Abstract
Lessons Learned
Low‐dose afatinib maintenance treatment among patients with EGFR‐mutated NSCLC achieved long‐time to treatment failure with fewer treatment‐related AEs without detracting from the therapeutic efficacy.
This modified regimen represents a practical usage that balances effectiveness and safety.
Background
Although afatinib is an effective therapy for patients with EGFR‐mutated non‐small cell lung cancer (NSCLC), drug‐related adverse events (AEs) have often necessitated dose reductions. In a post hoc analysis of the LUX‐Lung 3 and 6 trials, there was no difference in median progression‐free survival (PFS) between patients who had the dose of afatinib reduced and those who did not. We thus evaluated the efficacy and tolerability of low‐dose afatinib maintenance treatment among patients with NSCLC harboring EGFR mutations who had not been previously treated.
Methods
Eligible patients received afatinib 40 mg orally once daily. When prescribed grade ≥ 2 AEs, rash of grade ≥ 3, or unacceptable toxicity occurred, the afatinib dose was reduced from 40 to 30 mg and if needed from 30 to 20 mg. The primary endpoint was the 1‐year PFS rate. Secondary endpoints were PFS, overall response rate (ORR), and toxicity.
Results
Among 30 patients, 93% had adenocarcinoma, 53% had exon 19 deletion, 37% had L858R, and 10% had minor mutations. The 1‐year PFS rate was 50% (95% confidence interval [CI], 31.3–66.1) and the median PFS was 11.8 months (95% CI, 7.1–21.4). The incidence rate of grade ≥ 3 toxicities was 57%, including elevated aspartate aminotransferase/alanine aminotransferase level (13%), diarrhea (10%), and paronychia (10%).
Conclusion
Low‐dose afatinib maintenance treatment reduced treatment‐related AEs without detracting from the therapeutic efficacy.
Discussion
This prospective study was designed to investigate the efficacy and tolerability of dose modification of afatinib according to AEs among patients with advanced NSCLC harboring EGFR mutations not previously treated with a tyrosine kinase inhibitor (TKI) targeting the epidermal growth factor receptor (EGFR). In the LUX‐Lung 3 trial, the median PFS with afatinib was 11.1 months, the 1‐year PFS rate was approximately 45%, and the median overall survival (OS) was 28.2 months [1, 2]. The PFS of 11.8 months and the 1‐year PFS rate of 50% in this study compare favorably with the results of the LUX‐Lung 3 trial (Fig. 1). The swimmer plot of treatment duration among all patients showed a durable response with low‐dose afatinib maintenance treatment (Fig. 2).
Figure 1.
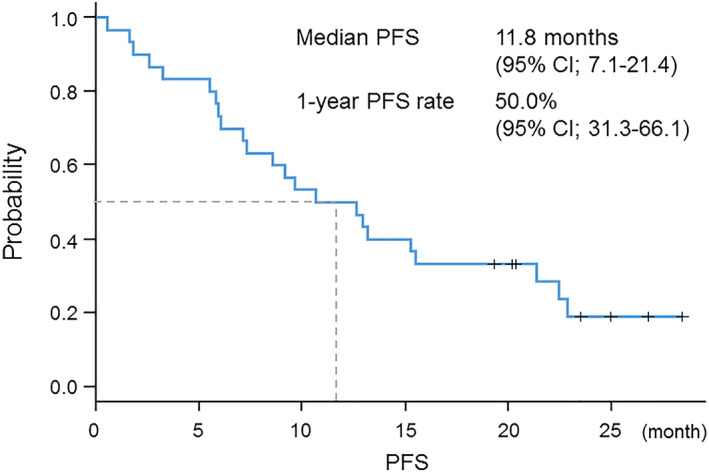
Kaplan‐Meier curve of progression‐free survival. Abbreviations: CI, confidence interval; PFS, progression‐free survival.
Figure 2.
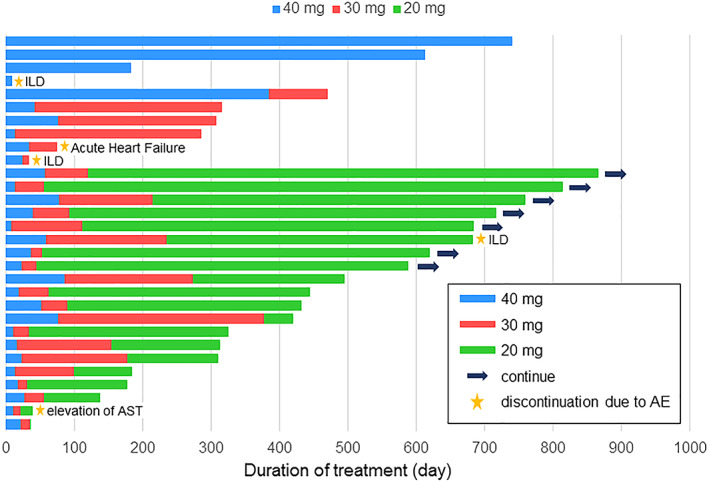
A swimmer plot of treatment duration in all patients. Stars represent discontinuation because of adverse events. Arrows represent the continuation at the time of data cutoff. Abbreviations: AE, adverse event; AST, aspartate aminotransferase; ILD, interstitial lung disease.
Although the subset analysis of Japanese patients in the LUX‐Lung 3 trial showed a median PFS of 13.8 months and a median OS of 46.9 months, there was a higher rate of AEs peculiar to EGFR‐TKI, such as diarrhea, rash/acne, and nail effects, and the incidence of AEs of grade ≥ 3 was approximately 70% in the Japanese subset [3]. Our study prescribed dose reduction among patients with treatment‐related AEs of grade ≥ 2, rash of grade ≥ 3, or any grade of unacceptable toxicity. Most patients required dose reduction, and two‐thirds of the patients required two dose reductions (Fig. 2). Regarding the toxicities of the modified dose, the incidence of all‐cause treatment‐related AEs of grade ≥ 3 decreased to 57%, which was tolerable compared with the incidence of AEs with afatinib in the LUX‐Lung 3 trial. Moreover, only a few patients experienced severe AEs after dose reduction. The incidence of interstitial lung disease, a severe AE of concern among Japanese patients, was relatively high, but it seemed to have occurred by chance considering the small size of our study sample. The most frequent AEs, such as diarrhea, rash/acne, paronychia, and stomatitis, were acceptable because of early dose reduction and enabled treatment to be accomplished without discontinuation after such AEs. Thus, low‐dose afatinib maintenance therapy reduced treatment‐related AEs without detracting from the therapeutic efficacy. Our data support the feasibility of modified dose reduction of afatinib among patients with EGFR‐mutated NSCLC. Low‐dose afatinib maintenance treatment may be an acceptable treatment option for patients with EGFR‐mutated NSCLC.
Trial Information
| Disease | Lung cancer – NSCLC |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | None |
| Type of Study | Phase II, single arm |
| Primary Endpoint | 1‐year progression‐free survival rate |
| Secondary Endpoints | |
| Progression‐free survival, overall response rate, toxicity, incidence of grade ≥ 3 adverse events | |
| Additional Details of Endpoints or Study Design | |
| Study design: This study was a single‐arm, phase II trial conducted at nine institutions in Japan. | |
| Inclusion criteria: Eligible patients were aged 20 years or older, with cytologically or histologically confirmed NSCLC that was classified as clinical stage IIIB–IV, or a postoperative recurrence harboring sensitive EGFR mutations except exon 20 insertion or T790M. The patients had not previously been treated with an EGFR‐TKI, nor had they received more than two cycles of cytotoxic anticancer therapy except adjuvant chemotherapy after operation or immune checkpoint inhibitor, and they had at least one measurable lesion according to RECIST, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, and an estimated life expectancy of ≥3 months. For radiation therapy, there was no history of radiation to the target lesion, ≥12 weeks had passed since the final dose of radiation had been administered to the chest, and ≥ 2 weeks had passed since the final dose of radiation had been administered to a body part other than the chest. Regarding surgery, ≥4 weeks had passed since the most recent day of operation. For patients with chest drainage or pleurodesis, ≥2 weeks had passed since the final treatment had been administered. Regarding anticancer agents, ≥3 weeks had passed since the last treatment had been administered. Laboratory criteria included a neutrophil count ≥1,500/mm3, a platelet count ≥100,000/mm3, a hemoglobin concentration ≥ 9 g/dL, total bilirubin level ≤ 1.5 mg/dL, aspartate transaminase and alanine transaminase levels ≤100 U/L, serum creatinine ≤1.5 mg/dL, and a partial pressure of arterial oxygen (PaO2) ≥60 mmHg. All enrolled patients provided written informed consent prior to enrolment in the present study. | |
| Exclusion criteria: We excluded patients who had pulmonary disorders such as idiopathic pulmonary fibrosis or interstitial pneumonia; symptomatic brain metastasis; pleural effusion, ascites, or pericardial fluid requiring drainage; active infectious disorders; active double cancer; unstable cardiac disorders such as angina pectoris, acute myocardial infarction within 3 months or cardiac failure; uncontrollable diabetes mellitus or hypertension; gastrointestinal disorders with serious diarrhea requiring glucocorticoid therapy or immunosuppressive agents; and those regarded as unsuitable for this study by the investigators. | |
| Treatment plan: The present study was conducted to evaluate the efficacy and tolerability of maintenance therapy with low‐dose afatinib among patients with NSCLC harboring an EGFR mutation not previously treated with an EGFR‐TKI. Patients initially received afatinib 40 mg orally once a day. The treatment was continued until disease progression, intolerable severe toxicity, or withdrawal of consent. Patients who experienced AEs of grade ≥ 2, rash of grade ≥ 3, or any grade of unacceptable toxicity could suspend treatment for up to 4 weeks. After suspension, treatment could be restarted based on the judgment of the investigators, and the dose of afatinib was decreased by 10 mg, initially to 30 mg/day and if needed down to 20 mg/day. After failure of afatinib, patients could receive any subsequent treatment, including continuation of afatinib, based on the judgment of the investigators. | |
| Endpoints: The primary endpoint was the 1‐year PFS rate. The secondary endpoints were PFS, ORR, toxicity profiles, and the incidence of AEs of grade ≥ 3. The follow‐up period was 12 months after the last patient enrolment. | |
| Statistical methods: For the primary endpoint, the minimum number of patients enrolled was 26, assuming a threshold 1‐year PFS rate of 42% and an expected 1‐year PFS rate of 63% with 90% power at a two‐sided alpha of .05. Considering that 10% of patients could be ineligible, the sample size was set at 30 patients. The 1‐year PFS rate and PFS were estimated using the Kaplan‐Meier method. Safety analyses were used to summarize AEs by maximum Common Terminology Criteria for Adverse Events grade at each dose of afatinib during the entire treatment period. | |
| Investigator's Analysis | Active and should be pursued further |
Drug Information
| Drug 1 | |
| Generic/Working Name | Afatinib |
| Company Name | Boehringer‐Ingelheim |
| Drug Type | Molecular targeting drug |
| Drug Class | EGFR |
| Dose | 40 milligrams (mg) per flat dose |
| Route | Oral (p.o.) |
| Schedule of Administration | Afatinib 40 mg was administered orally once a day until either disease progression or the incidence of prescribed AEs. |
Patient Characteristics
| Number of Patients, Male | 13 |
| Number of Patients, Female | 17 |
| Stage | Stage IV (n = 19), postoperative recurrence (n = 11) |
| Age | Median (range): 69 (46–79) |
| Number of Prior Systemic Therapies | Median: 0 |
| Performance Status: ECOG |
0 — 22 1 — 7 2 — 1 3 — 0 Unknown — 0 |
| Other | Exon 19 deletion (n = 16), L858R (n = 11), minor (n = 3: L861Q, n = 1; G719X, n = 2) |
| Cancer Types or Histologic Subtypes | Adenocarcinoma, 28; squamous cell carcinoma, 2 |
Primary Assessment Method
| Number of Patients Enrolled | 30 |
| Number of Patients Evaluable for Toxicity | 30 |
| Number of Patients Evaluated for Efficacy | 30 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 23 (77%) |
| Response Assessment SD | n = 3 (10%) |
| Response Assessment PD | n = 1 (3%) |
| Response Assessment OTHER | n = 3 (10%) |
| (Median) Duration Assessments PFS | 11.6 months, CI, 7.1–21.4 |
Kaplan‐Meier Time Units, Months
| Time of scheduled assessment and/or time of event | No. progressed (or deaths) | No. censored | Percent at start of evaluation period | Kaplan‐Meier % | No. at next evaluation/No. at risk |
|---|---|---|---|---|---|
| 0 | 100.00 | 100.00 | 30 | ||
| Outcome Notes | 1‐year PFS rate was 50% (95% CI, 31.3–66.1), which did not meet the statistical setting of this study. | ||||
Adverse Events
| Adverse event, (≥10%) | All treatment (n = 30) | Post‐reduction (n = 26) | ||
|---|---|---|---|---|
| All grade, n (%) | Grade 3–4, n (%) | All grade, n (%) | Grade 3–4, n (%) | |
| Any cause | 30 (100) | 14 (47) | 24 (92) | 4 (15) |
| Diarrhea | 29 (97) | 3 (10) | 7 (27) | 0 (0) |
| Rash/acne | 22 (73) | 2 (4) | 14 (54) | 1 (4) |
| Paronychia | 18 (60) | 4 (13) | 15 (58) | 1 (4) |
| Stomatitis | 18 (60) | 2 (7) | 5 (19) | 0 (0) |
| Elevation of AST/ALT | 11 (37) | 3 (10) | 6 (24) | 2 (8) |
| Anorexia | 8 (27) | 2 (7) | 0 (0) | 0 (0) |
| Pruritus | 6 (20) | 0 (0) | 2 (8) | 0 (0) |
| Dry skin | 4 (13) | 1 (3) | 3 (12) | 0 (0) |
| Anemia | 5 (17) | 0 (0) | 3 (12) | 0 (0) |
| Interstitial lung disease | 3 (10) | 1 (3) | 2 (8) | 0 (0) |
| Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase. | ||||
| Adverse Events Legend | ||||
| The rate of all‐cause AEs grade ≥ 3 was 57% (17 of 30). Interstitial lung disease occurred in three patients, and one patient died. Two treatment‐related deaths from interstitial lung disease, and acute cardiac failure were observed. | ||||
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
Lung cancer is the most common cause of cancer‐related deaths worldwide. Unfortunately, at the time of their original diagnosis most patients present with metastatic disease, and their standard therapeutic option is chemotherapy. The identification of targetable oncogenic gene mutations, including EGFR, ALK, ROS‐1, BRAF, and NTRK, have provided treatment options with molecular‐targeted therapies developed for each specific genetic mutation [4]. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have proven to be more effective than platinum doublets without reducing the quality of life among patients with non‐small cell lung cancer (NSCLC) harboring activating EGFR mutations [1, 5, 6].
Afatinib is a second‐generation ErbB family blocker that downregulates ErbB signaling by irreversibly binding to EGFR (ErbB1), HER2/ErbB2, ErbB4, and all relevant ErbB family dimers. The broad spectrum of activity and irreversible inhibition might be more potent and prolonged than those of the reversible first‐generation EGFR‐TKIs [7, 8]. Among patients with previously untreated advanced EGFR‐mutated NSCLC, first‐line afatinib treatment has shown longer progression‐free survival (PFS) and overall survival (OS) than platinum doublets [1, 9]. Moreover, in the LUX‐Lung 7 trial, afatinib produced significant benefits in PFS and time to treatment failure compared with gefitinib in the first‐line treatment of patients with previously untreated advanced EGFR‐mutated NSCLC. However, more serious drug‐related adverse events (AEs) were reported in the afatinib group than in the gefitinib group [10].
In the post hoc analyses of the LUX‐Lung 3 and 6 trials, dose adjustment of afatinib because of patient intolerability led to a reduction in afatinib‐related AEs. The median PFS for the patients with dose reduction in the first 6 months was not inferior to that of those without dose reduction in the first 6 months. Dose reduction was performed more commonly among Japanese patients, and most AEs caused by the administration of the EGFR‐TKI occurred more frequently in the Japanese subset [1, 3, 11]. Therefore, we conducted this multicenter, phase II trial to evaluate the efficacy and tolerability of low‐dose afatinib maintenance treatment with early dose reduction at grade ≥ 2 AEs among patients with NSCLC harboring EGFR mutations not previously treated with an EGFR‐TKI. Our study showed a favorable PFS and tolerable AEs in comparison with those of the LUX‐Lung 3 trial, although the 1‐year PFS rate did not meet our primary endpoint.
Our study included not only common mutations (exon 19 deletion [Del‐19] and L858R) but also uncommon mutations. Regarding the histological type, our study included not only patients with adenocarcinoma but also those with squamous cell carcinoma. The median PFS durations of patients with common and uncommon mutations were 12.8 and 7.4 months, respectively. Among patients with common mutations, the median PFS durations of Del‐19 and L858R were 11.6 and 13.6 months, respectively, in this trial. Although the Japanese subset analysis of the LUX‐Lung 3 trial showed more efficacy in the Del‐19 subset than the L858R subset, a similar tendency was not shown in this study, likely because of the small sample size. Uncommon mutations identified in three patients were G719X (n = 2) and L861Q (n = 1). In a combined post hoc analysis of the LUX‐Lung 2, 3, and 6 trials, the median PFS of afatinib in patients whose tumors harbored uncommon mutations was 10.7 months, which was shorter than that of patients with common mutations, and a similar tendency was shown in our study [12]. Regarding nonadenocarcinoma histology, there were a few reports about nonadenocarcinoma NSCLC harboring EGFR mutations but no data about treatment with afatinib. The median PFS of gefitinib was only 3.0 months among patients with nonadenocarcinoma NSCLC with EGFR mutation [13]. Our study included two patients with squamous cell carcinoma, and their PFS durations were only 6.1 and 8.7 months. The 1‐year PFS rate did not meet our primary endpoint because of early discontinuation caused by AEs and the early progression of disease among patients with less frequently occurring mutations and squamous histology. Further studies are needed to determine the efficacy among these patients.
The present study had several limitations. First, this study used a small sample size comprising a heterogeneous patient population, including uncommon mutations and squamous histology. Second, the present study did not investigate the OS. Although the FLAURA study demonstrated a prolonged PFS and OS among patients with advanced NSCLC harboring EGFR mutations and osimertinib is recommended as first‐line treatment, the median OS of the Asian subset in the FLAURA trial could not be proven to have statistical superiority [14]. The OS of afatinib is longer than that of gefitinib, and there has been no direct comparison between afatinib and osimertinib. It is possible that first‐line afatinib treatment is longer than that with osimertinib. Although only in cases of receiving sequential afatinib and osimertinib, the overall time on treatment with afatinib followed by osimertinib was 46.7 months among Asian patients [15]. Concerning this limitation, the Gio‐Tag Japan study, a prospective observational study of sequential treatment with afatinib followed by osimertinib for advanced EGFR‐mutated NSCLC, is ongoing in Japan.
In conclusion, low‐dose afatinib maintenance treatment for patients with NSCLC harboring EGFR mutations not previously treated with an EGFR‐TKI showed favorable efficacy and less toxicity. Based on these results, a modified afatinib dosage should be used in practice. Further investigation will be needed to evaluate the utility of this study.
Disclosures
Atsushi Nakamura: Merck Sharp & Dohme, Boehringer Ingelheim, Taiho Pharmaceutical, Kyowa Kirin (H); Shunichi Sugawara: Nippon Boehringer Ingelheim, AstraZeneca, Chugai Pharma, Pfizer, Eli Lilly and Company, Novartis, Bristol‐Myers Squibb, MSD, Ono Pharmaceutical, Taiho Pharmaceutical, Kyowa Hakko Kirin (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
The authors thank all patients, families, and investigators participating in the NJLCG1601 study.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- Trial Identifier: UMIN 000020688
- Sponsor: North Japan Lung Cancer Study Group
- Principal Investigator: Shunichi Sugawara
- IRB Approved: Yes
Contributor Information
Atsushi Nakamura, Email: atsu_shi_n@yahoo.co.jp.
Shunichi Sugawara, Email: swara357@sendai-kousei-hospital.jp.
References
- 1. Sequist LV, Yang JCH, Schuler M et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–3334. [DOI] [PubMed] [Google Scholar]
- 2. Yang JCH, Wu YL, Sequist LV et al. Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141–151. [DOI] [PubMed] [Google Scholar]
- 3. Kato T, Yoshioka H, Yamamoto N et al. Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non‐small cell lung cancer harboring activating EGFR mutations: Subgroup analysis of LUX‐Lung 3. Cancer Sci 2015;106:1202–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirsch FR, Scagliotti GV, Paz‐Ares L et al. Lung cancer: Current therapies and new targeted treatments. Lancet 2017;389:299–311. [DOI] [PubMed] [Google Scholar]
- 5. Maemondo M, Inoue A, Nukiwa T et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–2388. [DOI] [PubMed] [Google Scholar]
- 6. Mitsudomi T, Morita S, Fukuoka M et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–128. [DOI] [PubMed] [Google Scholar]
- 7. Li D, Ambrogio L, Wong KK et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solca F, Dahl G, Adolf GR et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 2012;343:342–350. [DOI] [PubMed] [Google Scholar]
- 9. Wu YL, Zhou C, Geater SL et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014;15:213–222. [DOI] [PubMed] [Google Scholar]
- 10. Paz‐Ares L, Tan EH, Park K et al. Afatinib versus gefitinib in patients with EGFR mutation‐positive advanced non‐small‐cell lung cancer: Overall survival data from the phase IIb LUX‐Lung 7 trial. Ann Oncol 2017;28:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang JCH, Sequist LV, Wu YL et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation‐positive lung adenocarcinoma: Post hoc analyses of the randomized LUX‐Lung 3 and 6 trials. Ann Oncol 2016;27:2103–2110. [DOI] [PubMed] [Google Scholar]
- 12. Yang JCH, Sequist LV, Wu YL et al. Clinical activity of afatinib in patients with advanced non‐small‐cell lung cancer harbouring uncommon EGFR mutations: A combined post‐hoc analysis of LUX‐Lung 2, LUX‐Lung 3, and LUX‐Lung 6. Lancet Oncol 2015;16:830–838. [DOI] [PubMed] [Google Scholar]
- 13. Shukuya T, Takahashi T, Yamamoto N et al. Efficacy of gefitinib for non‐adenocarcinoma non‐small‐cell lung cancer patients harboring epidermal growth factor receptor mutations: A pooled analysis of published reports. Cancer Sci 2011;102:1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramalingam SS, Vansteenkiste J, Soria JC et al. Overall survival with osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med 2020;382:41–50. [DOI] [PubMed] [Google Scholar]
- 15. Hochmair MJ, Morabito A, Cufer T et al. Sequential treatment with afatinib and osimertinib in patients with EGFR mutation‐positive non‐small‐cell lung cancer: An observational study. Future Oncol 2018;14:2861–2874. [DOI] [PubMed] [Google Scholar]


