Title | New assessment |
| Number of Patients Screened | 37 |
| Number of Patients Enrolled | 30 |
| Number of Patients Evaluable for Toxicity | 30 |
| Number of Patients Evaluated for Efficacy | 30 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 7 (23.3%) |
| Response Assessment SD | n = 11 (36.7%) |
| Response Assessment PD | n = 12 (40%) |
| Response Assessment OTHER | n = 0 (0%) |
| (Median) Duration Assessments PFS | 6.6 months, CI: 4.0–9.1 |
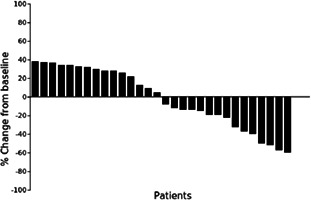
Waterfall plot showing the percentage of tumor diameter change for each patient at time of best response.
Kaplan‐Meier Time Units, Months
| Time of scheduled assessment and/or time of event | No. progressed (or deaths) | No. censored | Percent at start of evaluation period | Kaplan‐Meier % | No. at next evaluation/No. at risk |
|---|---|---|---|---|---|
| 0.87 | 01 | 0 | 100.00 | 96.67 | 29 |
| 2.90 | 01 | 0 | 96.67 | 93.33 | 28 |
| 2.97 | 01 | 0 | 93.33 | 90.00 | 27 |
| 3.07 | 01 | 0 | 90.00 | 86.67 | 26 |
| 3.30 | 01 | 0 | 86.67 | 83.33 | 25 |
| 3.50 | 01 | 0 | 83.33 | 80.00 | 24 |
| 3.57 | 01 | 0 | 80.00 | 76.67 | 23 |
| 3.70 | 01 | 0 | 76.67 | 73.33 | 22 |
| 3.73 | 01 | 0 | 73.33 | 70.00 | 21 |
| 4.17 | 01 | 0 | 70.00 | 66.67 | 20 |
| 4.30 | 01 | 0 | 66.67 | 63.33 | 19 |
| 5.40 | 01 | 0 | 63.33 | 60.00 | 18 |
| 5.60 | 01 | 0 | 60.00 | 56.67 | 17 |
| 6.30 | 01 | 0 | 56.67 | 53.33 | 16 |
| 6.57 | 01 | 0 | 53.33 | 50.00 | 15 |
| 7.33 | 01 | 0 | 50.00 | 46.67 | 14 |
| 7.50 | 01 | 0 | 46.67 | 43.33 | 13 |
| 8.53 | 01 | 0 | 43.33 | 40.00 | 12 |
| 9.40 | 01 | 0 | 40.00 | 36.67 | 11 |
| 9.50 | 01 | 0 | 36.67 | 33.33 | 10 |
| 9.80 | 0 | 0 | 33.33 | 33.33 | 10 |
| 10.27 | 1 | 0 | 33.33 | 30.00 | 9 |
| 10.53 | 1 | 0 | 30.00 | 26.67 | 8 |
| 11.97 | 0 | 0 | 26.67 | 26.67 | 8 |
| 12.30 | 0 | 0 | 26.67 | 26.67 | 8 |
| 14.37 | 0 | 0 | 26.67 | 26.67 | 8 |
| 15.30 | 0 | 0 | 26.67 | 26.67 | 8 |
| 15.87 | 01 | 0 | 26.67 | 23.33 | 7 |
| 16.70 | 0 | 0 | 23.33 | 23.33 | 7 |
| 17.27 | 0 | 0 | 23.33 | 23.33 | 7 |
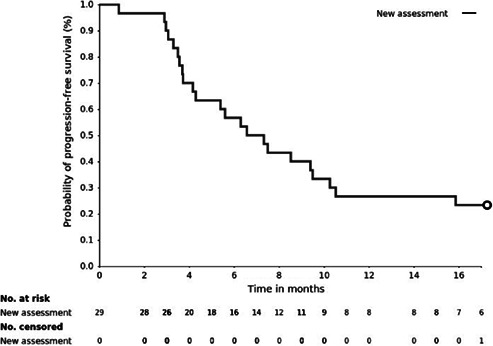
Progression‐free survival was calculated at the cutoff date using Kaplan‐Meier method of the intent‐to‐treat population.
Adverse Events, All Cycles
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
|---|---|---|---|---|---|---|---|
| Febrile neutropenia | 0% | 10% | 50% | 40% | 0% | 0% | 100% |
| Aspartate aminotransferase increased | 0% | 100% | 0% | 0% | 0% | 0% | 100% |
| Fatigue | 0% | 64% | 36% | 0% | 0% | 0% | 100% |
Adverse Events Legend
Toxicities occurring in each patient over the entire duration on study.
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
Despite advances in breast cancer over the past decades, most patients with metastatic breast cancer (MBC) still have poor life expectancy. Clear evidence for treatment recommendations after multiple lines of systemic treatment are limited. It was once believed that breast tissue was an almost sterile site, inaccessible to colonization by microbes. However, growing evidence suggested that breast tissue harbors its own unique microbiome, different from that found at other body sites 1. A variety of bacteria have been found in greater abundances in the breast cancer tissue compared with paired normal tissue, including Methylobacterium 2, Atopobium, Fusobacterium, Gluconacetobacter, Hydrogenophaga, Lactobacillus 3, Bacillus, Enterobacteriaceae, Staphylococcus 1, Haemophilus influenzae, and Streptococcus pyogenes 4. Previous studies suggested that bacteria are involved in tumor initiation, metastasis, and treatment response 5, 6. Recent studies have confirmed onco‐microbiota as an important contributor in cancer. For example, the abundance of F. nucleatum in colorectal cancer is abnormally elevated in cancer tissues as compared with normal adjacent tissues 7, 8, 9. In addition, Escherichia coli and Staphylococcus epidermidis cultured from tumor tissue in patients with breast cancer have been shown to induce DNA double‐stranded breaks in vitro 1. Moreover, quorum sensing peptides from Bacillus subtilis, Streptococcus mitis, and E. coli can promote tumor cell invasion and angiogenesis, thereby potentially leading to metastasis 10. Also, microbial dysbiosis could promote progression of pre‐existing cancer by triggering tumor‐elicited inflammation 11. Another possible contribution made by tumor‐associated bacteria is drug resistance. A previous study has found that bacteria could actively modify the efficacy of 16 out of 30 chemotherapeutic agents 11.
Currently, the causative role of Helicobacter pylori in gastric carcinoma is well established 12, 13. However, no specific bacterium has been found to be a carcinogen of breast cancer. In previous studies, an increase in the abundance of Blautia species, Clostridiaceae, Faecalibacterium and Ruminococcaceae in gut, and Methylobacterium, Atopobium, Fusobacterium, Gluconacetobacter, Hydrogenophaga, Lactobacillus, Bacillus, Enterobacteriaceae, Staphylococcus, H. influenzae, and S. pyogenes in tumor tissues were observed in patients with breast cancer, suggesting that a variety of bacteria may be involved in breast cancer progression 1, 2, 3, 4, 14, 15.
Elimination of tumor‐associated microbes by antibiotic treatment has demonstrated a dramatic reduction in tumor size and improved survival in animals 6, 16, 17. Bacterial enzyme cytidine deaminase was shown to mediate tumor resistance to gemcitabine in pancreatic carcinoma. Antibiotic treatment successfully abolished resistance and restored tumor sensitivity to agents 16. These results suggested that tumor‐associated bacteria could be a promising therapeutic target in patients with metastatic disease. A recent clinical study showed that short‐term preoperative treatment with oral doxycycline resulted in a reduction of cancer stem cells in patients with early breast cancer 18. Therefore, eliminating tumor‐associated bacteria by using broad‐spectrum antibiotics may be effective and feasible for patients with MBC.
Moxifloxacin is a fourth‐generation quinolone with broad‐spectrum coverage against gram‐positive bacteria, gram‐negative bacteria, atypical microorganisms, and anaerobes, which may effectively eliminate abnormally elevated bacteria in patients with MBC.
Although moxifloxacin itself has no direct antitumor effect, it may exert antitumor effects by eradicating tumor‐associated bacteria. Moreover, this new therapeutic strategy takes advantage of mild adverse reactions of moxifloxacin, as it has been used safely for long‐term treatment up to 6 months in patients with tuberculosis 19. Duration of moxifloxacin treatment varies from 7 to 21 days in different clinical settings 20. However, European guidelines recommend that the duration of antibacterial treatment should generally not exceed 8 days 21. Treatment guidelines from the American Thoracic Society and the Infectious Diseases Society of America recommend 5–7 days of moxifloxacin for patients with community‐acquired pneumonia 22. Based on the information above, we implemented a 7‐day use of moxifloxacin out of a 28‐day cycle in the present study.
In addition, not a lot of experience exists with the major inclusion criteria of the present study. When patients experience tumor enlargement, regardless of whether it meets the criteria for disease progression, it is likely that acquired resistance may have occurred and therefore result in treatment failure. However, National Comprehensive Cancer Network guidelines recommend that the regimens be changed only if progressive disease or unacceptable toxicity occurs. Therefore, out of ethical considerations, we determined to include patients who experienced stable disease with increased tumor size and treated them with moxifloxacin combined with ongoing treatment of physician's choice (TPC) in order to observe efficacy without compromising their benefits. In addition, treating breast cancer by the use of antibiotics is an emerging area and has never been reported in the metastatic setting. We adopted this inclusion standard for safety concerns as well.
We also observed tumor shrinkage after administration of moxifloxacin in all other subtypes except triple‐negative breast cancer (n = 4). Additionally, patients with hormone receptor (HR)‐positive/human epidermal growth receptor 2 (HER2)‐negative and HER2‐enriched subtypes were the majority of those who benefited from moxifloxacin. These results suggested that moxifloxacin may be more effective for HR‐ and HER2‐positive patients, which needs further validation in studies with large cohorts.
Known limitations of the present exploratory study included the modest sample size and the nature of single‐arm design. However, the present pilot study was intended as a precursor to a definitive randomized phase III trial examining the efficacy for TPC combined with or without moxifloxacin at our institute in the near future.
Disclosures
The authors indicated no financial relationships.
Table
Table 1.
Characteristics of 30 patients enrolled in the present study
| Characteristics | No. of cases (%) |
|---|---|
| Total | 30 |
| Age at diagnosis, median (range), years | 53.5 (29.0–72.0) |
| Molecular subtype | |
| Luminal A | 7 (23.3) |
| Luminal B (HER2 negative) | 12 (40.0) |
| Luminal B (HER2 positive) | 1 (3.3) |
| HER2‐enriched | 6 (20.0) |
| Triple negative | 4 (13.3) |
| Metastases at diagnosis | |
| No | 24 (80) |
| Yes | 6 (20.0) |
| Lines of prior therapy | |
| 0 | 6 (20.0) |
| 1 | 11 (36.7) |
| 2 | 5 (16.7) |
| 3–7 | 8 (26.7) |
| Therapy of physician's choice | |
| Chemotherapy | 23 (76.7) |
| Endocrine therapy | 17 (56.7) |
| Anti‐HER2 therapy | 7 (23.3) |
Abbreviation: HER2, human epidermal growth receptor 2.
Acknowledgments
We are grateful to the patients and their families for all their help in enabling the successful completion of this study. We thank Yongyi Zhong for her contributions to data management and quality control.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT03405168
- Sponsor: None
- Principal Investigator: Zhongyu Yuan
- IRB Approved: Yes
Contributor Information
Xiwen Bi, Email: bixw@sysucc.org.cn.
Zhongyu Yuan, Email: yuanshy@sysucc.org.cn.
References
- 1. Urbaniak C, Gloor GB, Brackstone M et al. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol 2016;82:5039–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xuan C, Shamonki JM, Chung A et al. Microbial dysbiosis is associated with human breast cancer. PLoS One 2014;9:e83744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hieken TJ, Chen J, Hoskin TL et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci Rep 2016;6:30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thompson KJ, Ingle JN, Tang X et al. A comprehensive analysis of breast cancer microbiota and host gene expression. PLoS One 2017;12:e0188873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grivennikov SI, Wang K, Mucida D et al. Adenoma‐linked barrier defects and microbial products drive IL‐23/IL‐17‐mediated tumour growth. Nature 2012;491:254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bullman S, Pedamallu CS, Sicinska E et al. Analysis of persistence and antibiotic response in colorectal cancer. Science 2017;358:1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fukase K, Kato M, Kikuchi S et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open‐label, randomised controlled trial. Lancet 2008;372:392–397. [DOI] [PubMed] [Google Scholar]
- 8. Castellarin M, Warren RL, Freeman JD et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2012;22:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kostic AD, Gevers D, Pedamallu CS et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 2012;22:292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Spiegeleer B, Verbeke F, D'Hondt M et al. The quorum sensing peptides PhrG, CSP and EDF promote angiogenesis and invasion of breast cancer cells in vitro. PLoS One 2015;10:e0119471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lehouritis P, Cummins J, Stanton M et al. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep 2015;5:14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fukase K, Kato M, Kikuchi S et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open‐label, randomised controlled trial. Lancet 2008;372:392–397. [DOI] [PubMed] [Google Scholar]
- 13. Wong BC, Lam SK, Wong WM et al. Helicobacter pylori eradication to prevent gastric cancer in a high‐risk region of China: A randomized controlled trial. JAMA 2004;291:187–194. [DOI] [PubMed] [Google Scholar]
- 14. Goedert JJ, Jones G, Hua X et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: A population‐based case‐control pilot study. J Natl Cancer Inst. 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bard JM, Luu HT, Dravet F et al. Relationship between intestinal microbiota and clinical characteristics of patients with early stage breast cancer. FASEB J 2015;29(suppl 1):914.2a. [Google Scholar]
- 16. Geller LT, Barzily‐Rokni M, Danino T et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017;357:1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zackular JP, Baxter NT, Iverson KD et al. The gut microbiome modulates colon tumorigenesis. mBio 2013;4:e00692‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scatena C, Roncella M, Di Paolo A et al. Doxycycline, an inhibitor of mitochondrial biogenesis, effectively reduces cancer stem cells (CSCs) in early breast cancer patients: A clinical pilot study. Front Oncol 2018;8:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jindani A, Harrison TS, Nunn AJ et al. High‐dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med 2014;371:1599–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. US Food and Drug Administration . Clinical Review (Avelox). Feb 2016. https://www.fda.gov/media/101211/download. Accessed December 2, 2019.
- 21. Woodhead M, Blasi F, Ewig S et al. Guidelines for the management of adult lower respiratory tract infections–Full version. Clin Microbiol Infect 2011;17(suppl 6):E1–E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mandell LA, Wunderink RG, Anzueto A et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis 2007;44(suppl 2):S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]


