Abstract
Lessons Learned
Treatment with the Aurora kinase A inhibitor yields often durable disease control, but limited tumor regression, in heavily pretreated patients with unresectable malignant pleural or peritoneal mesothelioma.
In a limited sample size, MYC copy‐number gain or gene amplification, a candidate predictive biomarker for alisertib, did not correlate with improved response numbers or patient outcomes.
Background
Malignant mesothelioma is an aggressive disease for which few effective therapies are available. The Aurora family kinases are critical for mitotic fidelity and highly expressed in mesothelioma, wherein their inhibition leads to growth arrest in vitro. We evaluated the efficacy of alisertib, an Aurora A kinase inhibitor, in relapsed malignant mesothelioma.
Methods
Twenty‐six patients with previously treated, unresectable pleural or peritoneal mesothelioma were enrolled on a single‐arm, single‐institution phase II trial of alisertib at a dosage of 50 mg twice daily for 7 of every 21 days. The primary endpoint was 4‐month disease control rate. Secondary endpoints included overall response rate, progression free survival, overall survival, safety/toxicity, and correlation of endpoints with MYC copy number.
Results
Of the 25 evaluable patients treated on study, 8 (32%) experienced 4‐month disease control, surpassing the futility endpoint. There were no confirmed partial or complete responses. Median progression‐free and overall survival were 2.8 months and 6.3 months, respectively. No associations between MYC copy number and outcomes were observed.
Conclusion
Alisertib has modest activity in this unselected malignant mesothelioma population. Several patients achieved durable disease control. Although the study did meet its prespecified futility endpoint, the sponsor elected to close the trial at the interim analysis.
Discussion
There are no Food and Drug Administration–approved therapies for patients with pretreated malignant mesothelioma, and this is a significant unmet need. Alisertib is a selective small molecule inhibitor of Aurora A kinase [1]. Preclinical studies have strongly suggested Aurora kinases to be relevant therapeutic targets in patients with mesothelioma. Aurora kinase gene expression is upregulated in mesothelioma tumor tissue and is a negative prognostic factor [2]. Aurora A kinase expression is strongly correlated with the expression of MYC oncogene, and increased expression of MYC, via overexpression or gene amplification, has been shown to predict sensitivity to Aurora kinase inhibitors [3]. Molecular profiling of mesothelioma identified MYC copy number gains (≥4 copies by fluorescence in situ hybridization [FISH]) in 23% of biphasic mesotheliomas and 13% of epithelioid mesotheliomas [4].
The present study is a single‐arm, single‐institution phase II trial to evaluate the efficacy and safety of alisertib in patients with unresectable pleural or peritoneal mesothelioma who had received at least one prior line of therapy, including platinum‐pemetrexed. The primary endpoint target was 4‐month disease control rate (DCR) of >30%. The observed 4‐month DCR was 32% (8/25), which met the prespecified efficacy endpoint for the interim analysis. Unfortunately, strategic changes at the sponsor level precluded expansion of this study as planned.
Among the overall evaluable population (n = 25), 1 patient (4.0%) had a documented minor response as best response, whereas an additional 13 patients (52.0%) experienced stable disease as best response. Median progression‐free and overall survival were 2.8 months (95% confidence interval [CI], 1.3–4.0) and 6.3 months (95% CI, 5.7–11.1), respectively (Fig. 1). The most common adverse events were fatigue, alopecia, anemia, nausea, and oral mucositis, and most events were grade 1 or 2. There were no grade 4 toxicities reported. There was one death on study, as a result of complications related to pre‐existing renal failure and pulmonary disease, including probable disease progression. An additional secondary endpoint related to MYC copy number identified between two and five patients with tumors bearing MYC copy number gain/amplification, depending on FISH criteria, but there was no correlation with response, duration of response, or survival in this small data set. Following relapse, patients with mesothelioma are typically re‐treated with platinum‐based chemotherapy or a variety of single‐agent immunotherapies or chemotherapies, each with modest and similar clinical benefit. An accepted standard of care for previously treated mesothelioma remains elusive, and additional, effective therapies are badly needed. Although alisertib, specifically, is unlikely to undergo further development for mesothelioma owing to shifting developmental strategies, several patients did achieve prolonged disease control. Further investigation is warranted to evaluate the role for Aurora kinase inhibitors and MYC as a biomarker in relapsed mesothelioma.
Figure 1.
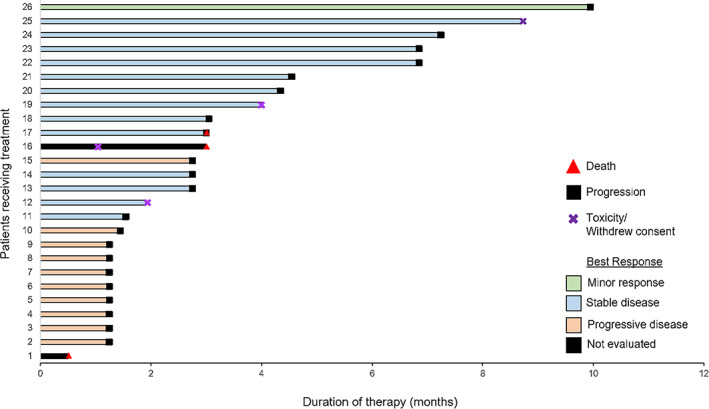
Individual swimmer plots for all patients receiving alisertib on protocol. Median progression‐free survival was 2.8 months (range: 0.5–10.0 months).
Trial Information
| Disease | Mesothelioma |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | 1 prior regimen |
| Type of Study | Phase II, single arm |
| Primary Endpoint | 4‐month disease control rate |
| Secondary Endpoints | Progression‐free survival, overall survival, overall response rate, safety, correlative endpoint |
| Additional Details of Endpoints or Study Design | |
| Treatment Plan: We conducted a single‐arm, single‐institution phase II trial of alisertib administered 50 mg twice daily for 7 days every 21 days at the University of Texas MD Anderson Cancer Center. Patient eligibility included unresectable pleural or peritoneal mesothelioma, up to four lines of prior therapy, including at least one prior platinum‐pemetrexed combination therapy, and adequate organ function. | |
| Assessments: Efficacy was assessed by either modified RECIST (mRECIST; preferred) or RECIST criteria (if mRECIST could not be performed) every 6 weeks. Archival or fresh tissue at baseline was collected to assess MYC copy number and evaluate the association between short‐term responses and subgroups defined based on FISH criteria for MYC gene analysis. | |
| Endpoints and Statistical Analysis: The primary endpoint was 4‐month disease control rate and secondary endpoints were survival (progression‐free and overall), overall response rate, duration of response, safety/toxicity, the association between responses and subgroups defined by MYC copy number gain. An interim analysis was planned after 24 evaluable patients were enrolled. Evaluable patients were identified as those who completed at least one cycle (i.e., 50‐mg doses twice daily for days 1–7 of first cycle) of alisertib treatment. The trial was conducted according to the Simon's minimax two‐stage design and the disease control rate at 4 months was estimated accordingly. It was assumed that the new regimen would have a target disease control rate of 50% at 4 months. A disease control rate of 30% or lower would be considered a failure and the new regimen would be rejected under this circumstance. When the probability of accepting a “bad” regimen (i.e., disease control rate ≤ 30%) is 0.05 and the probability of rejecting a “good” regimen (i.e., disease control rate ≥ 50%) is 0.10, Simon's minimax design requires entry of 24 patients in the first stage. If 7 or fewer patients are alive and free of disease progression at 4 months following initiation of treatment, the trial would be stopped and the regimen declared as ineffective. If 8 or more patients were alive and progression free at 4 months, 29 more patients would be entered in the study to reach a total of 53 patients. By the end of the study, the new regimen would be rejected if the disease control rate is less than or equal to 21/53 and would be accepted otherwise. | |
| Investigator's Analysis | Active but results overtaken by other developments |
Drug Information
| Generic/Working Name | Alisertib |
| Trade Name | MLN8237 |
| Company Name | Millennium/Takeda |
| Drug Type | Small molecule |
| Drug Class | Mitotic ‐ Aurora kinase |
| Dose | 50 mg per flat dose |
| Route | p.o. |
| Schedule of Administration | Twice daily days 1–7 of 21‐day cycle |
Patient Characteristics
| Number of Patients, Male | 24 |
| Number of Patients, Female | 2 |
| Stage | Advanced/metastatic |
| Age | Median (range): 69 (44–85) |
| Number of Prior Systemic Therapies | Median (range): 1 (1–4) |
| Performance Status: ECOG |
0 — 7 1 — 15 2 — 4 3 — Unknown — |
| Other | Prior radiation only: 2; prior surgery only: 4; prior radiation and surgery: 2 |
| Cancer Types or Histologic Subtypes | Epithelioid mesothelioma, 21; biphasic mesothelioma, 5 |
Primary Assessment Method: Experimental
| Title | 4‐month disease control rate |
| Number of Patients Screened | 28 |
| Number of Patients Enrolled | 26 |
| Number of Patients Evaluable for Toxicity | 25 |
| Number of Patients Evaluated for Efficacy | 25 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment OTHER | n = 8 (32%) |
| Primary endpoint of 4‐month disease control rate was defined as no more than 19% increase from baseline in tumor volume, and therefore, complete response, partial response, or stable disease, when assessed at 16 weeks |
Secondary Assessment Method: Experimental
| Title | Overall response rate |
| Number of Patients Screened | 28 |
| Number of Patients Enrolled | 26 |
| Number of Patients Evaluable for Toxicity | 25 |
| Number of Patients Evaluated for Efficacy | 25 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 0 (0%) |
| Response Assessment SD | n = 14 (56%) |
| Response Assessment PD | n = 10 (40%) |
| Response Assessment OTHER | n = 1 (4%) |
| Title | Progression‐free and overall survival |
| Number of Patients Screened | 28 |
| Number of Patients Enrolled | 26 |
| Number of Patients Evaluable for Toxicity | 25 |
| Number of Patients Evaluated for Efficacy | 25 |
| (Median) Duration Assessments PFS | 2.8 months, CI: 1.3–4.0 |
| (Median) Duration Assessments OS | 6.3 months, CI: 5.7–11.1 |
Primary Assessment Method: Control
| Title | 4‐month disease control rate |
| Number of Patients Screened | 28 |
| Number of Patients Enrolled | 26 |
| Number of Patients Evaluable for Toxicity | 25 |
| Number of Patients Evaluated for Efficacy | 25 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment OTHER | n = 8 (32%) |
Adverse Events
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
| Fatigue | 36% | 52% | 12% | 0% | 0% | 0% | 64% |
| Alopecia | 44% | 48% | 8% | 0% | 0% | 0% | 56% |
| Anemia | 48% | 12% | 24% | 16% | 0% | 0% | 52% |
| Nausea | 60% | 40% | 0% | 0% | 0% | 0% | 40% |
| Pharyngeal mucositis | 64% | 24% | 8% | 4% | 0% | 0% | 36% |
| Constipation | 72% | 28% | 0% | 0% | 0% | 0% | 28% |
| Platelet count decreased | 72% | 16% | 12% | 0% | 0% | 0% | 28% |
| Vomiting | 72% | 28% | 0% | 0% | 0% | 0% | 28% |
| Neutrophil count decreased | 80% | 0% | 8% | 12% | 0% | 0% | 20% |
| White blood cell decreased | 80% | 4% | 12% | 4% | 0% | 0% | 20% |
| Somnolence | 80% | 12% | 8% | 0% | 0% | 0% | 20% |
| Diarrhea | 84% | 12% | 0% | 4% | 0% | 0% | 16% |
| General disorders and administration site conditions ‐ Other, specify | 84% | 16% | 0% | 0% | 0% | 0% | 16% |
| Anorexia | 88% | 12% | 0% | 0% | 0% | 0% | 12% |
| Alanine aminotransferase increased | 92% | 8% | 0% | 0% | 0% | 0% | 8% |
| Aspartate aminotransferase increased | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Lung infection | 92% | 0% | 0% | 8% | 0% | 0% | 8% |
| Confusion | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Gastrointestinal disorders ‐ Other, specify | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Generalized muscle weakness | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Headache | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Hypokalemia | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Oral pain | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Hallucinations | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Skin and subcutaneous tissue disorders ‐ Other, specify | 96% | 4% | 0% | 0% | 0% | 0% | 4% |
| Hypersomnia | 96% | 0% | 4% | 0% | 0% | 0% | 4% |
| Adverse Events Legend | |||||||
| Abbreviation: NC/NA, no change from baseline/no adverse event. | |||||||
Assessment, Analysis, and Discussion
| Completion | Study terminated before completion |
| Terminated Reason | Company stopped development |
| Investigator's Assessment | Active but results overtaken by other developments |
Malignant mesothelioma, particularly when unresectable, has a very poor prognosis, with even the most favorable estimates suggesting a median overall survival of just over 1.5 years after the initiation of therapy [5]. The standard‐of‐care treatment for patients with newly diagnosed mesothelioma remains a combination of platinum and pemetrexed chemotherapy, with or without antiangiogenic therapy. The outlook after initial progression of disease is especially discouraging. Here, in the second line and beyond, there is no established standard of care based on a prospective, randomized trial. Instead, patients receive retreatment with first‐line therapy, presuming an initially durable response, or a selection of chemotherapies and immunotherapies. Preliminary results from a recent phase III trial in which patients with previously treated, unresectable mesothelioma were randomized to receive either pembrolizumab or gemcitabine plus vinorelbine highlight the challenges faced in the relapsed setting [6]. Although both arms represent acceptable options, progression‐free survival was similar and disappointing in each (2.5 months vs 3.4 months for the immunotherapy and chemotherapy arms, respectively). There is a clear, unmet need for additional and, especially, more efficacious alternatives in the relapsed setting.
Alisertib, also known as MLN8237, is a selective small molecule inhibitor of Aurora A kinase that has shown modest antitumor activity in a number of clinical settings [7]. The Aurora kinases are a highly conserved family of serine/threonine protein kinases that localize to centrosomes and the proximal mitotic spindle to ensure accurate mitosis [8]. As a result, inhibition of Aurora kinases lead to mitotic delays, severe chromosomal alignment and segregation defects, and, ultimately, apoptosis [1, 9, 10, 11]. Aurora kinases are functionally linked to the MYC oncogene—for example, Aurora A kinase binds directly to and stabilizes the MYC oncogene to promote tumor progression [3].
Unsurprisingly, Aurora A kinase expression is strongly correlated with MYC expression and, in other studies, MYC expression has been observed to predict sensitivity to Aurora kinase inhibitors [6, 8]. Overexpression of Aurora kinases and/or MYC is common in malignant mesothelioma, and in vitro inhibition of Aurora kinases leads to cell growth arrest in mesothelioma cell line models [2, 4, 12, 13, 14]. In light of these preclinical data and our own profiling studies, we hypothesized that alisertib was a feasible and relevant target in mesothelioma.
From June 2015 to August 2016, 26 patients were enrolled on a single‐arm, single‐institution phase II trial of alisertib administered 50 mg twice daily for 7 days every 21 days at the University of Texas MD Anderson Cancer Center (NCT02293005). Eligible patients had unresectable pleural or peritoneal mesothelioma, had received no more than four prior lines of systemic therapy (including one prior platinum‐pemetrexed combination), and had adequate organ function. Of the 26 patients who were enrolled, 25 were evaluable (1 patient did not complete one cycle of treatment—the criterion for evaluability). With respect to the primary endpoint of 4‐month disease control rate, 8 patients (32%) remained progression free at 4 months, which exceeded the prespecified futility endpoint of 30%.
As for other secondary endpoints related to efficacy and safety, there were no confirmed responses by RECIST/modified RECIST criteria. However, 1 patient (4.0%) did have a documented minor response as best response, whereas an additional 13 patients (52.0%) experienced stable disease as best response. Median progression‐free and overall survival were 2.8 months (95% confidence interval [CI], 1.3–4.0) and 6.3 months (95% CI, 5.7–11.1). Among the 26 total enrolled patients, 8 (30.8%) required a dose interruption and 3 (11.5%) required a dose reduction. There were 123 adverse events reported, with the majority grade 1 (69.1%) and 2 (21.1%). There was a 9.8% grade 3 toxicity rate, including four patients with grade 3 anemia, three with grade 3 neutropenia, two with grade 3 lung infections, and one each with grade 3 oral mucositis, diarrhea, and leukopenia. The most common adverse events were fatigue, alopecia, anemia, nausea/vomiting, oral mucositis, and constipation. Additionally, one patient expired while on study owing to a combination of respiratory failure and progression of pre‐existing renal failure. Radiographic evaluation performed just before this patient's death suggested possible progressive disease and questionable pneumonia, although the patient was afebrile, was not neutropenic, and had no causative organism identified in lower respiratory culture. The patient's respiratory failure and renal failure were both deemed not related to the study drug.
An additional secondary endpoint related to MYC copy number as a predictive biomarker for alisertib response in mesothelioma. Sufficient baseline archival or fresh tissue was available for 22 patients and analyzed for copy number gain by fluorescence in situ hybridization (FISH). Using four different FISH criteria for positivity, between two and five patient tumors were determined to have MYC copy number gain/amplification. However, there was no correlation with response, duration of response, or survival in this small data set using any of these cutoffs. For example, using the cutoff of ≥4 copies of the MYC locus [4] (Fig. 2), only 2 of 22 patients had MYC copy number gain, and both of these patients experienced progressive disease as their best response.
Figure 2.
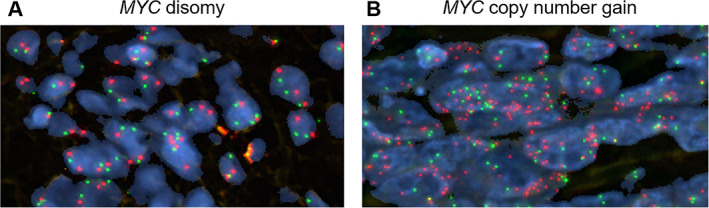
Representative images of MYC copy number examined by fluorescence in situ hybridization in patient samples. (A): Specimen with no copy number gain (disomy; n = 2). (B): Specimen with copy number gain (n ≥ 4). Red signals represent MYC gene probe, whereas green signals represent internal control probe (magnification ×1,000). Copy number analysis was performed in 50 nuclei per tumor in at least four areas.
Unfortunately, despite exceeding the prespecified futility cutoff for the primary endpoint of 4‐month disease control rate [15], the sponsor opted to conclude enrollment because of a shift in developmental strategy for alisertib. There were some limitations in the activity of alisertib in this population that may have limited its further development—most notably the lack of confirmed objective responses. However, it is worth noting that response rates are low in relapsed/refractory mesothelioma even for drugs considered standards of care, especially chemotherapy [6]. Moreover, the progression‐free survival in this trial was comparable to that seen in other trials evaluating accepted standards of care, including immunotherapy and chemotherapy, while acknowledging the limitations of cross‐study comparisons [6]. Although the biomarker analysis was ultimately underpowered owing to early termination of the study and few MYC copy number gains detected, there were no signals to support MYC status as a predictive biomarker in this context. This is in contrast to small cell lung cancer, where MYC status predicted improved clinical outcomes, although in that case detectable MYC expression by immunohistochemistry (rather than MYC copy number gain) was used as cutoff [7].
Although the activity of alisertib in this context was limited, the drug was well tolerated and safe, and further exploration of Aurora kinase inhibitors in mesothelioma is warranted. Future considerations may include the use of these agents in combination with immunotherapy, as preclinical data support additive or even synergistic activity of this combination [16], and alternative predictive biomarkers, including proteomic evaluation of MYC.
Disclosures
Carl M. Gay: AstraZeneca (RF); Renata Ferrarotto: AstraZeneca, Merck, Genentech, Pfizer (RF), Regeneron‐Sanofi, Ayala Pharma, Klus Pharm, Medscape, Cellestia Biotech (H); Don L. Gibbons: Astellas, Ribon Therapeutics, Alethia Biotherapeutics (C/A), AstraZeneca, Sanofi, Janssen, Ribon Therapeutics, Takeda (RF), Sanofi, Ribon Therapeutics (H); Bonnie S. Glisson: Pfizer Inc., ISA Pharmaceuticals, Cue Biopharma, MedImmune (RF); George R. Simon: Genentech, Eli Lilly, Takeda, AstraZeneca, Dava Oncology, Merck, Reflexion, Geneprex, Celgene, OncLive, Nexus Oncology, PER Oncology, IDP Pharma (C/A), Merck, Boehringer Ingelheim, Genentech, Takeda, AstraZeneca, Celgene (RF, H); John V. Heymach: AstraZeneca, Bristol‐Myers Squibb, GlaxoSmithKline, Guardant Health, Kairos Venture Investments, BrightPath Biotherapeutics, Hengrui Therapeutics, Eli Lilly, Spectrum, EMD Serono, Roche, Foundation One Medicine (C/A), AstraZeneca, Spectrum, Checkmate Pharmaceutics (RF), Spectrum & Bio‐Tree Systems, Inc. (Other: Royalties/Licensing Fees); Anne S. Tsao: Boehringer Ingelheim Pharmaceuticals, Bristol‐Myers Squibb, EMD Serono, Genentech BioOncology, Eli Lilly, Merck, Novartis, Roche Laboratories, Takeda Oncology, Ariad, Seattle Genetics, AstraZeneca, Sellas Life Science, Epizyme, Huron (C/A), Eli Lilly, Millennium, Polaris, Genentech, Merck, Boehringer Ingelheim, Bristol‐Myers Squibb, Ariad, Epizyme, Seattle Genetics, Takeda, EMD Serono (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This work was supported by Millennium Pharmaceuticals.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT02293005
- Sponsor: Millennium Pharmaceuticals
- Principal Investigator: Anne S. Tsao
- IRB Approved: Yes
Contributor Information
Carl M. Gay, Email: cgay@mdanderson.org.
Anne S. Tsao, Email: astsao@mdanderson.org.
References
- 1. Hoar K, Chakravarty A, Rabino C et al. MLN8054, a small‐molecule inhibitor of Aurora A, causes spindle pole and chromosome congression defects leading to aneuploidy. Mol Cell Biol 2007;27:4513–4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lopez‐Rios F, Chuai S, Flores R et al. Global gene expression profiling of pleural mesotheliomas: Overexpression of aurora kinases and P16/CDKN2A deletion as prognostic factors and critical evaluation of microarray‐based prognostic prediction. Cancer Res 2006;66:2970–2979. [DOI] [PubMed] [Google Scholar]
- 3. Dauch D, Rudalska R, Cossa G et al. A MYC‐aurora kinase A protein complex represents an actionable drug target in p53‐altered liver cancer. Nat Med 2016;22:744–753. [DOI] [PubMed] [Google Scholar]
- 4. Riquelme E, Suraokar MB, Rodriguez J et al. Frequent coamplification and cooperation between C‐MYC and PVT1 oncogenes promote malignant pleural mesothelioma. J Thorac Oncol 2014;9:998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zalcman G, Mazieres J, Margery J et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open‐label, phase 3 trial. Lancet 2016;387:1405–1414. [DOI] [PubMed] [Google Scholar]
- 6. Popat S, Curioni‐Fontecedro A, Polydoropoulou V et al. A multicentre, randomized phase III trial comparing pembrolizumab (P) vs single‐agent chemotherapy (CT) for advanced pre‐treated malignant pleural mesothelioma (MPM) ‐ Results from the European Thoracic Oncology Platform (ETOP 9‐15) PROMISE‐meso trial. Ann Oncol 2019;30(suppl 5):v851–v934. [DOI] [PubMed] [Google Scholar]
- 7. Owonikoko TK, Niu H, Nackaerts K et al. Randomized phase II study of paclitaxel plus alisertib versus paclitaxel plus placebo as second‐line therapy for SCLC: Primary and correlative biomarker analyses. J Thorac Oncol 2020;15:274–287. [DOI] [PubMed] [Google Scholar]
- 8. Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol 2001;2:21–32. [DOI] [PubMed] [Google Scholar]
- 9. Marumoto T, Honda S, Hara T et al. Aurora‐A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem 2003;278:51786–51795. [DOI] [PubMed] [Google Scholar]
- 10. Glover DM, Leibowitz MH, McLean DA et al. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell 1995;81:95–105. [DOI] [PubMed] [Google Scholar]
- 11. Katayama H, Zhou H, Li Q et al. Interaction and feedback regulation between STK15/BTAK/Aurora‐A kinase and protein phosphatase 1 through mitotic cell division cycle. J Biol Chem 2001;276:46219–46224. [DOI] [PubMed] [Google Scholar]
- 12. Romagnoli S, Fasoli E, Vaira V et al. Identification of potential therapeutic targets in malignant mesothelioma using cell‐cycle gene expression analysis. Am J Pathol 2009;174:762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crispi S, Fagliarone C, Biroccio A et al. Antiproliferative effect of Aurora kinase targeting in mesothelioma. Lung Cancer 2010;70:271–279. [DOI] [PubMed] [Google Scholar]
- 14. Kim KW, Mutter RW, Willey CD et al. Inhibition of survivin and aurora B kinase sensitizes mesothelioma cells by enhancing mitotic arrests. Int J Radiat Oncol Biol Phys 2007;67:1519–1525. [DOI] [PubMed] [Google Scholar]
- 15. Simon R. Optimal two‐stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10. [DOI] [PubMed] [Google Scholar]
- 16. Della Corte CM, Ajpacaja LL, Cardnell RJ et al. The Aurora kinase A‐inhibitor alisertib is a potential candidate for combination with immunotherapy in small cell lung cancer. Mol Cancer Ther 2019;18(suppl 12):B064a. [Google Scholar]


