To the Editor—The coronavirus disease (COVID) pandemic has been a threatening challenge to health systems worldwide [1]. In this context, the use of anti-inflammatory therapy to treat severe COVID patients has been controversial during the whole pandemics. General recommendations were against the use of corticosteroids [2], although some hope was placed on monoclonal anti interleukin 6 receptor antibodies in blocking the so-called cytokine storm produced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in selected patients [3, 4]. Recently published papers provide high-quality evidence that supports the use of corticosteroids in the early stage of severe COVID [5]. Nevertheless, a high rate of treatment failure was observed in both treatment subgroups (34.9% vs 54.3%).
In our experience, the early administration of corticosteroids associated to tocilizumab, according to the previously published recommendations by the Spanish Ministry of Health [6], may solve this high failure rate. In the HM Sanchinarro Hospital, a 200 bed teaching institution, we consecutively and ambispectively collected the outcome of 136 patients who received tocilizumab plus corticosteroids to treat severe COVID, defined as a SpO2/FiO2 <325 with bilateral pneumonia and a clinical diagnosis of infection by SARS-CoV-2. Patients were divided in 2 subgroups: those who received tocilizumab after 24 hours of admission and before SpO2/FiO2 decreased to ≤250 (early tocilizumab administration [ET], n = 38) and those who received the combination directly at admission or when SpO2/FiO2 was <250 (standard therapy [ST], n = 98). Statistical analysis was performed to predict a composite outcome of intensive care unit (ICU) admission and in-hospital mortality.
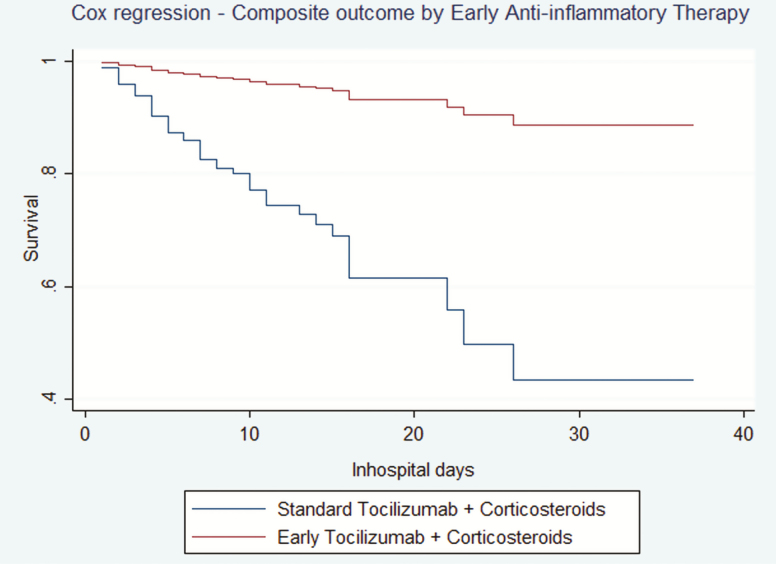
Mean age was 68 years (95% confidence interval [CI], 46–94), with a 72.8% of male individuals. 47 patients reached the composite outcome. Mean comorbidity burden was of 3.35 (95% CI, 2.96–3.75) points according to the Charlson comorbidity index (CCI). In the raw analysis, a 6.25% of patients in the ET group developed the composite outcome, whereas 45.3% of the patients in the ST group were admitted to the ICU and/or died during admission. A χ 2 analysis was performed to compare early and standard groups of the Fadel et al cohort with patients who received early and late combined anti-inflammatory therapy in our cohort. No differences were found in the late therapy groups (45.3% vs 54.3%, P = .54), although statistically different results were found between early therapy subgroups (6.25% vs 34.9%, P < .01). Generalized linear and Cox regression models, adjusted by age, sex, interaction between age and sex, CCI, time from onset to admission, dose of corticosteroids and tocilizumab and C-reactive protein and D-dimer levels at anti-inflammatory therapy administration, showed benefit in the use of ET in COVID patients (Relative Risk, 0.18, P = .01, and Hazard Ratio, 0.13, P = .01, respectively). Cox model results stratified by time to anti-inflammatory therapy administration are shown in (Figure 1).
Figure 1.
Multivariate Cox regression. Comparison between standard and early anti-inflammatory therapy in severe COVID-19.
The results of our cohort may reinforce the early administration of anti-inflammatory therapy published by Fadel et al. In addition, the promising results of the combination of corticosteroids and tocilizumab highlight the need of further investigations in the anti-inflammatory treatment of severe COVID.
Note
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. Coronavirusdisease 2019 (COVID-19) situation reports; 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed 21 June 2020.
- 2. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020; 395:473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capra R, De Rossi N, Mattioli F, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med 2020; 76:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fadel R, Morrison A, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drugs and Sanitary Products Spanish Agency, available treatments for management of SARS-CoV2 respiratory infection Available at: https://www.aemps.gob.es/la-aemps/ultima-informacion-de-la-aemps-acerca-del-covid%E2%80%9119/tratamientos-disponibles-para-el-manejo-de-la-infeccion-respiratoria-por-sars-cov-2/on19. May 2020.