Abstract
Background
The coronavirus disease 2019 (COVID‐19) pandemic has become a public health emergency affecting frail populations, including patients with cancer. This poses the question of whether cancer treatments can be postponed or modified without compromising their efficacy, especially for highly curable cancers such as germ cell tumors (GCTs).
Materials and Methods
To depict the state‐of‐the‐art management of GCTs during the COVID‐19 pandemic, a survey including 26 questions was circulated by e‐mail among the physicians belonging to three cooperative groups: (a) Italian Germ Cell Cancer Group; (b) European Reference Network–Rare Adult Solid Cancers, Domain G3 (rare male genitourinary cancers); and (c) Genitourinary Medical Oncologists of Canada. Percentages of agreement between Italian respondents (I) versus Canadian respondents (C), I versus European respondents (E), and E versus C were compared by using Fisher's exact tests for dichotomous answers and chi square test for trends for the questions with three or more options.
Results
Fifty‐three GCT experts responded to the survey: 20 Italian, 6 in other European countries, and 27 from Canada. Telemedicine was broadly used; there was high consensus to interrupt chemotherapy in COVID‐19–positive patients (I = 75%, C = 55%, and E = 83.3%) and for use of granulocyte colony‐stimulating factor primary prophylaxis for neutropenia (I = 65%, C = 62.9%, and E = 50%). The main differences emerged regarding the management of stage I and stage IIA disease, likely because of cultural and geographical differences.
Conclusion
Our study highlights the common efforts of GCT experts in Europe and Canada to maintain high standards of treatment for patients with GCT with few changes in their management during the COVID‐19 pandemic.
Implications for Practice
Despite the chaos, disruptions, and fears fomented by the COVID‐19 illness, oncology care teams in Italy, other European countries, and Canada are delivering the enormous promise of curative management strategies for patients with testicular cancer and other germ cell tumors. At the same time, these teams are applying safe and innovative solutions and sharing best practices to minimize frequency and intensity of patient contacts with thinly stretched health care capacity.
Keywords: COVID‐19, Curable tumors, Expert centers, Germ cell tumors, Pandemic, Testicular cancer
Short abstract
This article focuses on state‐of‐the‐art management of testicular germ cell tumors during the COVID‐19 pandemic, highlighting different approaches based on the pandemic experience in each individual country.
Introduction
The outbreak of coronavirus disease 2019 (COVID‐19) has become a public health emergency since the World Health Organization declared the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) a pandemic on March 11, 2020 [1]. First China, and, by the end of February 2020, Italy, experienced uncontrolled spread of SARS‐CoV‐2 and an increased number of cases of viral illness–related deaths [2]. There was higher recorded lethality in Italy compared with China (9% vs. 4.3%) [3]. Following the Chinese model, Italy was the second country to promptly activate containment measures and to restructure its health care system. Although the severity of the COVID‐19 illness and the risk of death appears to be associated with older age, male sex, and preexisting comorbidities (such as diabetes, obesity, cardiopulmonary disease, and immunosuppression) [4], patients with cancer and cancer survivors who often share these risk factors could represent additional high‐risk populations [5]. Moreover, most treatments in oncology cannot be postponed without compromising efficacy, especially for cancers with high cure rates, such as testicular germ cell tumors (GCTs). GCTs are the most prevalent solid malignancies in young men aged 15–35 years, with a rising incidence among whites [6]. To date, GCT remains one of the most curable solid cancers, even in cases of metastatic spread, with approximately 95% of men surviving at 5 years, because of the exquisite sensitivity of these cancers to cisplatin‐based chemotherapy [7, 8], with the highest cure rate for patients with clinical stage I (CSI) exceeding 99% [9, 10].
In the midst of the epidemic in Italy, physicians within the Italian Germ Cell Cancer Group initiated an international survey with colleagues involved in the European Reference Network–Rare Adult Solid Cancers (ERN‐EURACAN) Domain G3 network and Canadian cancer centers belonging to the Genitourinary Medical Oncologists of Canada (GUMOC), with the aim of depicting the actual state‐of‐art management of GCTs during the pandemic and to highlight relative different approaches based on each individual country's pandemic experience. Furthermore, the survey was intended to bring to light provider concerns regarding safely delivering the full promise of time‐tested management pathways for patients with and survivors of GCT.
Materials and Methods
The survey consists of 26 questions, focusing on COVID‐19 screening, treatment, follow‐up, supportive therapy, and surgery for patients with GCT during the COVID‐19 pandemic. The estimated time of completion was approximately 3 minutes. The questions selected for the survey were discussed within the Italian Germ Cell Cancer Group (IGG). A version of the survey is provided in supplemental online Appendix 1.
The survey was sent by e‐mail, from March 30 to April 17, 2020, to the physicians belonging to three high expertise GCT cooperative study groups: (a) IGG, (b) European Reference Network ERN EURACAN Domain G3 (Rare male genito‐urinary cancers), and (c) GUMOC. The answers were collected by one reference member for each group. The surveys were analyzed with blinding to the authors' identity and affiliation. The percentage of agreement between Italy versus Canada, Italy versus Europe, and Europe versus Canada were compared by using Fisher's exact tests for dichotomous answers and chi square test for trends for the questions with three or more options. All tests were two‐sided. Significance was assumed at p < .05. The data were analyzed using GraphPad Prism version 8.3.0 (GraphPad Software Inc. San Diego, CA). The epidemiology data describing the COVID‐19 prevalence and lethality were extracted from the worldometers Web site (https://www.worldometers.info/coronavirus/#countries); the datawrapper software (https://www.datawrapper.de) was used to create the epidemic graph maps.
Results
Characteristics of the Respondent
From March 30 to April 17, 2020, 53 experts in GCT treatments responded to the survey: 20 Italian (I), 6 in other European (E) countries, and 27 from Canada (C) (national and regional distributions are shown in Table 1. All the European, 95% of the Italian, and 89% of the Canadian physicians were medical oncologists, whereas 5% and 11% of the Italian and Canadian health professionals, respectively, were urologists. The list of the participating institutions is reported in supplemental online Tables 1–3. Figure 1 reports the epidemic graph maps of COVID‐19 pandemic at the time of the survey responses for each of the participant countries.
Table 1.
Regional and national distribution of the survey participants
| Location | n (%) |
|---|---|
| Italian regions | |
| North west | 5 (33.3) |
| North East | 4 (26.7) |
| Center | 1 (6.7) |
| South | 5 (33.3) |
| Canadian provinces | |
| Alberta | 6 (22.2) |
| British Columbia | 5 (18.5) |
| Manitoba | 3 (11.1) |
| New Brunswick | 1 (3.7) |
| Nova Scotia | 1 (3.7) |
| Ontario | 6 (22.2) |
| Prince Edward Island | 1 (3.7) |
| Quebec | 4 (14.8) |
| European countries | |
| France | 1 (16.6) |
| Germany | 1 (16.6) |
| Spain | 1 (16.6) |
| The Netherlands | 2 (33.3) |
| United Kingdom | 1 (16.6) |
Figure 1.
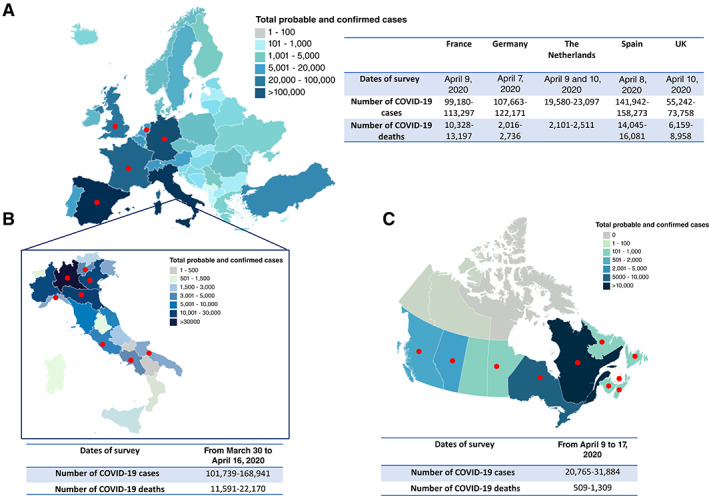
Epidemiology of COVID‐19. Epidemic graphic maps of Europe (A), Italy (B), and Canada (C) at the time the survey was circulated among the European Reference Network–Rare Adult Solid Cancers Domain G3, Italian Germ Cell Cancer Group, and Genitourinary Medical Oncologists of Canada. The maps report the prevalence of the patients positive for COVID‐19 corresponding to the last day of the survey. The tables report the survey dates and the prevalence of COVID‐19–positive patients and COVID‐19–related deaths at the time of the survey. The red dots mark the geographic areas of the respondent physicians.
Abbreviation: COVID‐19, coronavirus disease 2019.
Similarities and Differences Among the GCT Experts
The results of the survey are summarized in Figure 2. Patients with GCTs were preferentially assessed for COVID‐19–related symptoms by telephone consultation the day before their hospital appointment and then through physical examinations in dedicated areas (C = 51.8%; Italy = 40%). The other European participants used telephone screening in 80% of the cases. Only a minority of patients with GTC were tested for SARS‐CoV‐2 with nasopharyngeal swabs prior to starting chemotherapy (I = 15%, C = 7.4%, and E = 33.3%). It is recognized that reliability and availability of testing and analysis was very spotty at the time of the survey.
Figure 2.
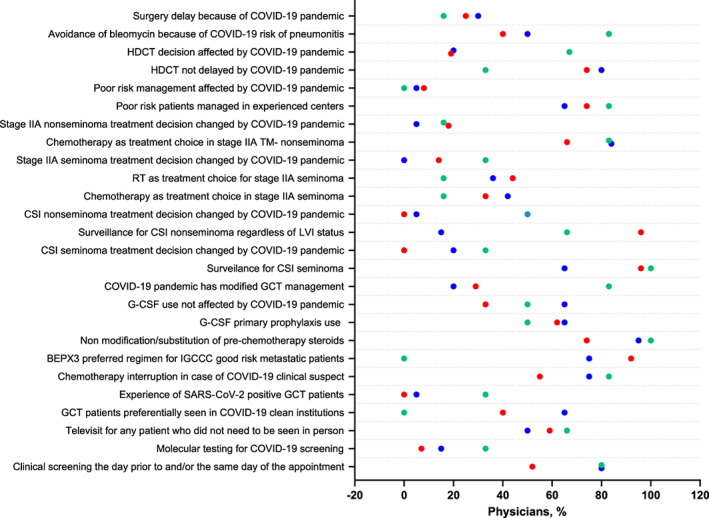
Testicular germ cell tumors management during COVID‐19 pandemic. Summary plots report the percentage of Italian (blue), Canadian (red), and European (green) responses to the GCT patient management survey during the COVID‐19 pandemic.
Abbreviations: BEP, bleomycin, etoposide, and platinum; COVID‐19, coronavirus disease 2019; CSI, clinical stage I; G‐CSF, granulocyte colony‐stimulating factor; GCT, germ cell tumor; HDCT, high‐dose chemotherapy; LVI, lymphovascular invasion; RT, radiotherapy; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TM, tumor markers.
Telemedicine was broadly used for any patient with GCT (including patients on active chemotherapy) who did not need to be seen in person (I = 50%, C = 60%, and E = 70%).
None of the Canadian physicians had a patient with GCT positive for SARS‐CoV‐2, whereas 5% and 33.3% of the Italian and European physicians had positive patients.
Despite these differences, there was high consensus about judicious chemotherapy interruptions based on International Germ Cell Cancer Collaborative Group (IGCCCG) risk and resuming the treatment after a SARS‐CoV‐2–negative result for patients with clinical suspicious COVID‐19 (I = 75%, C = 55%, and E = 83.3%).
Although 50% of the Italian, 44% of the Canadian, and 83% of the European respondents expressed some concerns about the use of bleomycin, there was a large consensus among the Italian and Canadian participants about the use of bleomycin, etoposide and cisplatinum (BEP) × 3 rather than etoposide and cisplatin (EP) × 4 in patients with IGCCCG good‐risk metastatic GCT (BEP × 3 preferred option for I = 75%, C = 92.5%). The European participants preferred to use EP × 4 rather than BEP × 3 because of the COVID‐19 pandemic (E = 100%; I vs. E, p = .02; C vs. E, p = .001).
In terms of supportive treatment, there was a consensus for the use of primary prophylactic granulocyte colony‐stimulating factor (G‐CSF) for the patients treated with chemotherapy (used by I = 65%, C = 62.9%, and E = 50%). Steroid modifications or substitutions were generally not adopted by the physicians surveyed.
Poor‐risk patients were followed in experienced centers in most cases (I = 65%, C = 74.07%, and E = 83.3%), and this strategy was not affected by the COVID‐19 pandemic.
Finally, surgical delay was reported by 30% of the Italian physicians, 25.9% of the Canadian physicians, and 16.6% of the European physicians. However, this difference was not statistically significant. Few Italian (20%) and Canadian (30%) physicians felt the COVID‐19 pandemic changed their treatment choice; however, 84.6% of European physicians believed the COVID‐19 pandemic did influence management of patients with GCT (I vs. E and C vs. E: p = .009 and p = .02, respectively).
The most discrepancies were observed for the management of CSI. Surveillance was the preferred options for patients with CSI seminoma in Canadian and European experiences, whereas 35% of the Italian participants opted for carboplatin (I vs. C, p = .0048). This decision was not affected by the pandemic for the Canadian physicians, whereas it was in 20% of Italian and European cases (I vs. C, p = .02; C vs. E, p = .02).
Similarly, for patients with CSI nonseminoma, the treatment management was variable among the different participating groups. Whereas surveillance, regardless of the lymphovascular invasion (LVI) status, was the preferred approach for 96.6% of the Canadian physicians, 70% of the Italian and 66.6% of the European doctors preferred a risk adjusted strategy reserving adjuvant chemotherapy for the LVI‐positive patients and surveillance for LVI‐negative patients (I vs. C, p = .002; C vs. E, p < .0001). In addition, 50% of the European physicians declared that this decision was affected by COVID‐19, whereas 5% of the Italian and none of the Canadian physicians interviewed felt the COVID‐19 pandemic influenced their treatment decision (I vs. E, p = .02; C vs. E, p = .003). Management of patients with stage IIA seminoma also differed among the participants: surveillance was the first choice of the European oncologists (66.6%), whereas the Italian oncologists chose preferentially chemotherapy (42.1%) and the Canadian oncologists radiation therapy (44.4%) (I vs. E, p = .04; C vs. E, p = .03). This decision was not affected by the COVID‐19 pandemic, according to most of the respondents.
Overall, 65% and 40% of the Italian and Canadian patients with GCT, respectively, were seen in COVID‐19–clean hospitals, compared with none for the other European participants (I vs. E, p < .0001).
Finally, there was a statistically significant difference between the Canadian and the European participants regarding the delay of high‐dose chemotherapy (HDCT) for the patients with cisplatin‐resistant/refractory GCT. Indeed, 66.6% of the European physicians reported a delay in HDCT compared with 20% and 18.51% of the Italian and Canadian participants (C vs. E, p = .04). This was related to the COVID‐19 pandemic according to 66.6% of the European, 20% of the Italian, and 14.81% of the Canadian doctors (C vs. E, p = .02).
Discussion
The unexpected and rapid spread of SARS‐CoV‐2 has left many oncologists facing unprecedented challenges. While the COVID‐19 pandemic advances, new cancer diagnoses and management thereof proceed unabated. The challenge of delivering highly reliable cancer management plans that often require frequent and prolonged interactions with the health care system and may or may not have synergistic toxicities with viral infection and viral illness is perhaps best exemplified by care of young patients with germ cell tumors [11].
Our study is a snapshot of the concerns of and measures adopted by GCT experts to deliver the best‐quality treatment without jeopardizing the success of cure.
Although the COVID‐19 pandemic has changed the health care structure, management strategies for patients with GCT largely have remained intact among the experienced centers reflected in our survey. This most likely reflects the priority to guarantee a high level of cure for patients with GCT, as also shown by the low rate of elective surgical delays as well as the management of patients with poor‐risk GCT in experienced centers. Conversely, the discrepancy between the use of HDCT in patients with cisplatin‐resistant/refractory GCT in Italy and Canada versus Europe may be related to the disruption of clinical trials enrollment (i.e., TIGER [12]) rather than the underuse or delay of HDCT.
The low rate of SARS‐CoV‐2 molecular testing for patients with GCT prior to chemotherapy is due to limited resources rather than an evidence‐based choice. As such, verbal COVID‐19 symptom and contact screening prior to the clinic appointment was the most common procedure.
COVID‐19–clean areas were most used in Italy. This discrepancy was likely related to the fact that Italy was the first Western country to face the pandemic, and as such, at the time of the survey, the infrastructure to create differential assistance pathways on the basis of the patients' viral status was already in place.
Interestingly, although most of the interviewed physicians were concerned about bleomycin lung toxicity, BEP × 3 was still the preferred option for patients with IGCCCG good‐risk metastatic GCT among the Italian and Canadian GCT experts, whereas the European GCT experts preferred to use EP × 4 because of the COVID‐19 pandemic. Although pneumonia and acute respiratory distress syndrome are considered the most severe and potentially fatal complications of COVID‐19 [13], there is no evidence that bleomycin increases the risk of COVID‐19 pneumonitis or respiratory failure. It is known that EP × 4 adds short‐ and long‐term toxicity while demonstrating higher rates of primary treatment failure and no difference in pulmonary toxicity compared with BEP × 3 [10, 14]. It is equally known that surveillance has excellent outcomes in both CSI seminoma and nonseminoma with no treatment‐related toxicity [15]. Although surveillance was the preferred option for CSI seminoma, in CSI nonseminoma the Italian and European centers were opting for chemotherapy for LVI‐positive patients. Those results emphasize the pre–COVID‐19 higher use in Canada of active surveillance for patients with CSI nonseminoma, irrespective of LVI status, compared with the European countries.
Active treatments (either radiotherapy or chemotherapy in seminoma and chemotherapy in nonseminoma) were also preferred to surveillance in stage IIA patients. Considering the high false positive clinical assessment rate in this patient population [16], surveillance or retroperitoneal lymph node dissection in nonseminomatous GCT and in experienced centers could be valid chemotherapy‐sparing options [17, 18].
All the experts shared their enthusiasm for the use virtual medicine, and it is possible that this will continue for certain patient populations even after the acute phase of the pandemic is over.
The oft‐used pandemic aphorism of “We are all in this together” is particularly apt when it comes to the global community of patients with germ cell tumor and their providers. Our community has been generally marked by a high degree of research collaboration, evidence sharing, and patient‐centered focus. COVID‐19 has swiftly and profoundly reordered priorities in global health care delivery, economies, and public health. As we move to an offensive posture regarding this crisis, it is imperative that we redouble our ability to collaborate, share evidence and best practices, and build resilient, low‐cost, rapid learning platforms for an uncertain future.
Recommendations
We have summarized the viewpoints of the 53 highly experienced germ cell tumor practitioners who participated to this survey in a brief list of recommendations that we believe are helpful for management of patients with GCT during the COVID‐19 pandemic. This is not a formally developed guideline but rather reflects the opinion of a robust number of expert physicians involved in GCT care.
Active surveillance should be the preferred option for patients with CSI seminoma.
Patients with CSI nonseminoma should preferably be offered active surveillance irrespective of lymphovascular invasion status.
Chemotherapy should be withheld until an active COVID‐19 infection has resolved or has been ruled out with highly accurate molecular testing.
Because of lacking data on increased lung toxicity in case of COVID‐19 infection, bleomycin should not routinely be omitted.
Patients with advanced GCT with IGCCCG good‐risk disease should not routinely be treated with four cycles of EP.
Primary G‐CSF prophylaxis during cisplatin‐based combination chemotherapy should be considered in any patients with advanced GCT receiving chemotherapy, including BEP.
Curative high‐dose chemotherapy should not be postponed or suspended. Consideration of high‐dose chemotherapy in the context of the TIGER clinical trial should be pursued within the institutions' trial capacity and regulation activated for the COVID‐19 pandemic.
Surgery, including residual tumor resection, should not be postponed.
Conclusion
With the limitations the geographical differences of the COVID‐19 epidemiology, the results of our study highlight the common efforts of GCT experts in Europe and Canada in maintaining high standards of treatment and therefore the expected cure rate for patients with GCT even during the COVID‐19 pandemic.
Author Contributions
Conception/design: Lucia Nappi, Margaret Ottaviano, Pasquale Rescigno, Christian Kollmannsberger, Craig Nichols, Giovannella Palmieri
Provision of study material or patients: Lucia Nappi, Margaret Ottaviano, Giuseppe L. Banna, Giuseppe L. Banna, Giulia Baciarello, Umberto Basso, Christina Canil, Alessia Cavo, Maria Cossu Rocca, Piotr Czaykowski, Ugo De Giorgi, Xavier Garcia del Muro, Marilena Di Napoli, Giuseppe Fornarini, Jourik A. Gietema, Daniel Y.C. Heng, Sebastien J. Hotte, Christian Kollmannsberger, Marco Maruzzo, Carlo Messina, Franco Morelli, Sasja Mulder, Franco Nolè, Christoph Oing, Teodoro Sava, Simona Secondino, Giuseppe Simone, Denis Soulieres, Bruno Vincenzi, Paolo A. Zucali, Giovannella Palmieri
Collection and/or assembly of data: Lucia Nappi, Margaret Ottaviano, Pasquale Rescigno, Marianna Tortora, Giovannella Palmieri
Data analysis and interpretation: Lucia Nappi, Margaret Ottaviano, Pasquale Rescigno, Giovannella Palmieri
Manuscript writing: Lucia Nappi, Margaret Ottaviano, Pasquale Rescigno, Christina Canil, Piotr Czaykowski, Jourik A. Gietema, Christian Kollmannsberger, Craig Nichols, Christoph Oing, Giovannella Palmieri
Final approval of manuscript: Lucia Nappi, Margaret Ottaviano, Pasquale Rescigno, Marianna Tortora, Giuseppe L. Banna, Giulia Baciarello, Umberto Basso, Christina Canil, Alessia Cavo, Maria Cossu Rocca, Piotr Czaykowski, Ugo De Giorgi, Xavier Garcia del Muro, Marilena Di Napoli, Giuseppe Fornarini, Jourik A. Gietema, Daniel Y.C. Heng, Sebastien J. Hotte, Christian Kollmannsberger, Marco Maruzzo, Carlo Messina, Franco Morelli, Sasja Mulder, Craig Nichols, Franco Nolè, Christoph Oing, Teodoro Sava, Simona Secondino, Giuseppe Simone, Denis Soulieres, Bruno Vincenzi, Paolo A. Zucali, Sabino De Placido, Giovannella Palmieri
Disclosures
Giulia Baciarello: Hoffmann‐La Roche Ltd, Astellas Pharma, Janssen Oncology, Sanofi (H), Hoffman‐La Roche Ltd, Astellas Pharma, Janssen Oncology, EuroPharma, Simon‐Kucher & Partners, Modra Pharmaceuticals, Sanofi (RF); Christina Canil: Roche, Bayer, Janssen, Eisai, Astellas, Merck, Ipsen, Pfizer, Bristol‐Myers Squibb (C/A), Sanofi Genzyme, Janssen (other—educational travel grant); Marilena Di Napoli: Tesaro, GlaxoSmithKline, AstraZeneca (SAB); Jourik A. Gietema: Roche, Siemens, Abbvie (RF—institution). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Coronavirus disease (COVID‐19) pandemic . World Health Organization Web site. Available at https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed March 22, 2020.
- 2. Lazzerini M, Putoto G. COVID‐19 in Italy: Momentous decisions and many uncertainties. Lancet Glob Health 2020;8:e641–e642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO coronavirus disease (COVID‐19) dashboard . World Health Organization Web site. Available at https://covid19.who.int/. Accessed March 19, 2020.
- 4. Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult in patients with COVID‐19 in Wuhan, China: A retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang W, Guan W, Chen R et al. Cancer patients in SARS‐CoV‐2 infection: A nationwide analysis in China. Lancet Oncol 2020;21:335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albers P, Albrecht W, Algaba F et al. Guidelines on testicular cancer: 2015 update. Eur Urol 2015;68:1054–1068. [DOI] [PubMed] [Google Scholar]
- 7. Verhoeven RH, Coebergh JW, Kiemeney LA et al. Testicular cancer: Trends in mortality are well explained by changes in treatment and survival in the southern Netherlands since 1970. Eur J Cancer 2007;43:2553–2558. [DOI] [PubMed] [Google Scholar]
- 8. Richiardi L, Scelo G, Boffetta P et al. Second malignancies among survivors of germ‐cell testicular cancer: A pooled analysis between 13 cancer registries. Int J Cancer 2007;120:623–631. [DOI] [PubMed] [Google Scholar]
- 9. Condello C, Rescigno P, Ottaviano M et al. Clinical features and psychological aspects of the decision‐making process in stage I testicular germ cell tumors. Future Oncol 2018;14:1591–1599. [DOI] [PubMed] [Google Scholar]
- 10. Kollmannsberger C, Tandstad T, Bedard PL et al. Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol 2015;33:51–57. [DOI] [PubMed] [Google Scholar]
- 11. Kutikov A, Weinberg DS, Edelman MJ et al. A war on two fronts: Cancer care in the time of COVID‐19. Ann Intern Med 2020;172:756–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Standard‐Dose Combination Chemotherapy or High‐Dose Combination Chemotherapy and Stem Cell Transplant in Treating Patients with Relapsed or Refractory Germ Cell Tumors. TIGER trial. ClinicalTrials.gov identifier: NCT0237504. Bethesda, MD: U.S. National Library of Medicine, March 2, 2015.
- 13. Grasselli G, Zangrillo A, Zanella A et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nichols C, Kollmannsberger C. Alternatives to standard BEP x 3 in good‐prognosis germ cell tumors–you bet your life. J Natl Cancer Inst 2010;102:1214–1215. [DOI] [PubMed] [Google Scholar]
- 15. Culine S, Kerbrat P, Kramar A et al. Refining the optimal chemotherapy regimen for good‐risk metastatic nonseminomatous germ‐cell tumors: A randomized trial of the Genito‐ Urinary Group of the French Federation of Cancer Centers (GETUG T93BP). Ann Oncol 2007;18:917–924. [DOI] [PubMed] [Google Scholar]
- 16. Stephenson AJ, Bosl GJ, Bajorin DF et al. Retroperitoneal lymph node dissection in patients with low stage testicular cancer with embryonal carcinoma predominance and/or lymphovascular invasion. J Urol 2005;174:557–560. [DOI] [PubMed] [Google Scholar]
- 17. Kollmannsberger CK, Nappi L, Nichols C. Management of stage II germ cell tumors: Be sure, be patient, be safe. J Clin Oncol 2019;37:1856–1862. [DOI] [PubMed] [Google Scholar]
- 18. Hamilton RJ, Nayan M, Anson‐Cartwright L et al. Treatment of relapse of clinical stage I nonseminomatous germ cell tumors on surveillance. J Clin Oncol 2019;37:1919–1926. [DOI] [PubMed] [Google Scholar]


