Abstract
Background
Bevacizumab treatment is subject to large interpatient variability in efficacy, which may partly be explained by differences in complex bevacizumab pharmacokinetic characteristics that influence bevacizumab exposure. Exposure–response relationships have been identified for other monoclonal antibodies. We aimed to identify possible exposure–survival relationships in bevacizumab‐treated patients with metastatic colorectal cancer (mCRC).
Materials and Methods
Patients with mCRC who started first‐line bevacizumab‐based chemotherapy between July 2012 and July 2014, and from whom serial blood samples and survival were prospectively collected, were included. Follow‐up was carried out until July 2018. Total bevacizumab trough concentrations were measured from cycle 2 to cycle 30 of treatment. The receiver operating characteristic (ROC) curve analysis and Cox analysis were used to identify the relationship between concentrations and overall survival (OS). In addition, OS was compared between different trough concentration groups.
Results
One hundred fifty‐seven blood samples from 46 patients were evaluable for analyses. ROC analysis showed a clear separation in survival based on trough levels (area under the curve = 0.739, p = .009). Cox regression also showed a strong positive correlation between trough levels and survival (p = .0004). Three distinct groups of exposure were identified: low (median trough concentration [Ctm] ≤41.9 mg/L); medium (Ctm 43–87.2 mg/L) with median OS of 12.8 and 36 months, respectively (p = .0003); and high (Ctm ≥7.9 mg/L), where the majority of patients were still alive 60 months after the initiation of treatment.
Conclusion
This study shows that survival was proportional to the magnitude of exposure in patients with mCRC. Further clinical research should focus on clarifying these exposure–outcome relationships in order to optimize dosing.
Implications for Practice
Bevacizumab‐based chemotherapy is standard first‐line treatment in metastatic colorectal cancer. Moreover, bevacizumab presents complicated pharmacokinetics, and in many cases, clinical outcomes can be highly variable, with some patients responding remarkably well and others not. This study's results show that patients who experienced longer overall survival also had significantly higher exposure to bevacizumab. Therefore, bevacizumab trough concentrations could be used both as a predictive biomarker and as a tool for treatment monitoring and optimization. Finally, the development of validated, rapid, and sensitive assays for bevacizumab concentration measurements in combination with these results may lead to a therapeutic drug monitoring–guided approach in bevacizumab treatment with better clinical outcomes.
Keywords: Therapeutic drug monitoring, Colorectal cancer, Survival, Bevacizumab, Predictive biomarkers
Short abstract
Bevacizumab concentrations as a predictive biomarker for clinical outcomes could represent an advance in therapeutics. This study investigated the relationship between bevacizumab exposure and overall survival in metastatic colorectal cancer.
Introduction
Colorectal cancer (CRC) is the second most commonly diagnosed cancer in women and third most common in men 1. It is estimated that approximately 20% of newly diagnosed patients have distant metastatic disease at the time of presentation 2. Major advances in the treatment of metastatic colorectal cancer (mCRC) have been made in the last 15 years. When fluorouracil was the only available active agent, overall survival (OS) was approximately 1 year. However, the average median survival duration is now 3 years, and 5‐year survival rates are as high as 20%. These improvements have been mainly driven by the availability of new active agents, which include conventional cytotoxic agents such as irinotecan and oxaliplatin, and biologic agents targeting angiogenesis and the epidermal growth factor receptor (EGFR) 3.
Bevacizumab, a recombinant humanized immunoglobulin G (IgG)1 monoclonal antibody, is the first approved antiangiogenetic agent for the treatment of mCRC 4. The mechanism of action of bevacizumab includes binding to circulating vascular endothelial growth factor A (VEGF‐A) and blocking of VEGF‐A binding to its receptors (VEGFR‐1 and VEGFR‐2) on the surface of endothelial cells, which results in the inhibition of tumor angiogenesis, growth, and metastases 5. In the absence of validated predictive factors for treatment benefit, the default position in the management of mCRC had been to add bevacizumab to the chemotherapy backbone chosen for the individual patient, particularly for patients with RAS mutated tumors, for whom an anti‐EGFR agent is contraindicated 6. Benefits from bevacizumab are often questioned and clinical outcomes are highly variable 7. Like most monoclonal antibodies, bevacizumab exhibits more complex and variable pharmacokinetic and pharmacodynamics characteristics than small molecules. Variability was associated with several factors such as tumor burden, binding to their molecular targets, and others 8, 9, 10, 11. This variability could cause significant differences in exposure to bevacizumab, which subsequently may affect clinical response, as already shown with other anticancer monoclonal antibodies. For example, patients with breast cancer treated with therapeutic drug monitoring (TDM)‐1 12, those with mCRC treated with cetuximab 13, patients with melanoma treated with ipilimumab 14, those with non‐small cell lung cancer treated with nivolumab 15, and patients with soft tissue sarcoma treated with olaratumab 16.
Bevacizumab concentrations as a predictive biomarker for clinical outcomes would represent a major advance in therapeutics. Therefore, the aim of the present study was to investigate the relationship between bevacizumab exposure and OS in mCRC.
Materials and Methods
Study Design and Patients
This was a prospective, real‐world study, conducted at the Department of Oncology, University Hospital of Patras, Greece. Forty‐six patients with mCRC who were treated with first‐line bevacizumab‐containing treatment between July 2012 and July 2014 were included in the study. Serial blood samples from cycle 2 up to cycle 30 and survival data were prospectively collected. Follow‐up was carried out until July 2018. Patients were 18 years of age or older, had Eastern Cooperative Oncology Group performance status 0–2 and histologically confirmed mCRC. All patients received standard of care first‐line treatment with bevacizumab in combination with oxaliplatin or irinotecan and fluoropyrimidine‐based chemotherapy. Bevacizumab was administered as an intravenous infusion at a dose of 5 mg/kg once every 2 weeks in combination with 5‐fluorouracil/leucovorin/irinotecan or oxaliplatin (BEV‐FOLFIRI or BEV‐FOLFOX, respectively) or at a dose of 7.5 mg/kg once every 3 weeks in combination with capecitabine/irinotecan or oxaliplatin (BEV‐CapIRI or BEV‐CapOX, respectively) in 3‐week cycles. Treatment was initially administered for six (BEV‐FOLFIRI, BEV‐FOLFOX) or four (BEV‐CapIRI, BEV‐CapOX) cycles; patients who responded to treatment continued with bevacizumab‐based maintenance treatment. Radiographic evaluation was performed every 8–12 weeks or when clinically indicated. The response was evaluated according to RECIST criteria version 1.1. The treating physicians decided maintenance treatment. The primary endpoint of the study was OS.
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice. Approval was obtained by the hospital's ethics committee. Prior to study enrollment, all patients provided signed informed consent.
Bevacizumab Serum Measurements
Trough (predose) concentrations of bevacizumab were measured in serum from cycle 2 up to cycle 30. This period was sufficient to account for the minimum time needed to investigate the response of each patient to the therapy while maintaining an acceptable number of follow‐up observations. A previously published and validated enzyme‐linked immunosorbent assay (ELISA) method by Panoilia et al. was used, where the detection limit was 0.033 mg/L and the range of linearity was between 5 and 75 mg/L with precision 5.6 % 8. Measurements were performed as follows: Microtiter Nunc Maxisorp 96‐well plates were coated with recombinant human VEGF165 (R&D Systems Europe, Abingdon, UK) at a concentration of 0.15 mg/L in carbonate–bicarbonate buffer (1 M, pH 9.6) overnight at 4°C (100 μL per well). After washing four times with phosphate‐buffered saline (PBS) containing 0.05 % Tween 20, the wells were blocked with PBS containing 1% bovine serum albumin (BSA; 200 μL per well) and were incubated for 2 hours at room temperature. Afterward, plates were washed and 100 μL of 1:100 diluted standards and samples in 1% PBS–BSA were added and were incubated for 1 hour at 37°C in an incubator shaker. Then, the plates were washed again, and 100 μL of peroxidase‐conjugated goat antihuman IgG specific for Fc fragment (AbD Serotec, A Bio‐Rad Company, Oxford, UK) diluted in 1% PBS–BSA was added to each well. After 1‐hour incubation at room temperature followed by washing, 100 μL OPD (Sigma‐Aldrich, Darmstadt, Germany) was added and the reaction could develop at room temperature in the dark. The color reaction was stopped with the addition of sulfuric acid (2 M, 50 μL per well). Optical density wasmeasured at 450 nm with a correction at 650 nm using an ELISA plate reader (ThermoMax, Molecular Devices, San Diego, CA, USA). Duplicate readings for 1:100 diluted standards and samples were performed. OriginPro 8.0 software (Origin‐Lab Corporation, Northampton, MA, USA) was used to determine the best‐fit line of the standard curve with regression analysis. The concentrations read from the standard curve were multiplied by the dilution factor.
The effect of exposure to bevacizumab on clinical outcome was defined by using the median trough concentration (Ctm) at steady state, occurring after cycle 2–4, during treatment. Trough concentration during a dosing interval reflects the lowest target saturation within this dosing interval. It should be sufficiently high to achieve desired pharmacodynamic effect (maximal VEGF inhibition) and clinical outcomes. Ctm was also selected, as it summarizes the serum exposure retrospectively and captures any missing doses or dose delays during treatment.
Statistical Analysis
All categorical data including therapy were tabulated and presented as frequencies and counts. The type I error rate or significance level was set to 0.05. Area under the receiver operating characteristic curve analysis was performed to evaluate our ability to classify the outcome (survival) depending on the bevacizumab levels. For the time‐to‐event analyses, we used the Kaplan‐Meier estimate, and all lost‐to‐follow‐up cases were censored up to the most recent available time point. The log‐rank test was used for the comparison of the OS distribution for different patient groups, whereas the effects of age, sex, and bevacizumab levels were analyzed using Cox regression. In terms of the sample size calculation, there were feasibility limitations in recruitment, and therefore, the investigators calculated the minimum effect size that this study could capture for a fixed number of patients. At a power level of 80% and alpha set as 0.05, for 46 patients with 60 months of follow‐up and 2 years of recruitment, a minimum of 1.5 years of difference in the median of OS between the compared groups can be statistically detected. Alternatively, this is approximately equal to a 0.34 hazard ratio θ under a Cox regression model, meaning that this study would able to identify predictors with large effect size. The Kaplan‐Meier graphs were generated using R version 3.5.2 for Windows (R Foundation for Statistical Computing, Vienna, Austria). The analyses and power calculations were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
A total of 46 consecutive patients with mCRC were enrolled in the study. Overall, patients had a mean age of 64.5 years (range 31–86) and were predominantly male (28/46; 61%). The most commonly used initial treatment was BEV‐FOLFOX (46%) and the most common maintenance treatment was bevacizumab monotherapy (30%). The mean number of metastatic sites was 2 (range 1–5; Table 1).
Table 1.
Baseline patient characteristics and therapeutic regimens used
| Characteristic | Total no. of patients (n = 46) | Frequency, % |
|---|---|---|
| Sex | ||
| Female | 18 | 39 |
| Male | 28 | 61 |
| Age, years | ||
| < 55 | 14 | 30 |
| 55–65 | 12 | 26 |
| > 65 | 20 | 44 |
| Metastatic site | ||
| 1 | 15 | 33 |
| 2 | 12 | 26 |
| > 2 | 19 | 41 |
| Initial treatment | ||
| Bevacizumab‐mFOLFOX6 | 22 | 48 |
| Bevacizumab‐FOLFIRI | 13 | 28 |
| Bevacizumab‐CapOX | 2 | 4 |
| Bevacizumab‐CapIRI | 9 | 20 |
| Maintenance treatment | ||
| Bevacizumab‐mFOLFOX6 | 4 | 9 |
| Bevacizumab‐FOLFIRI | 5 | 10 |
| Bevacizumab‐CapIRI | 9 | 20 |
| Bevacizumab‐De Gramont | 11 | 24 |
| Bevacizumab‐Capecitabine | 3 | 7 |
| Bevacizumab monotherapy | 14 | 30 |
| Response to treatment | ||
| Complete response | 1 | 2 |
| Partial response | 16 | 35 |
| Stable disease | 25 | 54 |
| Disease progression | 4 | 9 |
Bevacizumab‐mFOLFOX6 (bevacizumab 5 mg/kg, oxaliplatin 85 mg/m2, folinic acid 400 mg/m2, fluorouracil 400 mg/m2 bolus, fluorouracil 2,400 mg/m2 over 46 hours every 2 weeks), Bevacizumab‐FOLFIRI (bevacizumab 5 mg/kg, irinotecan 180 mg/m2, folinic acid 400 mg/m2, fluorouracil 400 mg/m2 bolus, fluorouracil 2,400 mg/m2 over 46 hours every 2 weeks), Bevacizumab‐CapOX (bevacizumab 7.5 mg/kg on d1, oxaliplatin 130 mg/m2 on d1, capecitabine 1,000 mg/m2 per 12 hours d1–14 every 3 weeks), Bevacizumab‐CapIRI (bevacizumab 7.5 mg/kg on d1, irenotecan 250 mg/m2 on d1, capecitabine 1,000 mg/m2 per 12 hours d1–14 every 3 weeks), Bevacizumab‐De Gramont (bevacizumab 5 mg/kg, folinic acid 200 mg/m2 on d1,2, fluorouracil 400 mg/m2 bolus on d1,2, fluorouracil 2,400 mg/m2 over 22 hours on d1,2 every 2 weeks), Bevacizumab‐Capecitabine (bevacizumab 7.5 mg/kg on d1, capecitabine 1,000 mg/m2 per 12 hours d1–14 every 3 weeks), Bevacizumab monotherapy (5 mg/kg every 2 weeks or 7.5 mg/kg every 3 weeks).
After a median follow‐up of 60 months, 88.1% of patients progressed and 58.0% died. For all patients, median PFS and OS were 9 and 18 months, respectively. In terms of response, complete response was achieved by one patient, partial response by 35% of patients, stable disease by 54% of patients, and progressive disease by 9% of patients, all at the first imaging evaluation.
In total, 157 trough concentration samples were analyzed. It was found that the median trough concentration at steady state was 74.6 mg/L, ranging from 10.9 to 195.5 mg/L.
The receiver operating characteristic curve analysis using the bevacizumab trough levels as the predictor and OS as the classification produced an area under the curve = 0.739 with p = .009. This suggests a distinct separation between the patients who did and did not survive in the studied cohort, depending on their bevacizumab trough levels.
This finding was further studied using Cox proportional hazards modeling with the survival status and time as the outcome and bevacizumab trough levels as the predictor (independent variable). The results of the Cox regression showed a strong positive correlation between bevacizumab Ctm and survival (p = .0004), verifying the previous findings and suggesting the continuous nature of the effect.
To quantify the results in a meaningful manner and demonstrate potentially translational aspects of these findings, patients were separated into three exposure groups depending on bevacizumab trough levels, namely, high with Ctm ≥87.9 mg/L, medium with Ctm between 43 and 87.2 mg/L, and low with Ctm ≤41.9 mg/L. Patients’ characteristics did not differ significantly between the three exposure groups (Table 2). It was found that median OS in patients of the low exposure group was 12.8 months, whereas that in the patients of the medium exposure group was 36 months (p = .0003). In the high exposure group, OS could not be determined, as more than 50% of patients were still alive after the 60 months follow‐up period (Fig. 1 and Table 1). Sex, age, and KRAS status were not associated with OS, as any combination did not add to the Cox model.
Table 2.
Baseline characteristics and OS in the three different groups of bevacizumab exposure
| Characteristic | Ctm ≤41.9 mg/L | Ctm 43–87.2 mg/L | Ctm ≥87.9 mg/L | p value |
|---|---|---|---|---|
| Median age, years | 63.3 | 63.4 | 61.3 | .89 |
| Sex (males) | 73% | 60% | 50% | .56 |
| KRAS (mutant) | 57% | 55% | 33% | .77 |
| Median OS (range), months | 12.8 (7.7–17.9) | 36 (23.4–48.5) | N/A | .0003 |
Median OS was not reached for the high exposure group.
Bolded p value is statistically significant.
Abbreviations: Ctm, median bevacizumab trough concentration at steady state; N/A, not applicable; OS, overall survival.
Figure 1.
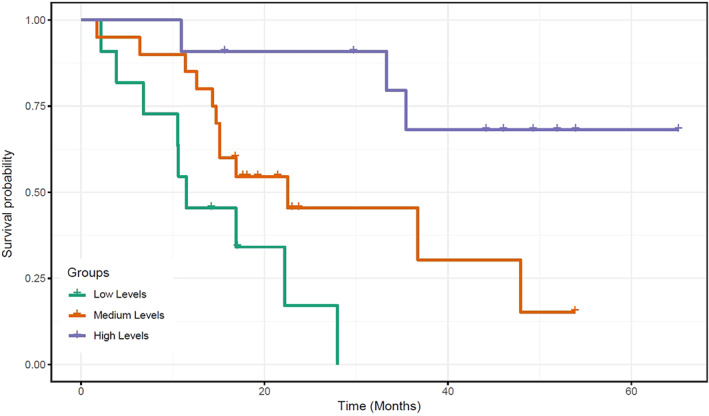
Kaplan‐Meier curves of overall survival according to median trough bevacizumab levels between cycles 2 and 30.
Discussion
Today, bevacizumab has become standard treatment in patients with mCRC. However, clinical outcomes can be highly variable, with some patients responding remarkably well and others not. Thus, because of this heterogeneous response, the real clinical impact of bevacizumab remains unclear. Pharmacokinetic parameters are among the most important factors, which influence drug action and clinical response. Bevacizumab presents very variable and complicated pharmacokinetics. Therefore, differences in the pharmacokinetic parameters could explain the interindividual variability observed in patients. The mean half‐life is approximately 20 days; however, large individual differences were noted ranging between 11 and 50 days, a variability that is anticipated to affect response to treatment 4.
In this study, we aimed to assess the relationship between bevacizumab exposure, measured as Ctm, and overall survival in patients with mCRC receiving first‐line bevacizumab in combination with oxaliplatin or irinotecan and fluoropyrimidine‐based chemotherapy. We demonstrated that patients who experienced longer OS also had significantly higher exposure to bevacizumab. Three distinct groups of patients were identified: the low exposure group (Ctm ≤41.9 mg/L) with median OS of 12.8 months, the medium exposure group (Ctm 43–87.2 mg/L) with median OS of 36 months (p = .0003), and the high exposure group (Ctm ≥87.9 mg/L), where the majority of patients were still alive 60 months after the initiation of treatment.
It has been previously reported that lower trough bevacizumab concentrations are associated with poorer clinical outcomes 17, 18. However, our study for the first time has identified three distinct groups of subpopulations within patients with mCRC, where bevacizumab exposure is directly and positively related to OS. These findings could be interpreted and used as prognostic markers for survival but more importantly could be used as guide for TDM in order to optimize dosing during the treatment; such an optimization will lead to better clinical outcomes and improved survival. TDM is a standard‐of‐care and cost‐effective practice for several classes of antibiotics, immunosuppressants, antiepileptics, human immunodeficiency virus agents, and many other drugs in clinical practice 19. Moreover, the TDM‐guided approach has been proved to improve safety and efficacy of both classic cytotoxic drugs and targeted therapies with small‐molecule tyrosine kinase inhibitors 20, 21, 22, 23, 24, 25, 26. Similarly, there are some studies that support the TDM approach for the treatment with monoclonal antibodies such as rituximab and cetuximab 27, 28, 29. In the case of bevacizumab in mCRC, the TDM approach has the practical advantage that trough concentrations provide the most relevant clinical information. Therefore, trough concentration measurement prior to the next dose could guide clinical decisions and potential dose adjustments in order to improve survival. In addition, implementation of such an approach seems feasible as the progress in the field for bevacizumab includes the development of validated, robust, rapid, and sensitive assays for bevacizumab levels measurements, which could be suitable for use in clinical routine as part of personalized treatment 17, 30.
Conclusion
A significant correlation between exposure to bevacizumab and survival was observed. Further studies for clarification of the dose, exposure, and clinical outcomes relationship in patients treated with bevacizumab are warranted. Considering the fundamental role of bevacizumab in the therapeutics of mCRC, the highly variable patient responses, and the wide range in clearance rates, the measurement of blood bevacizumab levels can be used to achieve long‐term responses and benefit from bevacizumab‐based therapies.
Author Contributions
Conception/design: Apostolos Papachristos, Gregory Sivolapenko
Provision of study material or patients: Apostolos Papachristos, Haralabos Kalofonos, Gregory Sivolapenko
Collection and/or assembly of data: Apostolos Papachristos, Polychronis Kemos
Data analysis and interpretation: Apostolos Papachristos, Polychronis Kemos, Haralabos Kalofonos, Gregory Sivolapenko
Manuscript writing: Apostolos Papachristos, Polychronis Kemos, Gregory Sivolapenko
Final approval of manuscript: Apostolos Papachristos, Polychronis Kemos, Haralabos Kalofonos, Gregory Sivolapenko
Disclosures
The authors indicated no financial relationships.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
For Further Reading: A. Bapsi Chakravarthy, Fengmin Zhao, Neal J. Meropol et al. Intergroup Randomized Phase III Study of Postoperative Oxaliplatin, 5‐Fluorouracil, and Leucovorin Versus Oxaliplatin, 5‐Fluorouracil, Leucovorin, and Bevacizumab for Patients with Stage II or III Rectal Cancer Receiving Preoperative Chemoradiation: A Trial of the ECOG‐ACRIN Research Group (E5204). The Oncologist 2020;25:e798–e807.
Implications for Practice: At 17% of its planned accrual, E5204 was terminated early owing to poor accrual. At a median follow‐up of 72 months, there was no significant difference in 5‐year overall survival (88.3% vs. 83.7%) or in 5‐year disease‐free survival (71.2% vs. 76.5%) between the two arms. Despite significant advances in the treatment of rectal cancer, especially in improving local control rates, the risk of distant metastases and the need to further improve quality of life remain a challenge. Strategies combining novel agents with chemoradiation to improve both distant and local control are needed.
References
- 1. Global Burden of Disease Cancer Collaboration , Fitzmaurice C, Allen C et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol 2017;3:524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 3. Heinemann V, von Weikersthal LF, Decker T et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first‐line treatment for patients with metastatic colorectal cancer (FIRE‐3): A randomised, open‐label, phase 3 trial. Lancet Oncol 2014;15:1065–1075. [DOI] [PubMed] [Google Scholar]
- 4. Avastin SmPC, EMC . Available at https://www.medicines.org.uk/emc/product/3885/smpc. Accessed July 10, 2018.
- 5. Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 2005;65:671–680. [PubMed] [Google Scholar]
- 6. Peeters M, Balfour J, Arnold D. Review article: Panitumumab–A fully human anti‐EGFR monoclonal antibody for treatment of metastatic colorectal cancer. Aliment Pharmacol Ther 2008;28:269–281. [DOI] [PubMed] [Google Scholar]
- 7. Giantonio BJ, Catalano PJ, Meropol NJ et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539–1544. [DOI] [PubMed] [Google Scholar]
- 8. Panoilia E, Schindler E, Samantas E et al. A pharmacokinetic binding model for bevacizumab and VEGF165 in colorectal cancer patients. Cancer Chemother Pharmacol 2015;75:791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu JF, Bruno R, Eppler S et al. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol 2008;62:779–786. [DOI] [PubMed] [Google Scholar]
- 10. Li J, Gupta M, Jin D et al. Characterization of the long‐term pharmacokinetics of bevacizumab following last dose in patients with resected stage II and III carcinoma of the colon. Cancer Chemother Pharmacol 2013;71:575–580. [DOI] [PubMed] [Google Scholar]
- 11. Caulet M, Lecomte T, Bouché O et al. Bevacizumab pharmacokinetics influence overall and progression‐free survival in metastatic colorectal cancer patients. Clin Pharmacokinet 2016;55:1381–1394. [DOI] [PubMed] [Google Scholar]
- 12. Wang J, Song P, Schrieber S et al. Exposure‐response relationship of T‐DM1: Insight into dose optimization for patients with HER2‐positive metastatic breast cancer. Clin Pharmacol Ther 2014;95:558–564. [DOI] [PubMed] [Google Scholar]
- 13. Azzopardi N, Lecomte T, Ternant D et al. Cetuximab pharmacokinetics influences progression‐free survival of metastatic colorectal cancer patients. Clin Cancer Res 2011;17:6329–6337. [DOI] [PubMed] [Google Scholar]
- 14. Feng Y, Roy A, Masson E et al. Exposure‐response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma. Clin Cancer Res 2019;19:3977–3986. [DOI] [PubMed] [Google Scholar]
- 15. Basak EA, Koolen SLW, Hurkmans DP et al. Correlation between nivolumab exposure and treatment outcomes in non‐small‐cell lung cancer. Eur J Cancer 2019;109:12–20. [DOI] [PubMed] [Google Scholar]
- 16. Jones RL, Mo G, Baldwin JR et al. Exposure‐response relationship of olaratumab for survival outcomes and safety when combined with doxorubicin in patients with soft tissue sarcoma. Cancer Chemother Pharmacol 2018;83:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nugue G, Bidart M, Arlotto M et al. Monitoring monoclonal antibody delivery in oncology: The example of bevacizumab. PLoS One 2013;8:e72021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caulet M, Lecomte T, Bouche O et al. Bevacizumab pharmacokinetics influence overall and progression‐free survival in metastatic colorectal cancer patients. Clin Pharmacokinet 2016;55:1381–1394. [DOI] [PubMed] [Google Scholar]
- 19. Touw DJ, Neef C, Thomson AH et al. Cost‐effectiveness of therapeutic drug monitoring: A systematic review. Ther Drug Monit 2005;27:10–17. [DOI] [PubMed] [Google Scholar]
- 20. Gao B, Yeap S, Clements A et al. Evidence for therapeutic drug monitoring of targeted anticancer therapies. J Clin Oncol 2012;30:4017–4025. [DOI] [PubMed] [Google Scholar]
- 21. Yu H, Steeghs N, Nijenhuis CM et al. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: Focus on the pharmacokinetic targets. Clin Pharmacokinet 2014;53:305–325. [DOI] [PubMed] [Google Scholar]
- 22. Patel JN, Papachristos A. Personalizing chemotherapy dosing using pharmacological methods. Cancer Chemother Pharmacol 2015;76:879–896. [DOI] [PubMed] [Google Scholar]
- 23. Patel JN, O'Neil BH, Deal AM et al. A community‐based multicenter trial of pharmacokinetically guided 5‐fluorouracil dosing for personalized colorectal cancer therapy. The Oncologist 2014;19:959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gotta V, Widmer N, Decosterd LA et al. Clinical usefulness of therapeutic concentration monitoring for imatinib dosage individualization: Results from a randomized controlled trial. Cancer Chemother Pharmacol 2014;74:1307–1319. [DOI] [PubMed] [Google Scholar]
- 25. Paci A, Veal G, Bardin C et al. Review of therapeutic drug monitoring of anticancer drugs part 1—Cytotoxics. Eur J Cancer 2014;50:2010–2019. [DOI] [PubMed] [Google Scholar]
- 26. Widmer N, Bardin C, Chatelut E et al. Review of therapeutic drug monitoring of anticancer drugs part two‐targeted therapies. Eur J Cancer 2014;50:2020–2036. [DOI] [PubMed] [Google Scholar]
- 27. Fracasso PM, Burris H 3rd, Arquette MA et al. A phase 1 escalating single‐dose and weekly fixed‐dose study of cetuximab: Pharmacokinetic and pharmacodynamic rationale for dosing. Clin Cancer Res 2007;13:986–993. [DOI] [PubMed] [Google Scholar]
- 28. Berinstein NL, Grillo‐López AJ, White CA et al. Association of serum Rituximab (IDEC‐C2B8) concentration and anti‐tumor response in the treatment of recurrent low‐grade or follicular non‐Hodgkin's lymphoma. Ann Oncol 1998;9:995–1001. [DOI] [PubMed] [Google Scholar]
- 29. McLaughlin P, Grillo‐Lopez AJ, Link BK et al. Rituximab chimeric anti‐CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four‐dose treatment program. J Clin Oncol 1998;16:2825–2833. [DOI] [PubMed] [Google Scholar]
- 30. Ternant D, Cézé N, Lecomte T et al. An enzyme‐linked immunosorbent assay to study bevacizumab pharmacokinetics. Ther Drug Monit 2010;32:647–652. [DOI] [PubMed] [Google Scholar]


