Abstract
Background
There is limited understanding of the characteristics of patients with coronavirus disease 2019 (COVID-19) requiring hospitalization in Japan.
Methods
This study included 2638 cases enrolled from 227 healthcare facilities that participated in the COVID-19 Registry Japan (COVIREGI-JP). The inclusion criteria for enrollment of a case in COVIREGI-JP are both (1) a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test and (2) inpatient treatment at a healthcare facility.
Results
The median age of hospitalized patients with COVID-19 was 56 years (interquartile range [IQR], 40–71 years). More than half of cases were male (58.9%, 1542/2619). Nearly 60% of the cases had close contact to confirmed or suspected cases of COVID-19. The median duration of symptoms before admission was 7 days (IQR, 4–10 days). The most common comorbidities were hypertension (15%, 396/2638) and diabetes without complications (14.2%, 374/2638). The number of nonsevere cases (68.2%, n = 1798) was twice the number of severe cases (31.8%, n = 840) at admission. The respiratory support during hospitalization includes those who received no oxygen support (61.6%, 1623/2636) followed by those who received supplemental oxygen (29.9%, 788/2636) and invasive mechanical ventilation/extracorporeal membrane oxygenation (8.5%, 225/2636). Overall, 66.9% (1762/2634) of patients were discharged home, while 7.5% (197/2634) died.
Conclusions
We identified the clinical epidemiological features of COVID-19 in hospitalized patients in Japan. When compared with existing inpatient studies in other countries, these results demonstrated fewer comorbidities and a trend towards lower mortality.
Keywords: SARS-CoV-2, inpatients, mortality, epidemiology
Registry data of 2638 hospitalized COVID-19 cases revealed that mechanical ventilation was required in 8.5% of cases, and the overall mortality was 7.5%. Results of this study demonstrated fewer comorbidities and lower mortality than existing inpatient studies in other countries.
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global public health crisis [1, 2]. In Japan, the first case of coronavirus disease 2019 (COVID-19) was reported on 16 January 2020, and COVID-19 was classified as a designated infectious disease on 1 February [3]. “Three pillars” of COVID-19 countermeasures including the early response to clusters have been formulated [4, 5].
The number of cases increased in late March, triggered by the influx of infected patients from overseas, and a state of emergency was declared on 7 April. In May, the spread of the disease began to be temporarily controlled, and the declaration of a state of emergency was lifted on 25 May. With the reactivation of social activities, the number of infected people has again increased since late June of 2020 [6].
In Japan, as of 7 July 2020, there have been 19 816 cases and 979 deaths, with fewer cases and deaths than in Western countries [6, 7]. The number of cases may be affected by the number of tests conducted: there were 6.0 tests performed per 1000 population, which was approximately 5–10% of tests performed in the United States or European countries [6, 7]. A total of 7.73 confirmed COVID-19 deaths per million in the population is about 2% the death rate of the United States, less than 1.4% that of Italy and Spain, and less than 1.25% that of the United Kingdom [6–8]. The poor prognosis of COVID-19 may be related to a complex combination of factors, including number of patients, infrastructure of medical facilities, resources of medical personnel, and patient background. To determine the reasons for these differences in mortalities and to improve the management of COVID-19, a complete picture of the COVID-19–affected population in Japan is needed.
A nationwide COVID-19 inpatient registry, “COVID-19 Registry Japan” (COVIREGI-JP), was started on 2 March 2020 [9]. Using data from COVIREGI-JP, we conducted this study to identify the clinical epidemiological characteristics of inpatients with COVID-19 in Japan.
METHODS
Study Design and Patients
This was an observational study. Healthcare facilities that voluntarily participated in COVIREGI-JP enrolled the patients. Research collaborators in each facility manually input the data into the registry. The research collaborators received funding from the research grant for each patient enrolled. The inclusion criteria for enrollment were as follows: (1) a positive SARS-CoV-2 test [10] and (2) inpatient treatment at a healthcare facility. Some eligible inpatients might not have been registered in COVIREGI-JP by the principal investigator’s decision (eg, patients participating in other clinical studies whose data registration in COVIREGI-JP was deemed inappropriate, patients who refused to participate in the study by opting out, etc). If a patient had a history of multiple COVID-19 hospitalizations and met the aforementioned inclusion criteria, each admission was included in the registry.
Data Collection and Case Report Form
We modified the case report form of the ISARIC (International Severe Acute Respiratory and Emerging Infection Consortium) to enable the collection of clinical epidemiological information and treatment data in Japan [11]. The study data were collected and managed using REDCap (Research Electronic Data Capture), a secure, Web-based data-capture application hosted at the JCRAC (Joint Center for Researchers, Associates and Clinicians) Data Center of the National Center for Global Health and Medicine [12].
Dataset
We used data from cases for whom all of the following major items as of 7 July 2020 had been entered: demographic and epidemiological characteristics, comorbidities, signs and symptoms at admission, outcome at discharge, supportive care, history of drug administration, and complications during hospitalization. We did not treat parameters with the option “unknown” as missing values.
Definitions of Severity at Admission and Respiratory Support During Hospitalization
Severity at Admission
“Severe disease at admission” was defined as participants meeting 1 or more of the following criteria: requiring invasive or noninvasive mechanical ventilation, requiring supplemental oxygen, oxygen saturation (SpO2) of 94% or less on room air, or tachypnea (respiratory rate ≥24 breaths per minute) [13].
Respiratory Support During Hospitalization
“Respiratory support” during hospitalization was defined as follows—no oxygen: patients who were never supported with supplemental oxygen during hospitalization; oxygen: patients who were supported with noninvasive mechanical ventilation or supplemental oxygen (including high-flow oxygen devices) during hospitalization; and invasive mechanical ventilation (IMV)/extracorporeal membrane oxygenation (ECMO): patients who were supported with IMV or ECMO.
History of Drug Administration During Hospitalization
Information on medications with antiviral effect against SARS-CoV-2, immunomodulatory and/or immunosuppressive effects against COVID-19, antibiotics, antifungal drugs, neuraminidase inhibitors, and anticoagulants was collected. These medications were included in the analysis if they were administered at least once during the hospitalization period. If more than 1 drug was used in a patient, each drug was counted separately regardless of whether they were administered concurrently. If 1 drug was administered during different periods of hospitalization, it was counted as “1”.
Statistical Analysis
Continuous variables are described as medians and interquartile range (IQR). As the number of missing values differed for each parameter, the number of cases in each parameter’s severity/respiratory support category was used as the denominator for calculating the percentage. All statistical analyses were conducted using R, version 3.5.1 (R Core Team).
Ethics
This study was approved by the National Center for Global Health and Medicine (NCGM) ethics review (NCGM-G-003494-0). Information regarding opting out of our study is available on the registry website.
RESULTS
Data from 2638 cases from 227 facilities were included for the analysis. The available numbers of cases were different depending on each parameter due to the missing data. Nonsevere cases at admission (68.2%, n = 1798) were more than twice as prevalent as severe cases (31.8%, n = 840). Among the 2636 cases, respiratory support during hospitalization comprised patients who received no oxygen (61.6%, 1623/2636), patients who received oxygen (29.9%, 788/2636), and patients who received IMV/ECMO (8.5%, 225/2636). Respiratory support during hospitalization at each category of severity at admission was as follows; nonsevere (no oxygen, 1463 [81.5%]; oxygen, 302 [16.8%]; IMV/ECMO, 31 [1.7%]), severe (no oxygen, 160 [19%]; oxygen, 486 [57.9%]; IMV/ECMO, 194 [23.1%]).
Patients’ Demographic and Epidemiological Characteristics
More than half of the cases were male (58.9%, 1542/2619), and male cases were more prevalent in severe categories (Table 1). The median age of the whole cohort was 56 years (IQR, 40–71 years), which was higher in severe cases (67; 54–78 years). The numbers of cases in each age group in each respiratory support category during hospitalization are shown in Supplementary Figure 1. The majority of cases (95.9%, 2528/2635) were Japanese, and most of the non-Japanese cases were East Asian (48/106), followed by white (22/106), South Asian (18/106), and Latin American (7/106).
Table 1.
Patients’ Demographic and Epidemiological Characteristics
| Severity at Admission | Respiratory Support During Hospitalization | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of Cases | Subcategories | Total | Nonsevere | Severe | No Oxygen | Oxygen | IMV/ECMO | |
| Demographics | ||||||||
| Sex | 2619 | Male | 1542 (58.9) | 975 (54.7)a | 567 (67.7) | 856 (53.2) | 508 (64.8) | 178 (79.1) |
| Female | 1077 (41.1) | 807 (45.3) | 270 (32.3) | 752 (46.8) | 276 (35.2) | 47 (20.9) | ||
| Age, median [IQR], years | 2625 | 56 [40, 71] | 51.5 [35, 66] | 67 [54, 78] | 49 [33, 63] | 68 [54, 80] | 66 [56, 77] | |
| Age (class) | 2625 | 0–9 years | 23 (0.9) | 19 (1.1) | 4 (0.5) | 21 (1.3) | 2 (0.3) | 0 |
| 10–19 years | 44 (1.7) | 41 (2.3) | 3 (0.4) | 42 (2.6) | 2 (0.3) | 0 | ||
| 20–29 years | 271 (10.3) | 245 (13.7) | 26 (3.1) | 250 (15.5) | 15 (1.9) | 6 (2.7) | ||
| 30–39 years | 298 (11.4) | 256 (14.3) | 42 (5) | 257 (15.9) | 34 (4.3) | 6 (2.7) | ||
| 40–49 years | 358 (13.6) | 275 (15.4) | 83 (9.9) | 254 (15.7) | 85 (10.8) | 19 (8.4) | ||
| 50–59 years | 478 (18.2) | 342 (19.1) | 136 (16.2) | 309 (19.2) | 126 (16.1) | 42 (18.7) | ||
| 60–69 years | 422 (16.1) | 242 (13.5) | 180 (21.5) | 206 (12.8) | 152 (19.4) | 64 (28.4) | ||
| 70–79 years | 392 (14.9) | 208 (11.6) | 184 (21.9) | 167 (10.4) | 168 (21.4) | 57 (25.3) | ||
| ≥80 years | 339 (12.9) | 158 (8.8) | 181 (21.6) | 107 (6.6) | 201 (25.6) | 31 (13.8) | ||
| Smoking history | 2627 | Currently smoking | 358 (13.6) | 266 (14.9) | 92 (11) | 241 (14.9) | 85 (10.8) | 31 (13.8) |
| Smoking in the past | 596 (22.7) | 334 (18.7) | 262 (31.3) | 276 (17.1) | 251 (32) | 69 (30.7) | ||
| Never | 1216 (46.3) | 886 (49.5) | 330 (39.4) | 832 (51.5) | 304 (38.8) | 79 (35.1) | ||
| Unknown | 457 (17.4) | 303 (16.9) | 154 (18.4) | 267 (16.5) | 144 (18.4) | 46 (20.4) | ||
| Drinking alcoholb | 2086 | Daily | 166 (8) | 103 (7.4) | 63 (9) | 90 (7.1) | 59 (9.2) | 17 (9.2) |
| Occasional | 676 (32.4) | 472 (34) | 204 (29.2) | 449 (35.7) | 164 (25.5) | 63 (34.1) | ||
| None | 654 (31.4) | 443 (31.9) | 211 (30.2) | 413 (32.8) | 198 (30.8) | 43 (23.2) | ||
| Unknown | 590 (28.3) | 370 (26.7) | 220 (31.5) | 307 (24.4) | 221 (34.4) | 62 (33.5) | ||
| BMI, median [IQR], kg/m2 | 2024 | 23.1 [20.5, 26] | 22.7 [20.2, 25.4] | 24 [21.5, 26.9] | 22.4 [20.1, 25.3] | 24 [21.5, 26.9] | 24.4 [22.5, 27.5] | |
| Exposures within 14 daysc | ||||||||
| Travel to countries with COVID-19 cases | 2634 | Yes | 192 (7.3) | 145 (8.1) | 47 (5.6) | 136 (8.4) | 40 (5.1) | 16 (7.1) |
| No | 2319 (88) | 1566 (87.2) | 753 (89.7) | 1406 (86.8) | 714 (90.7) | 197 (87.6) | ||
| Unknown | 123 (4.7) | 84 (4.7) | 39 (4.6) | 78 (4.8) | 33 (4.2) | 12 (5.3) | ||
| Close contactd with COVID-19 cases (including probable cases) | 2638 | Yes | 1537 (58.3) | 1118 (62.2) | 419 (49.9) | 1027 (63.3) | 427 (54.2) | 82 (36.4) |
| No | 776 (29.4) | 483 (26.9) | 293 (34.9) | 428 (26.4) | 249 (31.6) | 98 (43.6) | ||
| Unknown | 325 (12.3) | 197 (11) | 128 (15.2) | 168 (10.4) | 112 (14.2) | 45 (20) | ||
| Contact detailse | 1257 | Family | 414 (32.9) | 301 (33.4) | 113 (31.7) | 284 (34) | 97 (27.6) | 33 (47.1) |
| Workplace | 285 (22.7) | 226 (25.1) | 59 (16.5) | 221 (26.5) | 55 (15.6) | 9 (12.9) | ||
| Healthcare facility | 397 (31.6) | 245 (27.2) | 152 (42.6) | 208 (24.9) | 173 (49.1) | 16 (22.9) | ||
| Education facility | 4 (0.3) | 4 (0.4) | 0 | 3 (0.4) | 1 (0.3) | 0 | ||
| Presence in a healthcare facility where COVID-19 infections have been managed | 2637 | Yes | 304 (11.5) | 207 (11.5) | 97 (11.6) | 164 (10.1) | 125 (15.9) | 15 (6.7) |
| No | 2222 (84.3) | 1521 (84.6) | 701 (83.6) | 1391 (85.7) | 633 (80.4) | 196 (87.1) | ||
| Unknown | 111 (4.2) | 70 (3.9) | 41 (4.9) | 68 (4.2) | 29 (3.7) | 14 (6.2) | ||
| Presence in a cruise ship where COVID-19 outbreak occurred | 2634 | Yes | 82 (3.1) | 59 (3.3) | 23 (2.7) | 52 (3.2) | 21 (2.7) | 9 (4) |
| No | 2505 (95.1) | 1707 (95.1) | 798 (95.1) | 1535 (94.8) | 758 (96.3) | 210 (93.3) | ||
| Unknown | 47 (1.8) | 29 (1.6) | 18 (2.1) | 33 (2) | 8 (1) | 6 (2.7) | ||
| Had meal with ≥3 persons (excluding family members) | 2121 | Yes | 291 (13.7) | 207 (14.7) | 84 (11.8) | 198 (15.4) | 75 (11.6) | 18 (9.5) |
| No | 1033 (48.7) | 673 (47.7) | 360 (50.7) | 601 (46.8) | 342 (52.8) | 90 (47.4) | ||
| Unknown | 797 (37.6) | 531 (37.6) | 266 (37.5) | 484 (37.7) | 231 (35.6) | 82 (43.2) | ||
| Presence in “three Cs” (close contact in a closed and crowded space)f | 2122 | Yes | 320 (15.1) | 223 (15.8) | 97 (13.7) | 209 (16.3) | 77 (11.9) | 34 (18) |
| No | 1109 (52.3) | 728 (51.6) | 381 (53.7) | 651 (50.7) | 372 (57.3) | 86 (45.5) | ||
| Unknown | 693 (32.7) | 461 (32.6) | 232 (32.7) | 424 (33) | 200 (30.8) | 69 (36.5) | ||
| Occupationg | ||||||||
| Healthcare worker | 2133 | Yes | 176 (8.3) | 142 (10) | 34 (4.8) | 141 (10.9) | 25 (3.8) | 10 (5.3) |
| No | 1888 (88.5) | 1231 (86.8) | 657 (92) | 1103 (85.6) | 615 (93.9) | 170 (89.5) | ||
| Unknown | 69 (3.2) | 46 (3.2) | 23 (3.2) | 44 (3.4) | 15 (2.3) | 10 (5.3) | ||
| Restaurant worker | 2130 | Yes | 71 (3.3) | 50 (3.5) | 21 (2.9) | 46 (3.6) | 18 (2.8) | 7 (3.7) |
| No | 1913 (89.8) | 1269 (89.6) | 644 (90.3) | 1154 (89.6) | 591 (90.6) | 168 (88.4) | ||
| Unknown | 146 (6.9) | 98 (6.9) | 48 (6.7) | 88 (6.8) | 43 (6.6) | 15 (7.9) | ||
| Specific service workersh | 2132 | Yes | 55 (2.6) | 44 (3.1) | 11 (1.5) | 42 (3.3) | 9 (1.4) | 4 (2.1) |
| No | 1927 (90.4) | 1272 (89.7) | 655 (91.7) | 1152 (89.4) | 602 (92) | 173 (91.1) | ||
| Unknown | 150 (7) | 102 (7.2) | 48 (6.7) | 94 (7.3) | 43 (6.6) | 13 (6.8) |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; IMV/ECMO, invasive mechanical ventilation/extracorporeal membrane oxygenation; IQR, interquartile range; ISARIC, International Severe Acute Respiratory and Emerging Infection Consortium; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aAs the number of missing values differed for each parameter, the number of cases in each parameter’s severity/respiratory support category was used as the denominator for calculating the percentage.
bDaily: ≥3 cans beer per day, occasional includes daily alcohol intake <3 cans beer per day.
cPresence in a laboratory handling suspected or confirmed SARS-CoV-2 samples: 25/2638 (0.9%).
d“Close contact” was defined as in the ISARIC case record form [11].
eClose contact with nonfamily roommates: 18/1257 (1.4%).
fClose contact in a closed and crowded space such as sports gym, live music club, karaoke, game center, buffet, indoor party, conference, nightclub/bar, etc [14].
gRegardless of the contact with a confirmed COVID-19 case or a history of handling specimens from a confirmed COVID-19 case. Microbiology laboratory workers represented 5/2131 (0.2%). Two cases (2/2131: 0.1%) were commercial sex workers.
hIncluded nightlife business workers at bars and pubs, host and hostess clubs, nightclubs, and other similar work.
For exposures within 14 days of admission, contact with positive or probable COVID-19 cases comprised 58.3% (1537/2638) of cases. Cases with a history of presence in a healthcare facility where COVID-19 infections were managed represented 11.5% of cases (304/2637). Healthcare workers comprised 8.3% (176/2133) of cases.
Condition at Admission and Comorbidities
Compared with nonsevere cases, severe cases tended to have a higher temperature, heart rate, and respiratory rate on admission, and were less alert (Table 2). Pneumonia was much more prevalent by X-ray in severe cases than in nonsevere cases (86.9% [623/717] vs 52.1% [679/1303], respectively).
Table 2.
Condition at Admission and Comorbidities
| Severity at Admission | Respiratory Support During Hospitalization | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | n | Subcategories | Total | Nonsevere | Severe | No Oxygen | Oxygen | IMV/ECMO |
| Condition at admissiona | ||||||||
| Days from symptom onset to hospitalization, median [IQR] | 2324 | … | 7 [4, 10] | 6 [4, 10] | 7 [4, 10] | 7 [4, 10] | 6 [3, 9] | 7 [4.3, 9] |
| Body temperature, median [IQR], °C | 2635 | … | 37 [36.6, 37.7] | 36.9 [36.5, 37.5] | 37.5 [36.8, 38.3] | 36.8 [36.5, 37.4] | 37.5 [36.8, 38.3] | 37.7 [36.9, 38.4] |
| Heart rate, median [IQR], beats/minute | 2587 | … | 85 [76, 97] | 84 [75, 95] | 88 [78, 100] | 83.5 [75, 94.8] | 88 [78, 100] | 90 [80, 103.8] |
| Respiration rate, median [IQR], breaths/minute | 1683 | … | 18 [16, 22] | 17 [16, 18] | 23 [18, 26] | 18 [16, 20] | 20 [17, 24] | 22 [18, 28] |
| Systolic blood pressure, median [IQR], mm Hg | 2557 | … | 124 [112, 139] | 124 [112, 137] | 126 [112, 142] | 123 [112, 135] | 128 [113, 142] | 129 [114, 148] |
| Diastolic blood pressure, median [IQR], mm Hg | 2531 | … | 78 [69, 88] | 78 [69, 88] | 76 [67, 86] | 78 [70, 88] | 77 [67, 87] | 76 [67, 88.5] |
| AVPU scale | 1912 | A (alert) | 1793 (93.8) | 1235 (97.4)b | 558 (86.6) | 1150 (97.6) | 497 (89.2) | 146 (82.5) |
| V (verbal) | 88 (4.6) | 27 (2.1) | 61 (9.5) | 22 (1.9) | 47 (8.4) | 19 (10.7) | ||
| P (pain) | 19 (1) | 4 (0.3) | 15 (2.3) | 5 (0.4) | 9 (1.6) | 5 (2.8) | ||
| U (unresponsive) | 12 (0.6) | 2 (0.2) | 10 (1.6) | 1 (0.1) | 4 (0.7) | 7 (4) | ||
| SpO2 under room air, median [IQR], | 2185 | 97 [96, 98] | 97 [96, 98] | 94 [92, 95] | 97 [96, 98] | 95 [94, 97] | 94 [91, 96] | |
| Oxygen support | 2595 | None (room air) | 2185 (84.2) | 1755 (100) | 430 (51.2) | 1587 (100) | 520 (66.5) | 76 (33.9) |
| Noninvasive oxygen therapy | 349 (13.4) | 0 | 349 (41.5) | 0 | 261 (33.4) | 88 (39.3) | ||
| Invasive mechanical ventilation | 61 (2.4) | 0 | 61 (7.3) | 0 | 1 (0.1) | 60 (26.8) | ||
| Route of noninvasive O2 administration | 349 | Nasal cannula | 225 (64.5) | 0 | 225 (64.5) | 0 | 192 (73.6) | 33 (37.5) |
| Face mask | 67 (19.2) | 0 | 67 (19.2) | 0 | 38 (14.6) | 29 (33) | ||
| Reservoir mask | 52 (14.9) | 0 | 52 (14.9) | 0 | 27 (10.3) | 25 (28.4) | ||
| High-flow oxygen device | 5 (1.4) | 0 | 5 (1.4) | 0 | 4 (1.5) | 1 (1.1) | ||
| X-ray imaging findingc | 2020 | No abnormality | 683 (33.8) | 607 (46.6) | 76 (10.6) | 595 (50.2) | 82 (13) | 6 (2.9) |
| Pneumonia | 1302 (64.5) | 679 (52.1) | 623 (86.9) | 579 (48.9) | 538 (85.5) | 185 (89.8) | ||
| Abnormality (excl. pneumonia) | 35 (1.7) | 17 (1.3) | 18 (2.5) | 11 (0.9) | 9 (1.4) | 15 (7.3) | ||
| Finding by CTc | 1864 | No abnormality | 209 (11.2) | 190 (15.7) | 19 (2.9) | 192 (17.9) | 15 (2.5) | 2 (1) |
| Pneumonia | 1615 (86.6) | 1000 (82.6) | 615 (94) | 861 (80.3) | 575 (96.3) | 178 (91.8) | ||
| Abnormality (excluding pneumonia) | 40 (2.1) | 20 (1.7) | 20 (3.1) | 19 (1.8) | 7 (1.2) | 14 (7.2) | ||
| Comorbiditiesd | 2638 | n = 2638 | n = 1798 | n = 840 | n = 1623 | n = 788 | n = 225 | |
| Any comorbidity | 1235 (46.8) | 683 (38) | 552 (65.7) | 539 (33.2) | 527 (66.9) | 169 (75.1) | ||
| Myocardial infarction | 39 (1.5) | 19 (1.1) | 20 (2.4) | 15 (0.9) | 21 (2.7) | 3 (1.3) | ||
| Congestive heart failure | 96 (3.6) | 40 (2.2) | 56 (6.7) | 24 (1.5) | 64 (8.1) | 8 (3.6) | ||
| Peripheral vascular disease | 47 (1.8) | 21 (1.2) | 26 (3.1) | 18 (1.1) | 17 (2.2) | 12 (5.3) | ||
| Cerebrovascular disease | 145 (5.5) | 72 (4) | 73 (8.7) | 51 (3.1) | 78 (9.9) | 16 (7.1) | ||
| Paralysis | 30 (1.1) | 13 (0.7) | 17 (2) | 11 (0.7) | 17 (2.2) | 2 (0.9) | ||
| Dementia | 155 (5.9) | 85 (4.7) | 70 (8.3) | 68 (4.2) | 81 (10.3) | 6 (2.7) | ||
| COPD | 44 (1.7) | 11 (0.6) | 33 (3.9) | 9 (0.6) | 24 (3) | 11 (4.9) | ||
| Chronic lung disease (excluding COPD) | 66 (2.5) | 18 (1) | 48 (5.7) | 14 (0.9) | 40 (5.1) | 12 (5.3) | ||
| Bronchial asthma | 116 (4.4) | 74 (4.1) | 42 (5) | 64 (3.9) | 39 (4.9) | 13 (5.8) | ||
| Mild liver disease | 69 (2.6) | 40 (2.2) | 29 (3.5) | 29 (1.8) | 25 (3.2) | 15 (6.7) | ||
| Moderate to severe liver dysfunction | 6 (0.2) | 4 (0.2) | 2 (0.2) | 3 (0.2) | 3 (0.4) | 0 | ||
| Peptic ulcer | 22 (0.8) | 8 (0.4) | 14 (1.7) | 5 (0.3) | 14 (1.8) | 3 (1.3) | ||
| Hypertension | 396 (15) | 192 (10.7) | 204 (24.3) | 153 (9.4) | 180 (22.8) | 63 (28) | ||
| Hyperlipidemia | 216 (8.2) | 121 (6.7) | 95 (11.3) | 98 (6) | 82 (10.4) | 36 (16) | ||
| Diabetes without complications | 374 (14.2) | 185 (10.3) | 189 (22.5) | 142 (8.7) | 158 (20.1) | 74 (32.9) | ||
| Diabetes with complications | 67 (2.5) | 33 (1.8) | 34 (4) | 23 (1.4) | 36 (4.6) | 8 (3.6) | ||
| Obesity | 146 (5.5) | 85 (4.7) | 61 (7.3) | 58 (3.6) | 66 (8.4) | 22 (9.8) | ||
| Moderate to severe renal dysfunction | 37 (1.4) | 21 (1.2) | 16 (1.9) | 11 (0.7) | 20 (2.5) | 6 (2.7) | ||
| Hemodialysis before admission | 16 (0.6) | 10 (0.6) | 6 (0.7) | 5 (0.3) | 10 (1.3) | 1 (0.4) | ||
| Solid tumor | 103 (3.9) | 52 (2.9) | 51 (6.1) | 39 (2.4) | 48 (6.1) | 16 (7.1) | ||
| Leukemia | 17 (0.6) | 14 (0.8) | 3 (0.4) | 9 (0.6) | 7 (0.9) | 1 (0.4) | ||
| Lymphoma | 28 (1.1) | 21 (1.2) | 7 (0.8) | 9 (0.6) | 17 (2.2) | 2 (0.9) | ||
| Metastatic solid tumor | 33 (1.3) | 20 (1.1) | 13 (1.5) | 19 (1.2) | 11 (1.4) | 3 (1.3) | ||
| Collagen disease | 29 (1.1) | 13 (0.7) | 16 (1.9) | 12 (0.7) | 10 (1.3) | 7 (3.1) | ||
| HIV | 2 (0.1) | 1 (0.1) | 1 (0.1) | 1 (0.1) | 1 (0.1) | 0 | ||
| Immunosuppressione | 2593 | Yes | 95 (3.7) | 61 (3.4) | 34 (4.2) | 38 (2.4) | 43 (5.6) | 14 (6.5) |
| No | 2498 (96.3) | 1717 (96.6) | 781 (95.8) | 1569 (97.6) | 726 (94.4) | 201 (93.5) | ||
| Immunosuppression detailsf | 95 | Neutropenia | 8 (8.4) | 6 (9.8) | 2 (5.9) | 2 (5.3) | 6 (14) | 0 |
| Use of steroid in the past 1 month | 15 (15.8) | 7 (11.5) | 8 (23.5) | 3 (7.9) | 9 (20.9) | 3 (21.4) | ||
| Chemotherapy in the past 3 months | 66 (69.5) | 42 (68.9) | 24 (70.6) | 25 (65.8) | 35 (81.4) | 6 (42.9) | ||
| Radiotherapy in the past 3 months | 4 (4.2) | 4 (6.6) | 0 | 1 (2.6) | 2 (4.7) | 1 (7.1) | ||
| Solid-organ transplantation | 2 (2.1) | 2 (3.3) | 0 | 2 (5.3) | 0 | 0 | ||
| Immunosuppressant use in the past 3 months | 36 (37.9) | 27 (44.3) | 9 (26.5) | 18 (47.4) | 12 (27.9) | 6 (42.9) |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; CT, computed tomography; HIV, human immunodeficiency virus; IMV/ECMO, invasive mechanical ventilation/extracorporeal membrane oxygenation; IQR, interquartile range; SpO2, oxygen saturation.
aFirst available data at presentation/admission within 24 hours.
bAs the number of missing values differed for each parameter, the number of cases in each parameter’s severity/respiratory support category was used as the denominator for calculating the percentage.
cChest X-ray and chest CT were performed on admission in 2040/2634 (77.4%) (nonsevere: 1318/1794 [73.5%]; severe: 722/840 [86%]); and 1868/2630 (71%) (nonsevere: 1212/1791 [67.7%]; severe: 656/839 [78.2%]) cases, respectively.
dComorbidities that existed prior to the hospitalization for COVID-19 and are present; new onset diseases associated with COVID-19 are not included. Definitions were based on Charlson’s score unless otherwise specified [15]. Chronic lung disease (excluding COPD) was defined as patients with pulmonary diseases who are dyspneic with slight activity. Bronchial asthma and obesity were based on physicians’ diagnosis. No case was on peritoneal dialysis. Only 1 case and 2 cases had congenital heart disease and congenital malformation, respectively.
eImmunosuppression includes neutropenia (<500 neutrophils/μL), glucocorticoid/steroid use within 1 month (doses greater or equal to an equivalent of 20 mg of prednisone per day for at least 1 month), chemotherapy, radiation therapy, or immunosuppressant use (such as anti-tumor necrosis factor α therapy, anti–interleukin-6 receptor/anti-CD20 monoclonal antibodies, selective T-cell co-stimulation blocker, methotrexate, tacrolimus) in the past 3 months, post-transplantation, asplenia, primary immunodeficiency syndrome.
fMultiple immunosuppressions exist in some cases. No case had previous blood transplant, asplenia, or primary immunodeficiency syndrome.
Overall, patients with severe disease on admission and those who received supplemental oxygen or IMV/ECMO during hospitalization tended to have more comorbid conditions than patients who did not have severe disease/received no oxygen. In particular, patients with underlying cardiovascular diseases, diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD)/chronic lung diseases, and obesity were more frequently categorized as severe cases than those without these comorbidities. Additionally, 3.7% (95/2593) cases had an immunosuppressive status.
Signs and Symptoms at Hospital Admission
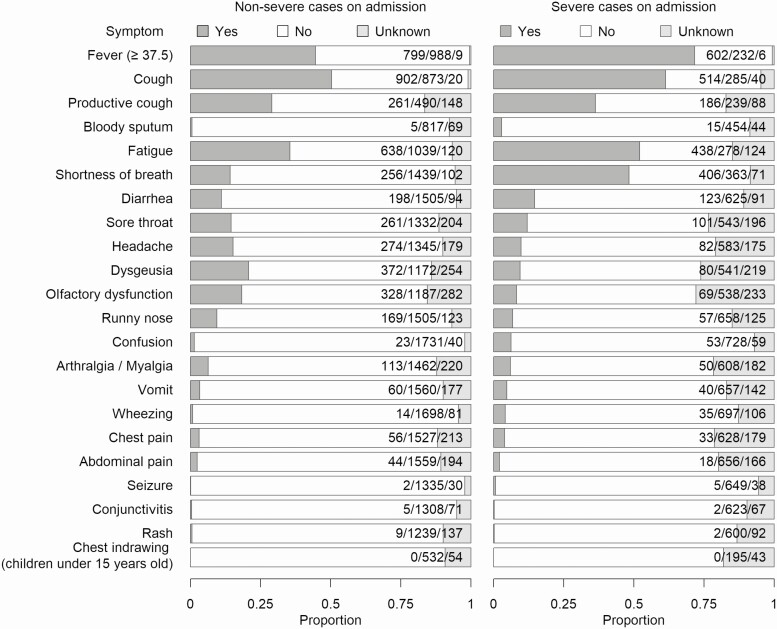
Of the 2113 cases, 6.9% (145) reported no symptoms, which accounted for 9.3% (132) of nonsevere cases and 1.9% (13) of severe cases (Figure 1, Supplementary Table 1). Approximately half of the patients had fever, cough, and fatigue. Compared with nonsevere cases, severe cases tended to present with fever, fatigue, cough, and shortness of breath. Conversely, dysgeusia and olfactory dysfunction were more common in nonsevere cases than in severe cases.
Figure 1. .
Signs and symptoms observed/reported at hospital admission and associated with the presented episode of acute illness. The proportions are shown by severity at admission.
Supportive Care and Outcome
Of the patients who were designated as severe at admission, 26.3% (221/840) required admission to the intensive care unit during their stay (Table 3). A tracheotomy was conducted in 14.4% (32/222) of patients who received IMV/ECMO. Of the patients who received IMV/ECMO, 13.8% (31/225) and 15.2% (34/224) required ECMO or renal replacement therapy/dialysis, respectively.
Table 3.
Supportive Care and Outcome
| Severity at Admission | Respiratory Support During Hospitalization | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Number of Cases | Subcategories | Total | Nonsevere | Severe | No Oxygen | Oxygen | IMV/ECMO |
| Supportive care during admissiona | ||||||||
| Admission to ICUb | 2638 | Yes | 282 (10.7) | 61 (3.4)c | 221 (26.3) | 19 (1.2) | 81 (10.3) | 182 (80.9) |
| No | 2356 (89.3) | 1737 (96.6) | 619 (73.7) | 1604 (98.8) | 707 (89.7) | 43 (19.1) | ||
| Length of ICU stay, median [IQR], days | 280 | … | 10 [5, 17] | 7 [4, 13.3] | 10.5 [5, 19] | 8 [3.5, 9.8] | 4 [2, 9] | 13 [8, 22] |
| High-flow oxygen device use | 2634 | Yes | 85 (3.2) | 19 (1.1) | 66 (7.9) | 0 | 54 (6.9) | 32 (14.3) |
| No | 2549 (96.8) | 1776 (98.9) | 773 (92.1) | 1622 (100) | 734 (93.1) | 191 (85.7) | ||
| Noninvasive mechanical ventilationd | 2634 | Yes | 38 (1.4) | 12 (0.7) | 26 (3.1) | 0 | 18 (2.3) | 20 (8.9) |
| No | 2596 (98.6) | 1783 (99.3) | 813 (96.9) | 1622 (100) | 769 (97.7) | 204 (91.1) | ||
| IMV | 2633 | Yes | 222 (8.4) | 31 (1.7) | 191 (22.8) | 0 | 0 | 224 (99.6) |
| No | 2411 (91.6) | 1764 (98.3) | 647 (77.2) | 1621 (100) | 785 (100) | 1 (0.4) | ||
| Days from admission to IMV, median [IQR] | 206 | … | 1 [0, 3] | 4 [4, 7] | 1 [0, 3] | NA | NA | 1 [0, 3] |
| Duration of IMV, median [IQR], days | 197 | … | 11 [7, 18] | 8 [4, 14] | 11 [7, 18] | NA | NA | 11 [7, 18] |
| Prone positioning | 221 | Yes | 52 (23.5) | 7 (22.6) | 45 (23.7) | 0 | 0 | 52 (23.5) |
| No | 169 (76.5) | 24 (77.4) | 145 (76.3) | 0 | 0 | 169 (76.5) | ||
| Nitric oxide inhalation | 221 | Yes | 4 (1.8) | 0 | 4 (2.1) | 0 | 0 | 4 (1.8) |
| No | 217 (98.2) | 31 (100) | 186 (97.9) | 0 | 0 | 217 (98.2) | ||
| Tracheotomy | 222 | Yes | 32 (14.4) | 3 (9.7) | 29 (15.2) | 0 | 0 | 32 (14.4) |
| No | 190 (85.6) | 28 (90.3) | 162 (84.8) | 0 | 0 | 190 (85.6) | ||
| Neuromuscular blocking agent | 168 | Yes | 71 (42.3) | 11 (52.4) | 60 (40.8) | 0 | 0 | 71 (42.3) |
| No | 97 (57.7) | 10 (47.6) | 87 (59.2) | 0 | 0 | 97 (57.7) | ||
| ECMO | 2635 | Yes | 31 (1.2) | 2 (0.1) | 29 (3.5) | 0 | 0 | 31 (13.8) |
| No | 2604 (98.8) | 1795 (99.9) | 809 (96.5) | 1623 (100) | 786 (100) | 194 (86.2) | ||
| Days from admission to ECMO, median [IQR] | 26 | … | 5 [3, 7.5] | 4.5 [4.3, 4.8] | 5 [2.5, 8] | NA | NA | 5 [3, 7.5] |
| Duration of ECMO, median [IQR], days | 26 | … | 9.5 [8, 14.5] | 6 [4.5, 7.5] | 10.5 [8, 15] | NA | NA | 9.5 [8, 14.5] |
| Vasopressor/inotropic support | 2637 | Yes | 121 (4.6) | 13 (0.7) | 108 (12.9) | 0 | 7 (0.9) | 114 (50.7) |
| No | 2516 (95.4) | 1784 (99.3) | 732 (87.1) | 1623 (100) | 781 (99.1) | 111 (49.3) | ||
| RRT or dialysis | 2628 | Yes | 50 (1.9) | 14 (0.8) | 36 (4.3) | 8 (0.5) | 8 (1) | 34 (15.2) |
| No | 2578 (98.1) | 1776 (99.2) | 802 (95.7) | 1611 (99.5) | 776 (99) | 190 (84.8) | ||
| Type of RRT or dialysis | 50 | Hemodialysis | 22 (44) | 10 (76.9) | 12 (32.4) | 6 (85.7) | 8 (88.9) | 8 (23.5) |
| CRRT | 28 (56) | 3 (23.1) | 25 (67.6) | 1 (14.3) | 1 (11.1) | 26 (76.5) | ||
| Blood transfusion | 2631 | Yes | 126 (4.8) | 29 (1.6) | 97 (11.6) | 7 (0.4) | 25 (3.2) | 94 (42) |
| No | 2505 (95.2) | 1764 (98.4) | 741 (88.4) | 1613 (99.6) | 761 (96.8) | 130 (58) | ||
| Immunoglobulin | 2631 | Yes | 69 (2.6) | 28 (1.6) | 41 (4.9) | 6 (0.4) | 45 (5.7) | 18 (8) |
| No | 2562 (97.4) | 1764 (98.4) | 798 (95.1) | 1613 (99.6) | 741 (94.3) | 207 (92) | ||
| Outcome at discharge | ||||||||
| Outcome | 2634 | Discharge to home | 1762 (66.9) | 1320 (73.5) | 442 (52.7) | 1249 (77.1) | 442 (56.2) | 69 (30.8) |
| Transfer to another hospital | 437 (16.6) | 244 (13.6) | 193 (23) | 184 (11.4) | 180 (22.9) | 73 (32.6) | ||
| Transfer to nonmedical facilitye | 194 (7.4) | 160 (8.9) | 34 (4.1) | 157 (9.7) | 32 (4.1) | 5 (2.2) | ||
| Transfer to long-term care facility | 44 (1.7) | 23 (1.3) | 21 (2.5) | 24 (1.5) | 18 (2.3) | 2 (0.9) | ||
| Death | 197 (7.5) | 49 (2.7) | 148 (17.7) | 7 (0.4) | 115 (14.6) | 75 (33.5) | ||
| Duration of hospitalization, median [IQR], days | 2586 | … | 15 [9, 23] | 14 [9, 22] | 17 [10, 26] | 14 [9, 20] | 19 [11, 27] | 19 [10, 33] |
| Days from symptom onset to death, median [IQR] | 190 | … | 17 [11, 24] | 15 [11, 33] | 18 [12, 24] | 12.5 [5.5, 30] | 14 [9, 22] | 21 [15.8, 30] |
| Finding by X-rayf | 950 | No abnormality | 418 (44) | 301 (52.2) | 117 (31.4) | 287 (60.3) | 112 (30.9) | 19 (17) |
| Pneumonia | 499 (52.5) | 263 (45.6) | 236 (63.3) | 181 (38) | 237 (65.5) | 81 (72.3) | ||
| Abnormality (excluding pneumonia) | 33 (3.5) | 13 (2.3) | 20 (5.4) | 8 (1.7) | 13 (3.6) | 12 (10.7) | ||
| Finding by CTf | 465 | No abnormality | 81 (17.4) | 60 (21.1) | 21 (11.7) | 60 (24.3) | 15 (9.8) | 6 (9.2) |
| Pneumonia | 365 (78.5) | 219 (76.8) | 146 (81.1) | 183 (74.1) | 133 (86.9) | 49 (75.4) | ||
| Abnormality (excluding pneumonia) | 19 (4.1) | 6 (2.1) | 13 (7.2) | 4 (1.6) | 5 (3.3) | 10 (15.4) | ||
| Body temperature,g median [IQR], °C | 2475 | … | 36.7 [36.4, 36.9] | 36.7 [36.4, 36.9] | 36.8 [36.5, 37.1] | 36.6 [36.4, 36.8] | 36.7 [36.5, 37] | 37.1 [36.7, 37.9] |
| Tracheotomyh | 2409 | Yes | 28 (1.2) | 7 (0.4) | 21 (3.1) | 5 (0.3) | 6 (0.9) | 17 (11.6) |
| No | 2381 (98.8) | 1722 (99.6) | 659 (96.9) | 1593 (99.7) | 657 (99.1) | 129 (88.4) | ||
| Self-care abilityh | 2425 | Same as before the onset of COVID-19 | 2045 (84.3) | 1575 (90.6) | 470 (68.4) | 1489 (92.8) | 500 (74.6) | 54 (36.2) |
| Worsened | 237 (9.8) | 83 (4.8) | 154 (22.4) | 34 (2.1) | 119 (17.8) | 84 (56.4) | ||
| Improved | 106 (4.4) | 64 (3.7) | 42 (6.1) | 68 (4.2) | 35 (5.2) | 3 (2) | ||
| Unknown | 37 (1.5) | 16 (0.9) | 21 (3.1) | 13 (0.8) | 16 (2.4) | 8 (5.4) | ||
| Walking abilityh | 1849 | Same as before the onset of COVID-19 | 1542 (83.4) | 1174 (90.7) | 368 (66.4) | 1121 (93.3) | 379 (72.6) | 42 (33.6) |
| Worsened | 204 (11) | 59 (4.6) | 145 (26.2) | 27 (2.2) | 105 (20.1) | 72 (57.6) | ||
| Improved | 58 (3.1) | 36 (2.8) | 22 (4) | 35 (2.9) | 20 (3.8) | 3 (2.4) | ||
| Unknown | 45 (2.4) | 26 (2) | 19 (3.4) | 19 (1.6) | 18 (3.4) | 8 (6.4) | ||
| Oxygen therapy requiredh | 2430 | Yes | 182 (7.5) | 54 (3.1) | 128 (18.6) | 2 (0.1) | 115 (17.2) | 65 (43.6) |
| No | 2248 (92.5) | 1689 (96.9) | 559 (81.4) | 1608 (99.9) | 554 (82.8) | 84 (56.4) | ||
| RRT or dialysish | 2431 | Yes | 16 (0.7) | 11 (0.6) | 5 (0.7) | 8 (0.5) | 6 (0.9) | 2 (1.3) |
| No | 2415 (99.3) | 1731 (99.4) | 684 (99.3) | 1604 (99.5) | 662 (99.1) | 147 (98.7) |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: COVID-19, coronavirus disease 2019; CRRT, continuous renal replacement therapy; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IMV, invasive mechanical ventilation; IQR, interquartile range; NA, not available; RRT, renal replacement therapy.
aIf the patient received these treatments at any time during hospitalization, the treatment was included in analysis. Plasmapheresis (PMX-DHP [polymyxin B-immobilized fiber column-direct hemoperfusion]) was conducted in 3 IMV/ECMO cases.
bReadmission to ICU and reintubation were rare (3/282 [1.1%]: 1 case with oxygen and 2 IMV/ECMO cases, 5/220 [2.3%]: all IMV/ECMO cases, respectively).
cAs the number of missing values differed for each parameter, the number of cases in each parameter’s severity/respiratory support category was used as the denominator for calculating the percentage.
dNoninvasive mechanical ventilation includes BIPAP (biphasic positive airway pressure) or CPAP (continuous positive airway pressure).
eFacilities for isolation purposes for whom no medical or nursing care is necessary.
fData on chest X-ray and chest CT were for those performed within 1 week of discharge and closest to the date of discharge. Chest X-ray and chest CT were performed in 986/2428 (40.6%) (no oxygen: 492/1608 [30.6%]; oxygen: 378/670 [56.4%]; IMV/ECMO: 116/148 [78.4%]) and 470/2428 (19.4%) (no oxygen: 251/1611 [15.6%]; oxygen: 154/667 [23.1%]; IMV/ECMO: 65/148 [43.9%]) cases, respectively.
gMaximum body temperature within 24 hours of discharge from hospital.
hData were counted only on the patients who were alive at discharge.
The majority in the no-oxygen group (1249/1621 [77.1%]), more than half in the oxygen group (442/787 [56.2%]), and approximately one-third in the IMV/ECMO group (69/224 [30.8%]) were discharged home. Seven cases (7/1621 [0.4%]) in the no-oxygen group, 115 (115/787 [14.6%]) in the oxygen group, and 75 (75/224 [33.5%]) in the IMV/ECMO group died in the hospital. Approximately 10% (17/146) of patients who received IMV/ECMO had a tracheotomy at discharge and 43.6% (65/149) needed oxygen. Self-care ability and walking ability were worsened in more than half of the patients who received IMV/ECMO upon discharge (84/149 [56.4%] and 72/125 [57.6%], respectively).
History of Drug Administration During Hospitalization
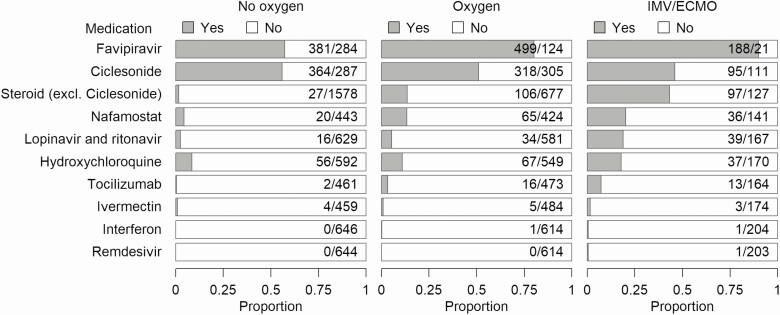
The most common medications used in this cohort were favipiravir, and ciclesonide (only available as an inhalant) (Figure 2, Supplementary Table 2). Favipiravir, lopinavir/ritonavir, and hydroxychloroquine were more frequently used as the severity of the disease increased, while ciclesonide was used in half of the patients regardless of the severity of the disease. Steroids (excluding ciclesonid), nafamostat, and tocilizumab tended to be used more commonly in patients who received IMV/ECMO; this trend was particularly pronounced with steroid treatment (no oxygen, 1.7% [27/1605]; oxygen, 13.5% [106/783]; IMV/ECMO, 43.3% [97/224]). Ivermectin, remdesivir, and interferon were rarely used in this cohort.
Figure 2. .
History of drug administration during hospitalization. Medications with antiviral effect against SARS-CoV-2, immunomodulatory effect against COVID-19, and/or immunosuppressive effect against COVID-19 were included. Proportions are shown by respiratory support during hospitalization. Ciclesonide is available only as an inhalant. Abbreviations: COVID-19, coronavirus disease 2019; excl., excluding; IMV/ECMO, invasive mechanical ventilation/extracorporeal membrane oxygenation; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Antimicrobials were used in more than half of the patients who received oxygen (58.5%, 456/780) and in the majority of patients who received IMV/ECMO (92.8%, 207/223). Antifungal drugs and anticoagulants were used in 9.4% (21/224) and 47.6% (107/225) of the patients who received IMV/ECMO, respectively.
Complications During Hospitalization
Acute respiratory distress syndrome (ARDS) was the most common complication in patients who received IMV/ECMO (70.2%, 158/225) (Supplementary Table 3). Bacterial pneumonia was also prevalent (6.9%, 181/2633), but methicillin-resistant Staphylococcus aureus pneumonia (n = 13) and Pseudomonas aeruginosa pneumonia (n = 10) were much less common. Diagnosed deep-vein thrombosis (0.9%, 19/1873) or pulmonary embolism (PE) (0.5%, 11/2046) accounted for less than 1% of all cases. Bacteremia accounted for 7.1% (16/225) of patients who received IMV/ECMO, and gastrointestinal bleeding accounted for 5.4% (12/224) of patients who received IMV/ECMO.
Discussion
This is the largest study to date describing the clinical epidemiological characteristics of hospitalized patients with COVID-19 in Japan. Although this study did not capture all hospitalized COVID-19 cases due to voluntary participation, the dataset represents approximately 13% (2638/19 816) of the number of confirmed COVID-19 cases in Japan as of 7 July 2020 [7].
Our findings related to demographics showed that severe cases tended to be males, patients with past smoking history, and older patients, which are consistent with existing reports of risk of severe disease and death in patients with COVID-19 [16–18]. Although the median age of our cases (56 [40–71] years) was comparable to or slightly lower than existing inpatient data [17, 19, 20], 12.9% of the cohort was aged 80 or older, reflecting the long life expectancy in Japan [21]. In patients aged 60 years or older, the proportions of severely ill patients increased; however, the proportion of patients who received IMV/ECMO was lower among patients aged 80 or older than patients in their 60s and 70s. This might partly be due to the less aggressive treatment approaches for this population.
As more than half of the inpatients had a history of close contact with confirmed or suspected COVID-19 cases, the strategy to target clusters of patients with a history of close contacts was considered beneficial in terms of identifying the risk of infection. Although this does not necessarily imply healthcare-associated transmission, presence in a healthcare facility where COVID-19 infections have been managed was found in 11.5% of the cases; therefore, healthcare-associated facilities also seem to be an important target for controlling the spread of COVID-19. The proportion of healthcare workers in our cohort is higher than in other reports (3.8–5.1%) [22]. This may be partly due to different patient populations (eg, cases other than inpatients) included.
Comorbidities were also studied in this patient population. Cardiovascular disease, DM, chronic lung disease/COPD, and obesity were more prevalent in severe cases, consistent with previous reports [2, 17, 20]. The frequency of cardiovascular complications, DM (16.7%), and obesity (5.5%) was lower than that reported in the United States (DM, 28.3–34.7%; body mass index >30 kg/m2: 39.6–48.3%) [17, 23], in the United Kingdom (DM without complications, 20.7%; obesity, 10.5%) [16], and in an inpatient cohort in China (DM, 19–20%) [2, 20].
In this study, fever, cough, dyspnea, and fatigue were more likely to be present in severely ill patients, whereas olfactory dysfunction and dysgeusia were more likely to be present in those with nonsevere disease. The incidence of fever, cough, dyspnea, and fatigue was lower in our study than in the existing inpatient studies but this could be influenced by the phase of the epidemic and admission criteria in each country [16, 23]. Different prevalences have been reported on olfactory and taste disturbances [24, 25]. These symptoms have been increasingly recognized in Japan as symptoms of COVID-19 since the end of March [26], which could have influenced the testing and identification of patients who presented with these specific symptoms.
In this cohort, which included the early phase of cases of COVID-19 in Japan, favipiravir and ciclesonide were the predominant antiviral drugs administered. Patients who received IMV/ECMO tended to be treated more frequently with steroids and nafamostat. In Japan, as of 29 July 2020, some steroids (prednisolone and dexamethasone) were indicated for severe infections, but only remdesivir is approved for the treatment of COVID-19 (approved on 7 May 2020). All other drugs (listed in Figure 2) were used off-label. Regardless of whether or not the drug was used, inpatients with COVID-19 could be enrolled voluntarily in this registry. However, favipiravir users were more likely to be included in the registry because healthcare providers are encouraged to participate when they contact the Ministry of Health, Labor, and Welfare about the provision of favipiravir. The preliminary report of the Favipiravir Observational Study in Japan included more elderly and more comorbid patients than that observed for this registry [27], and reported higher in-hospital mortality (11.6%). As favipiravir users were more likely to be included in this registry, data of patients in this cohort may have been slightly more severely skewed than those of the general Japanese population hospitalized with COVID-19. As noted in the Methods section, patients enrolled in other clinical studies may not have been included in this registry. It is also important to note that we did not count concomitant therapies in the current analysis. Evaluation of the efficacy of antiviral and immunomodulatory agents is outside the scope of this study and requires further studies. Anticoagulants were used in less than half of the patients who received IMV/ECMO; however, their use is expected to increase as the evidence for anticoagulation therapy is being recognized.
In our inpatient cohort, the mortality rate was 7.5% of cases, and 33.5% of the patients who received IMV/ECMO. The mortality rate in this study was lower than those in previously reported inpatient cohorts (approximately 15% to >20%) [2, 16, 17, 19, 20, 28]. Additionally, mortality was lower even among intubated patients [16, 17, 20]. As some of these studies included patients with an undetermined outcome during hospitalization, the quantification of mortality may be even higher. The lower rate of mechanical ventilation than in previous studies [16, 17, 19, 20] and the lower number of severe cases at admission than in a study using the same criteria for severity [13] suggest lower overall severity of hospitalized patients in Japan. Some of the studies with significantly higher mortality, especially among mechanically ventilated patients [17, 20], may reflect the impact on healthcare infrastructure (eg, unavailability to intubate in a timely manner) due to the rapid increase in patients in the region. The reasons for the relatively lower mortality in Japan might be due to factors such as a lower number of comorbid patients or a lack of a drastic increase in patient numbers during the period of patient enrollment. Approximately 70% of the patients in our cohort had nonsevere disease on admission, and they would have been managed as outpatients in other countries. This may have also contributed to the low mortality observed in our cohort compared with that in other countries. Further investigation into the factors associated with severity of the disease would be necessary to expand on this.
Although this registry does not include the information on the exact cause of death, cases of patient death despite no oxygen use during hospitalization had underlying diseases: 5 patients had malignancy (3/5 were metastatic tumors) and 2 patients were in their 80s and 90s with dementia.
Some caveats with regard to the interpretation of the results of this study are warranted. Although this study covered a large number of inpatients with COVID-19 in Japan, there may have been some selection bias in their inclusion, as noted above, and also due to the manual input of the data. Thus, the study results may not completely reflect those of the general Japanese population hospitalized with COVID-19. Deep-vein thrombosis and PE were quite low compared with existing reports and were likely to be underreported as they were based on diagnoses of each institution [29]. Future validation using objective indicators such as D-dimer is therefore necessary. Although the data center provides a data-input manual, an input check function, and inquiries to institutions, the accuracy of the data may be lower than that of clinical trials. In addition, due to the nature of the registry, data are updated daily, and it is possible that the findings using this registry may diverge from the presented results in the future. National policies for COVID-19 are in flux, and changes to regulations such as discharge criteria (ie, from mandatory SARS-CoV-2 negativity to criteria based on the days from the disease onset) were made during the collection of these registry data, which may have affected the duration of hospitalization [30, 31].
We reveal the characteristics of diverse clinical epidemiological features of COVID-19 in hospitalized patients in Japan. Compared with existing inpatient studies in other countries, these data show that the patient population with COVID-19 in Japan had fewer comorbidities, and there was a trend toward lower mortality.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Part of these data was presented at a media seminar at the National Center for Global Health and Medicine on 6 August 2020 in Tokyo, Japan.
Acknowledgments. The authors thank all the participating facilities for their care of patients with COVID-19 and their cooperation on data entry to the registry.
Financial support. This research was funded by the Health and Labor Sciences Research grant, “Research for risk assessment and implementation of crisis management functions for emerging and re-emerging infectious diseases.”
Potential conflicts of interest. H. O. reports personal fees as a statistician and as an external consultant for clinical trials from EPS International, outside the submitted work. S. S. reports grants from Shionogi, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect 2020; 80:e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ministry of Health, Labour and Welfare. Current status of the novel coronavirus infection and the response of the MHLW. 4 February 2020. Available at: https://www.mhlw.go.jp/stf/newpage_09290.html. Accessed 15 September 2020.
- 4. Expert Meeting on the Novel Coronavirus Disease Control. Views on the novel coronavirus disease control. 9 March 2020. Available at: https://www.mhlw.go.jp/content/10900000/000608425.pdf. Accessed 15 September 2020.
- 5. Inoue H. Japanese strategy to COVID-19: How does it work? J Glob Health 2020; 2:131–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Our World in Data. Japan: coronavirus pandemic. Available at: https://ourworldindata.org/coronavirus/country/japan?country=~JPN. Accessed 15 September 2020.
- 7. Ministry of Health, Labour and Welfare. Current status of the novel coronavirus infection and the response of the MHLW. 2020. Available at: https://www.mhlw.go.jp/stf/newpage_12312.html. Accessed 15 September 2020.
- 8. Statistic Bureau, Ministry of Internal Affairs and Communications. Population projection 2020 June report. Available at: https://www.stat.go.jp/data/jinsui/pdf/202006.pdf. Accessed 15 September 2020.
- 9. National Center for Global Health and Medicine. COVID-19 Registry Japan. Available at: https://covid-registry.ncgm.go.jp. Accessed 15 September 2020.
- 10. Ministry of Health, Labour and Welfare. Notification of physicians and veterinarians under the Infectious Diseases Act. Available at: https://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou11/01-shitei-01.html. Accessed 15 September 2020.
- 11.International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC). COVID-19 clinical research resources. Available at: https://isaric.tghn.org/covid-19-clinical-research-resources/. Accessed 15 September 2020.
- 12. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Co vid-19—Final Report. New Engl J Med 2020; doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 14. Expert Meeting on the Novel Coronavirus Disease Control. Analysis and recommendations of the response to the novel coronavirus (COVID-19). 1 April, 2020. Available at: https://www.mhlw.go.jp/content/10900000/000620826.pdf. Accessed 15 September 2020.
- 15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 16. Docherty AB, Harrison EM, Green CA, et al. ; ISARIC4C Investigators . Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020; 369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020; 369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet 2017; 389:1323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med 2020; 173:120–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lechien JR, Chiesa-Estomba CM, Hans S, Barillari MR, Jouffe L, Saussez S. Loss of smell and taste in 2013 European patients with mild to moderate COVID-19. Ann Intern Med 2020; doi: 10.7326/M20-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance—United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The Japan Times. Lo ss of smell and taste may be symptoms of coronavirus, experts say. Available at: https://www.japantimes.co.jp/news/2020/03/29/national/science-health/loss-smell-taste-may-symptoms-coronavirus-infection-experts/. Accessed 15 September 2020.
- 27. Favipiravir Observational Study Group. Preliminary report of the Favipiravir Observational Study in Japan. Available at: http://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_casereport_en_200529.pdf. Accessed 15 September 2020.
- 28. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 324:782–93. [DOI] [PubMed] [Google Scholar]
- 29. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemostasis 2020; 18:1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ministry of Health, Labour and Welfare. Handling of patients with novel coronavirus infections discharged from the hospital and restriction of work under the Act on Prevention of Infectious Diseases and Medical Care for Patients With Infectious Diseases. 29 May 2020. Available at: https://www.mhlw.go.jp/content/000635398.pdf. Accessed 15 September 2020.
- 31. Ministry of Health, Labour and Welfare. Handling of patients with novel coronavirus infections discharged from the hospital and restriction of work under the act on prevention of infectious diseases and medical care for patients with infectious diseases. 12 June, 2020. Available at: https://www.mhlw.go.jp/content/000639691.pdf. Accessed 15 September 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.