Abstract
Background
Although trastuzumab (T) represents the standard of care for the adjuvant treatment of HER2‐positive early‐stage breast cancer, contrasting results are available about the cardiac toxicity associated to its use. We conducted a multiregional population‐based cohort investigation aimed to assess both the short‐ and long‐term cardiovascular (CV) outcomes in women with early breast cancer treated with T‐based or standard adjuvant chemotherapy (CT).
Materials and Methods
We used health care use databases of six Italian regions, overall accounting for 42% of the Italian population. The study cohort was made by all women surgically treated for breast cancer who started a first‐line adjuvant T‐based or CT treatment. Patients treated with T were 1:2 matched to those treated with CT based on date of treatment start, age, and presence of CV risk factors. Short‐ and long‐term CV outcomes (heart failure and cardiomyopathy) were measured, respectively, after 1 year and at the end of follow‐up.
Results
Among 28,599 women who met the inclusion criteria, 6,208 T users were matched to 12,416 CT users. After a mean follow‐up of 5.88 years, short‐ and long‐term cumulative CV risk were 0.8% and 2.6% in patients treated with T and 0.2% and 2.8% in those treated with CT, respectively. Adjusted hazard ratios were 4.6 (95% confidence interval [CI], 2.6–8.0) for short‐term and 1.2 (95% CI, 0.9–1.6) for long‐term CV risk.
Discussion
In our large real‐world investigation, T‐associated cardiotoxicity was limited to the treatment period. The addition of T to adjuvant CT did not result in long‐term worsening of CV events.
Implications for Practice
Adjuvant trastuzumab‐based chemotherapy represents the backbone therapy in patients with HER2‐positive early breast cancer. Although well tolerated, cardiovascular events can manifest during or after therapy because of treatment‐related toxicities. In this wide multicenter and unselected cohort, long‐term symptomatic cardiotoxicity was low and limited to the treatment period. The findings suggest that developing tools that would be adequately able to predict cardiac toxicity at an early stage remains an important area in which additional research efforts are needed.
Keywords: Early breast cancer, Trastuzumab, Cardiotoxicity, Real world
Short abstract
With breast cancer patients experiencing longer survival, emphasizing their overall health through management of late and long‐term treatment effects is becoming increasingly important. This article reports a multiregional real‐world population‐based cohort investigation carried out to compare short‐term and long‐term cardiovascular risk of symptomatic cardiovascular events in women with localized breast cancer treated with trastuzumab‐based or standard adjuvant therapy in clinical practice.
Introduction
Trastuzumab (Herceptin, Roche; Basel, Switzerland) was the first humanized monoclonal antibody against the extracellular domain of HER2 approved in 1998 by the U.S. Food and Drug Administration (FDA) in patients with HER2 overexpressed and/or amplified (i.e., HER2‐positive) metastatic breast cancer [1, 2]. Later on, in 2006, adjuvant treatment with 1 year of trastuzumab was also approved in patients with HER2‐positive early (localized) breast cancer [3, 4]. Given its well‐documented efficacy in reducing recurrence and death, as compared with standard chemotherapy alone, trastuzumab became the standard of care for HER2‐positive breast cancer [5].
With longer survival, emphasizing overall health through management of late and long‐term treatment effects is increasingly important. Trastuzumab is well tolerated; nevertheless, cardiovascular (CV) events, such as congestive heart failure (CHF) or decreased left ventricular ejection fraction, can manifest during or after therapy because of treatment‐related toxicities [6, 7, 8]. However, randomized clinical trials (RCTs) [9] and observational studies [10] showed that cardiac toxicity remains low and occurs mostly during the treatment phase. Moreover, the results of two RCTs showed that CV events were asymptomatic and reversible in most patients, who reached recovery within than 6 months after stopping therapy [11, 12]. Conversely, other observational studies showed a prolonged risk of CV events, even after the treatment stopped [13, 14, 15]. Therefore, CV safety in long‐term survivors of adjuvant chemotherapy with or without trastuzumab is still a debated issue, also because many of the studies with trastuzumab have been carried out in selected patients, whereas, at present, the drug has entered the vademecum of oncologists for years and is offered on a large scale.
With these premises, a wide multiregional real‐world population‐based cohort investigation was carried out to compare short‐ and long‐term cardiovascular risk of symptomatic CV events in women with localized breast cancer treated with trastuzumab‐based or standard adjuvant therapy in clinical practice. The study is a part of an Italian project funded by the Italian Medicines Agency (Agenzia Italiana del Farmaco) and the Health Department of the Sardinia Region, which supported the Biological Drugs in Oncology (Farmaci Biologici in Oncologia; FABIO) program.
Materials and Methods
Data Sources
The Italian National Health Service (NHS) provides universal and mostly free of charge health care services, including medicines for cancer. The service is administered within each of the 21 Italian regions by an automated system of health care use (HCU) databases that collect a variety of information, including at least (a) demographic and administrative data on residents who receive NHS assistance (almost the whole resident population); (b) hospital discharge records providing information on primary diagnosis, coexisting conditions, and procedures performed to inpatients admitted in public and private hospitals and coded according to the International Classification of Diseases, 9th Revision Clinical Modification classification system; (c) drugs dispensed by territorial pharmacies and medicines directly administered in the outpatient setting and day‐hospital coded according to the Anatomical Therapeutic Chemical (ATC) classification system; (d) data on outpatient services, including specialist visits, laboratory tests, and diagnostic imaging; and (e) copayment exemption database, including exemption for cancer, the latter two both coded according to the National nomenclature. Record linkage between databases is allowed through a single identification code (Regional Health Code). To preserve privacy, each identification code is automatically converted into an anonymous code, and the inverse process is prevented by deletion of the conversion table.
The FABIO Network
The FABIO program was conducted by retrieving HCU data from six Italian regions localized at Northern (Lombardy), Central (Lazio and Marche), Southern (Abruzzo), and Insular (Sardinia and Sicily) Italy. The corresponding resident population overall accounts for about 25.5 million inhabitants, representing 42.1% of the entire Italian population. A detailed description of the procedure adopted from designing, harmonizing and achieving regional cancer research platforms is given in supplementary online Appendix 1.
Cohort Selection and Exposure Definition
During the recruitment period, in a time‐span ranging between 2007 and 2016 based on data availability of participating regions, female NHS beneficiaries with an intervention of breast surgery were selected. The date of the first hospital admission for breast surgery was defined as the “index date.” Among these patients, those who had a diagnosis of invasive breast cancer during the year before the index date were included in the study cohort. Women were excluded if they (a) already underwent breast surgery during the year before the index date; (b) received a diagnosis of secondary cancer, distant lymph nodes, or distant metastases during the year before or 6 months after the index date; (c) received diagnosis of CHF or cardiomyopathy (i.e., the outcomes of interest) in the 3 years before the index date; (d) were younger than 18 years; (e) died during the index hospitalization; or (f) did not receive drug therapy with trastuzumab or standard chemotherapy within 6 months after the index date. Specific diagnostic and therapeutic codes used for the current study are given in supplemental online Table 1.
The date of the first cancer drug dispensation after discharge from the index hospitalization was defined as “treatment start.” As trastuzumab is indicated for the treatment of patients with early breast cancer in association or following adjuvant chemotherapy [16], cohort members were classified as exposed to trastuzumab (i.e., belonging to the trastuzumab arm), or to standard chemotherapy alone (i.e., belonging to the standard arm), according to whether they received or not at least one trastuzumab dispensation (ATC L01XC03) during the year following treatment start, respectively.
Outcome and Covariates
The endpoint of interest was the first hospitalization for CHF or cardiomyopathy identified during follow‐up. The admission date of the first hospitalization for these causes was defined as the date of outcome onset. Several covariates were assessed at index date for each cohort member, including age, year of cohort inclusion, nodal status, type of surgery (i.e., radical or conservative), and presence of CV risk factors (i.e., diabetes, hypertension, cardiac arrhythmia, and renal failure) recorded within 3 years before index date.
Matching Cohort Arms
To reduce the between‐exposure arms heterogeneity, each cohort member exposed to trastuzumab (index case) was matched with two cohort members on standard chemotherapy alone. The latter were randomly selected from those who started therapy at the same date of the index case (±30 days). Matching was based on age at surgical intervention (±1 year) and presence of at least one CV risk factor. Then, in each 1:2 matched risk set, patients accumulated person‐years of follow‐up from the date of the first trastuzumab prescription of the index case until the outcome onset, or censoring (death, emigration, or endpoint of follow‐up), whichever came first. As we were interested in both short‐ and long‐term effects of exposure to trastuzumab, endpoint of follow‐up was fixed at 1 year or at the last date with data available within each region (i.e. from December 31, 2016, to June 30, 2018, depending on regional data availability), respectively.
Statistical Analysis
Between‐arm differences in baseline characteristics were tested by the χ2 statistics. The cumulative risk of occurrence of CV events was estimated by using the Kaplan‐Meier (KM) estimator. The Gehan‐Breslow‐Wilcoxon test was used for testing between‐arm CV risk differences because it does not require proportional hazards and gives more weight to events at early time points [17, 18]. To increase the precision of the estimates, between‐region summarized KM curves were estimated. However, as regional data were not available to be analyzed in a pooled analysis, a method for reconstructing individual‐patient data starting from each regional KM curve was applied. Briefly, a digital software was used to read the coordinates of the KM curves within each region. Information on the number of women still at risk at each year of follow‐up and the total number of outcome events was used to solve the inverted KM equation, which allowed for reconstructing the regional KM data for each arm, thus obtaining pooled individual‐patient data [19].
The associations between adjuvant treatment with trastuzumab‐based therapy and short‐term or long‐term outcome occurrence were estimated by means of a Cox proportional hazard model and expressed as hazard ratio (HR), along with its 95% confidence interval (CI). As patients treated with chemotherapy alone may have switched to trastuzumab during follow‐up, the exposure of interest was considered as a dichotomous time‐dependent covariate. Beyond the exposure of interest, the model included nodal status and type of surgery. Between‐arm differences in terms of age, CV risk factors, and date of treatment start were intrinsically accounted for by the matched study design. Again, to increase the precision of the estimates, and because regional data were not available to be analyzed in a pooled analysis, the so‐called two‐stage meta‐analysis was performed [20]. Briefly, the proportional hazard model was separately fitted within each region, and between‐region summarized HR was estimated by means of a fixed‐effect model, using the inverse variance weighting [21]. Between‐regions heterogeneity was evaluated by using the χ2 statistics [22] and was measured through the I 2 index [23], the latter measuring the percentage variation across the regions due to heterogeneity.
In a secondary analysis limited to data from the Lombardy Region (i.e., the largest Region among those included in the FABIO program), the synergy between trastuzumab and radiotherapy in increasing the risk of occurrence of CV was assessed. Exposure to radiotherapy, evaluated in both inpatient and outpatient setting, was considered as a time‐dependent covariate.
For all the tested hypotheses, two‐tailed p values < .05 were considered statistically significant.
Ethical Issues
The Ethical Committee of the University of Milano‐Bicocca evaluated the protocol and established that the study (a) was exempt from informed consent (according to General Authorization for the Processing of Personal Data for Scientific Research Purposes issued by the Italian Privacy Authority on December 15, 2016; http://www.garanteprivacy.it/web/guest/home/docweb/-/docweb-display/docweb/5805552), (b) provides sufficient guarantees of individual records anonymity, and (c) was designed according to quality standards of good practice of observational research based on secondary data.
Results
The process of selection of the study cohort in each participating region, as well as in all regions together, is reported in Figure 1. Among the almost 112,062 patients who underwent breast surgery identified during the study period across all participating regions, 82,376 met the inclusion criteria and constituted the study cohort. Of these, 28,599 women received a cancer drug therapy within 6 months from the surgical intervention, of whom 6,728 (23.5%) had trastuzumab‐based therapy and 21,871 (76.5%) had standard chemotherapy alone. Finally, 6,208 trastuzumab users were matched to 12,416 standard therapy users.
Figure 1.
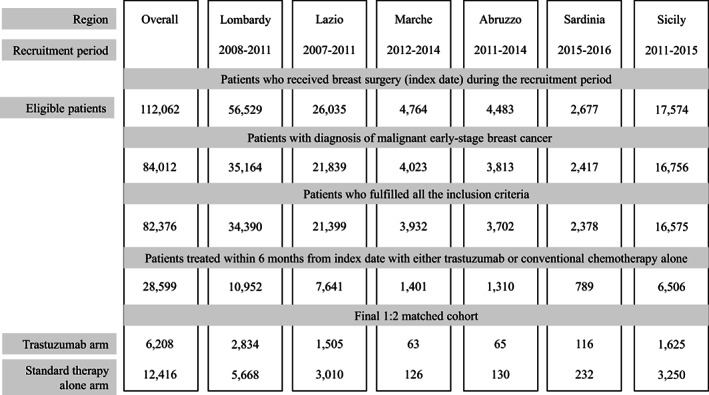
Flow‐chart of inclusion and exclusion criteria for each participant region, as well as in the entire investigated population
As shown in Table 1, matched patients had a median age of 55 years and 37% prevalence of CV risk factors. Compared with patients belonging to the standard arm, those belonging to the trastuzumab arm were less likely to have a positive nodal status (41% vs. 44%, p < .001) and to receive a conservative surgery (64% vs. 69%, p < .001).
Table 1.
Baseline characteristics (%) of 6,208 patients treated with T and 12,416 patients treated with CT alone
| Characteristics | Overall | Lombardy | Lazio | Marche | Abruzzo | Sardinia | Sicily | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | CT | T | CT | T | CT | T | CT | T | CT | T | CT | T | CT | |
| No. of patients | 6,208 | 12,416 | 2,834 | 5,668 | 1,505 | 3,010 | 63 | 126 | 65 | 130 | 116 | 232 | 1,625 | 3,250 |
| Age, yr, a % | ||||||||||||||
| 18–54 | 47.9 | 47.8 | 45.7 | 45.8 | 51.6 | 50.8 | 44.8 | 49.2 | 55.4 | 55.4 | 55.2 | 54.1 | 46.6 | 48.0 |
| 55–64 | 29.5 | 29.6 | 30.1 | 29.8 | 28.8 | 29.6 | 32.8 | 28.6 | 33.9 | 31.5 | 32.8 | 34.0 | 28.7 | 28.7 |
| 65–74 | 18.9 | 19.1 | 20.2 | 20.4 | 16.6 | 16.9 | 19.2 | 19.0 | 10.8 | 13.1 | 11.2 | 10.2 | 19.7 | 19.7 |
| ≥ 75 | 3.7 | 3.5 | 4.0 | 4.0 | 3.1 | 2.7 | 3.2 | 3.2 | 0 | 0 | 0.9 | 1.7 | 4.0 | 3.6 |
| Median | 55 | 55 | 56 | 56 | 54 | 54 | 55 | 55 | 53 | 53 | 54 | 53 | 55 | 55 |
| CV risk factors,a % | ||||||||||||||
| At least one | 37.4 | 37.4 | 34.1 | 34.1 | 37.0 | 37.0 | 33.3 | 33.3 | 38.5 | 38.5 | 28.5 | 28.5 | 44.3 | 44.3 |
| Hypertension | 29.5 | 30.2 | 33.2 | 32.8 | 35.1 | 36.5 | 31.8 | 32.8 | 36.9 | 37.7 | 27.6 | 26.8 | 42.4 | 42.6 |
| Diabetes | 8.2 | 6.3 | 4.7 | 5.3 | 6.6 | 6.8 | 6.4 | 5.6 | 7.7 | 7.7 | 3.5 | 5.5 | 10.5 | 10.9 |
| Renal failure | 0.2 | 0.1 | 0.2 | 0.1 | 0.3 | 0.1 | 0 | 0 | 0 | 0.8 | 0.9 | 0 | 0.1 | 0.0 |
| Cardiac arrhythmia | 0.7 | 0.5 | 0.8 | 0.4 | 1.1 | 0.7 | 1.6 | 0.8 | 1.5 | 0.8 | 0.9 | 0.4 | 0.1 | 0.4 |
| Nodal status, % | ||||||||||||||
| Positive | 40.8 | 44.2 | 40.4 | 50.6 | 26.0 | 32.1 | 44.4 | 30.4 | 21.5 | 28.5 | 26.7 | 37.9 | 43.3 | 45.5 |
| Negative | 59.2 | 55.8 | 59.6 | 49.4 | 74.0 | 67.9 | 55.6 | 69.6 | 78.5 | 71.5 | 73.3 | 62.1 | 56.7 | 54.5 |
| p value, a % | <.001 | <.001 | <.001 | .057 | .300 | .038 | <.001 | |||||||
| Type of surgery | ||||||||||||||
| Conservative | 63.9 | 69.4 | 61.6 | 65.7 | 64.2 | 68.2 | 57.1 | 72.8 | 73.8 | 83.8 | 68.1 | 60.4 | 67.6 | 76.7 |
| Radical | 36.1 | 30.6 | 38.4 | 34.3 | 35.8 | 31.8 | 42.9 | 27.2 | 26.2 | 16.2 | 31.9 | 39.6 | 32.4 | 23.3 |
| p value a | <.001 | <.001 | .008 | .030 | .010 | .0161 | <.001 | |||||||
Chi‐square test for homogeneity
Matching variables
Abbreviations: CT, standard chemotherapy alone; T, trastuzumab‐based chemotherapy.
During a mean follow‐up of 5.84 and 5.90 years, 109 (1.8%) and 199 (1.6%) cohort members experienced the outcome among patients belonging to the trastuzumab arm and standard arm respectively, with the corresponding long‐term incident rates 3.0 (95% CI, 2.4–3.6) and 2.7 (2.4–3.1) every 1,000 women‐years (p = .206). For short‐term incidence rates (i.e., by restricting the analysis only to the first years of follow‐up), the corresponding figures were 7.8 (5.6–10.0) and 1.9 (1.1–2.7) every 1,000 women‐years (p < .001).
Different patterns in the cumulative CV outcomes were observed according to therapeutic arm (Fig. 2). Women belonging to the trastuzumab arm had a rapid increment of heart failure or cardiomyopathy up to reach 0.8% during the first year after starting therapy (i.e., during the expected period of treatment with trastuzumab), whereas a lower slope was observed from the second year on, until reaching 2.6% along the entire 8‐year follow‐up. Interestingly, among the 109 CV outcomes experienced by patients on treatment with trastuzumab, 48 (44%) occurred during the first year of follow‐up, and the remaining 61 occurred in the following 7 years. In contrast, in patients belonging to the standard harm, the CV risk increased almost linearly during follow‐up, reaching figures of 0.2% and 2.8% after the first year of follow‐up and among the entire 8‐year follow‐up, respectively. However, considering the entire follow‐up, there was no significant evidence of between‐treatment differences in the CV risk (p = .134). Region‐specific KM curves are shown in supplemental online Figure 1.
Figure 2.
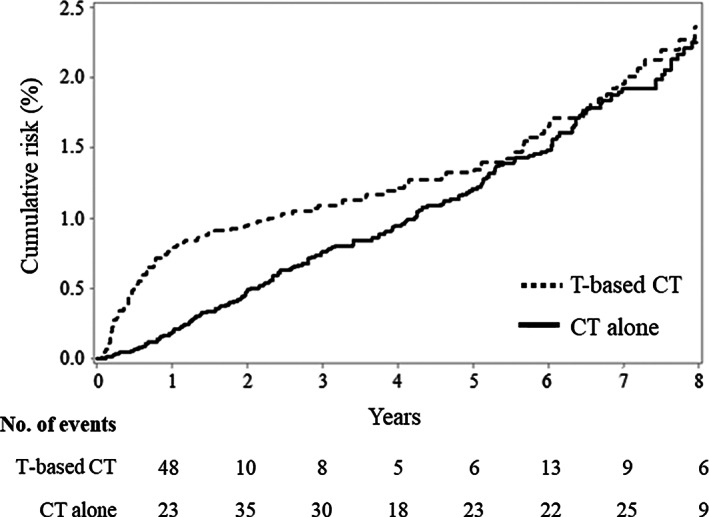
Kaplan‐Meier estimates of the cumulative risk of hospitalization for heart failure or cardiomyopathy in 6,208 and 12,416 breast cancer women on adjuvant therapy respectively with T‐based CT and CT alone. Abbreviations: CT, standard chemotherapy; T, trastuzumab.
Forrest plots of region‐specific and between‐region summarized HR of short‐term (upper plot) and long‐term (lower plot) CV outcomes are shown in Figure 3. As far as short‐term effects are concerned (upper plot), there was no evidence of between‐region heterogeneity in HR (p = .43; I 2 = 0%). Summarized HR was 4.6 (95% CI, 2.6–8.0). As far as long‐term effects are concerned (lower plot), even in this case there was no evidence of between‐region heterogeneity in HR (p = .71; I 2 = 0%), whereas summarized HR was 1.2 (95% CI, 0.9–1.6).
Figure 3.
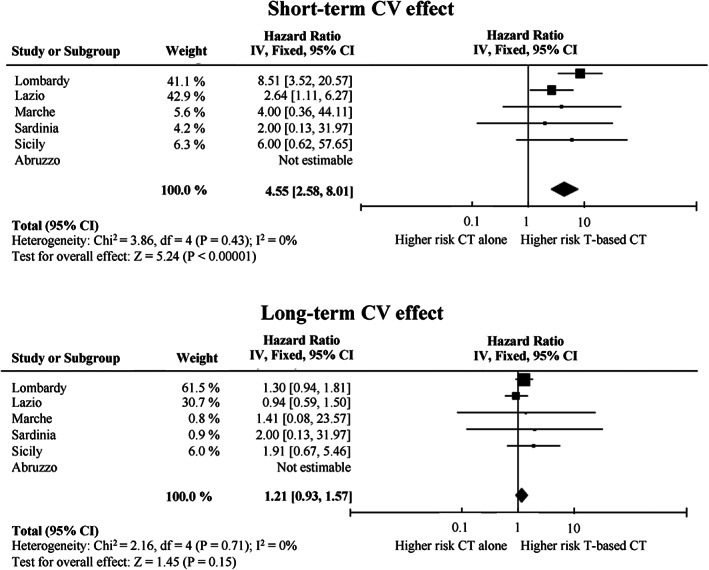
Forest plot of the summarized associations between trastuzumab‐based adjuvant therapy and the short‐term and long‐term risk of hospitalization for heart failure or cardiomyopathy, compared use of standard chemotherapy alone. Estimates are shown for each participant region, and by summarizing region‐specific hazard ratios. Abbreviations: CI, confidence interval; CT, standard chemotherapy; IV, inverse variance; T, trastuzumab.
Secondary analysis showed that the proportion of patients treated with radiotherapy was similar in the two treatment arms (72.4% and 75.8%, respectively, in the trastuzumab arm and in the standard arm, p < .001). Short‐term and long‐term HRs were 2.38 (95% CI, 0.61–9.33) and 1.16 (95% CI, 0.71–1.92), respectively, for those treated with radiotherapy, as compared with those who did not. No interaction was found between trastuzumab and radiotherapy treatment, neither related to short‐term (p = .202) nor to long‐term (p = .239) CV risk assessment.
Discussion
This Italian multiregional real‐world study evaluated the cardiotoxicity of trastuzumab adjuvant therapy for treating early‐stage breast cancer. The study suggests that women treated with trastuzumab had high risk of hospitalization for CHF and cardiomyopathy during the treatment period, up to 4.6 times higher than women treated with standard chemotherapy alone during the first year after the start of drug treatment. Of note, the risk diminishes after the end of treatment and becomes comparable to that of women treated with standard chemotherapy alone after 8 years of follow‐up. These results are consistent with both RCTs [9, 11, 12] and observational studies [10, 24] that showed that trastuzumab‐based chemotherapy does not result in long‐term worsening of CV events.
As compared with observational studies, our retrospective cohort study showed a lower incidence of CV events. Indeed, we estimated that women on treatment with trastuzumab had 0.8% and 2.4% cumulative incidence of heart failure and cardiomyopathy after 1 year and 8 years of follow‐up, respectively. Higher figures of 1.8% and 2.8% after 1 and 3 years of follow‐up were reported by a recent Italian real‐world study [10]. Endpoints considered by the latter study, however, regarded myocardial infarction, rhythm disorders, cardiac dysfunctions, and cardiac death, other than heart failure and cardiomyopathy as in our study. Similarly, 1 year and 8 years cumulative incidence of CHF, respectively, of 3.0% and greater than 5% were observed in a population‐based Canadian study of women who underwent surgery for breast cancer [24]. Endpoints considered by this study, however, regarded both outpatient and inpatient diagnoses of CHF, rather than the severe CHF episodes leading to hospital admission as in our study, likely generating an underestimate of the risk of CV events.
Other population‐based observational studies have raised concerns that trastuzumab‐related cardiac toxicity in routine practice may be greater than that seen in clinical trials, especially in the elderly and in patients previously exposed to anthracyclines. In particular, a nationwide Danish retrospective cohort study reported that, compared with the CV risk of 6,695 patients treated with standard chemotherapy alone, that of 2,117 women treated with trastuzumab‐based therapy was increased 3.6‐fold during the first 18 months of observation and 1.9‐fold in the following 18 months [13]. Similarly, a real‐world study using the U.S. SEER‐Medicare database showed that patients with breast cancer aged more than 67 years and treated with trastuzumab with or without anthracyclines had a 78% increased risk of CHF and cardiomyopathy compared with patients treated with no adjuvant trastuzumab or chemotherapy during a 3‐year follow‐up (HR, 1.78; 95% CI, 1.43–2.21) [15]. Finally, a population‐based retrospective cohort study in the U.S. showed a linear trend of long‐term CV risk of patients both treated with trastuzumab only and with trastuzumab plus anthracyclines during a 5.5 years follow‐up. In particular, cumulative incidence was 3.6%, 7.8%, and 12.1% after, respectively, 1, 3, and 5 years follow‐up in patients treated with trastuzumab only and 6.2%, 13.2%, and 20.1% in patients treated with trastuzumab plus anthracyclines [14].
Our study has several strengths. First, the target population from which we selected the final cohort was representative of the routine clinical practice in Italy. Indeed, all beneficiaries of the NHS hospitalized with a diagnostic code of breast cancer during the recruitment period were included in the study, with no restrictions on age and concomitant diseases. Moreover, participating regions covered approximately 42% of the Italian population and likely reflected the heterogeneity in clinical practice for treating breast cancer and caring CV outcomes. Second, to the best of our knowledge, this is the largest study performed so far on cardiotoxicity of trastuzumab in terms of patients enrolled. Third, the median follow‐up was almost 6 years, with more than 15% of cohort patients still at risk of experiencing a CV event after 8 years of observation. This allowed studying cardiotoxicity associated to trastuzumab both during the treatment period and, more importantly, during several years after ceasing the treatment, giving important evidence on the reversibility of the CV risk.
In contrast, the main limitation of the study is the paucity of data on individual characteristics, clinical features and drug patterns and regimens. Consequently, the increased CV risk associated with trastuzumab might have been biased by factors influencing both chemotherapeutic regimen and outcome onset. Factors such as ethnicity or socioeconomic status can be confidently ruled out because the Italian population is largely white, and free‐of‐pay access to cancer care is ensured for all NHS beneficiaries. Furthermore, it is unlikely that between‐arm structural differences may affect CV risk only during the period in treatment with trastuzumab. Finally, our analyses were adjusted for nodal status and type of surgery, which may be considered proxies of breast cancer stage. Other unmeasured factors, however, might affect our findings. For example, it is known that anthracyclines increase the risk of congestive CHF [25] and that the harmful effect of anthracyclines may be amplified when they are administered in combination with trastuzumab [26]. In contrast, the Canadian observational study cited above did not provide evidence that the use of anthracyclines, at dosages used in the current chemotherapy protocols for adjuvant breast cancer therapy, increases the risk of CHF in patients treated with trastuzumab [24]. In our study, we could not assess the combined effect of anthracyclines and trastuzumab because anthracycline use was not traceable from out HCU database. Other unintended residual confounders (i.e., family history and smoking status) could affect our findings. Moreover, data on asymptomatic decline in left ventricular ejection fraction or symptomatic CHF not requiring hospitalization were not available, because our databases did not include details about physical examinations, symptoms, blood tests (i.e., brain natriuretic peptide), or echocardiography results.
Conclusion
In conclusion, our large real‐world investigation offers further evidence that trastuzumab‐associated severe cardiotoxicity is limited to the treatment period and that in breast cancer survivors, the addition of trastuzumab to adjuvant chemotherapy does not result in long‐term worsening of CV events. Developing tools that would be adequately able to predict cardiac toxicity at an early stage remains an important area in which additional research efforts are needed. Therefore, in view of the success of the treatment in terms of absolute survival, our results underline the importance of the collaboration between oncologists and cardiologists to ensure optimal care to women with breast cancer treated with cardiotoxic agents.
Author Contributions
Conception/design: Matteo Franchi, Trama A, Giovanni Apolone, Giovanni Corrao
Provision of study material or patients: Garau D, Ursula Kirchmayer, Marilena Romero, Ilenia De Carlo, Salvatore Scondotto
Collection and/or assembly of data: Matteo Franchi
Data analysis: Matteo Franchi, Ivan Merlo
Data interpretation: Matteo Franchi, Annalisa Trama, Ivan Merlo, Pamela Minicozzi, Luigi Tarantini Donatella Garau Ursula Kirchmayer, Mirko Di Martino, Marilena Romero, Ilenia De Carlo, Salvatore Scondotto, Giovanni Apolone, Giovanni Corrao
Manuscript writing: Matteo Franchi, Giovanni Corrao
Final approval of manuscript: Matteo Franchi, Annalisa Trama, Ivan Merlo, Pamela Minicozzi, Luigi Tarantini Donatella Garau Ursula Kirchmayer, Mirko Di Martino, Marilena Romero, Ilenia De Carlo, Salvatore Scondotto, Giovanni Apolone, Giovanni Corrao
Disclosures
Giovanni Corrao: Novartis, GlaxoSmithKline, Roche, AMGEN, Bristol‐Myers Squibb (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supplementary Information
Acknowledgments
The “FArmaci BIologici in Oncologia” (FABIO) working group includes the following: Giovanni Corrao, Matteo Franchi and Ivan Merlo (Lab Healthcare Research & Pharmacoepidemiology, Department of Statistic and Quantitative Methods, University of Milano‐Bicocca, Milan, Italy); Luca Merlino (Epidemiologic Observatory, Regional Welfare Service, Lombardy Region, Milan, Italy); Annalisa Trama, Pamella Minicozzi, and Giovanni Apolone (Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy); Luigi Tarantini (Department of Cardiology, Azienda Ospedale San Martino, ASL n. 1, Belluno, Italy); Donatella Garau (General Directorate for Health, Sardinia Region, Italy); Ursula Kirchmayer, Mirko Di Martino and Adele Lallo (Department of Epidemiology ASL Roma 1, Lazio Regional Health Service, Rome, Italy); Marilena Romero (Department of Medical, Oral and Biotechnological Sciences – Section of Pharmacology and Toxicology, University of Chieti, Italy), Antonio D'Ettorre, Antonia Petrucci (Regional Health Authority, Abruzzo Region); Ilenia De Carlo, Luigi Patregnani, and Christian Bogino (Regional Health Authority, Marche Region); Salvatore Scondotto and Walter Pollina Addario (Department of Health Services and Epidemiological Observatory, Regional Health Authority, Palermo, Sicily Region, Italy), Gianluca Trifirò (Department of Biomedical and Dental Sciences and Morphofunctional Imaging, University of Messina, Messina, Italy); Anna Rosa Marra (Italian Medicine Agency (Agenzia Italiana del Farmaco, Rome, Italy).
This work was supported by a research grant from the AIFA ‐ the Italian Medicines Agency – (project AIFA ‐ Fondi Farmacovigilanza Attiva; CUP code H56J16000500005) and from the Sardinia Region (CUP code H56D15000030005). Data analyses were performed at the Laboratory of Healthcare Research & Pharmacoepidemiology, University of Milano‐Bicocca with grants from the Italian Ministry of Education, University and Research (‘Fondo d'Ateneo per la Ricerca’ portion, year 2018).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Slamon DJ. Addition of Herceptin (humanized anti‐HER2 antibody) to first line chemotherapy for HER2 overexpressing metastatic breast cancer (HER27MBC) markedly increases anti‐cancer activity: A randomized, multinational controlled phase III trial. Proc Amer Soc Clin Oncol 1998;17:377a. [Google Scholar]
- 2. Slamon DJ, Leyland‐Jones B, Shak S et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 3. Piccart‐Gebhart MJ, Procter M, Leyland‐Jones B et al; Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer. N Engl J Med 2005;353:1659–1672. [DOI] [PubMed] [Google Scholar]
- 4. Romond EH, Perez EA, Bryant J et al. Trastuzumab plus adjuvant chemotherapy for operable HER2‐positive breast cancer. N Engl J Med 2005;353:1673–1684. [DOI] [PubMed] [Google Scholar]
- 5. Yin W, Jiang Y, Shen Z et al. Trastuzumab in the adjuvant treatment of HER2‐positive early breast cancer patients: A meta‐analysis of published randomized controlled trials. PLoS One 2011;6:e21030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahabreh IJ, Linardou H, Siannis F et al. Trastuzumab in the adjuvant treatment of early‐stage breast cancer: a systematic review and meta‐analysis of randomized controlled trials. The Oncologist 2008;13:620–630. [DOI] [PubMed] [Google Scholar]
- 7. Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Saf 2008;31:459–467. [DOI] [PubMed] [Google Scholar]
- 8. Moja L, Tagliabue L, Balduzzi S et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 2012:CD006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cameron D, Piccart‐Gebhart MJ, Gelber RD et al; Herceptin Adjuvant (HERA) Trial Study Team. 11 years’ follow‐up of trastuzumab after adjuvant chemotherapy in HER2‐positive early breast cancer: Final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017;389:1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonifazi M, Franchi M, Rossi M et al. Long term survival of HER2‐positive early breast cancer treated with trastuzumab‐based adjuvant regimen: A large cohort study from clinical practice. Breast 2014;23:573–578. [DOI] [PubMed] [Google Scholar]
- 11. de Azambuja E, Procter MJ, van Veldhuisen DJ et al. Trastuzumab‐associated cardiac events at 8 years of median follow‐up in the Herceptin Adjuvant trial (BIG 1‐01). J Clin Oncol 2014;32:2159–2165. [DOI] [PubMed] [Google Scholar]
- 12. Suter TM, Procter M, van Veldhuisen DJ et al. Trastuzumab‐associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol 2007;25:3859–3865. [DOI] [PubMed] [Google Scholar]
- 13. Banke A, Fosbøl EL, Ewertz M et al. Long‐term risk of heart failure in breast cancer patients after adjuvant chemotherapy with or without trastuzumab. JACC Heart Fail 2019;7:217–224. [DOI] [PubMed] [Google Scholar]
- 14. Bowles EJ, Wellman R, Feigelson HS et al; Pharmacovigilance Study Team. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study. J Natl Cancer Inst 2012;104:1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen J, Long JB, Hurria A et al. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol 2012;60:2504–2512. [DOI] [PubMed] [Google Scholar]
- 16. European Medicine Agency . Herceptin: Summary of product characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/herceptin-epar-product-information_en.pdf. Accessed March 16, 2020.
- 17. Gehan EA. A generalized Wilcoxon test for comparing arbitrarily singly‐censored samples. Biometrika 1965;52:203–223. [PubMed] [Google Scholar]
- 18. Breslow N. A generalized Kruskal‐Wallis test for comparing K samples subject to unequal patterns of censorship. Biometrika 1970;57:579–594. [Google Scholar]
- 19. Guyot P, Ades AE, Ouwens MJ et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan‐Meier survival curves. BMC Med Res Methodol 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scotti L, Rea F, Corrao G. One‐stage and two‐stage meta‐analysis of individual participant data led to consistent summarized evidence: Lessons learned from combining multiple databases. J Clin Epidemiol 2018;95:19–27. [DOI] [PubMed] [Google Scholar]
- 21. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- 22. Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–129. [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldhar HA, Yan AT, Ko DT et al. The temporal risk of heart failure associated with adjuvant trastuzumab in breast cancer patients: A population study. J Natl Cancer Inst 2015;djv301. [DOI] [PubMed] [Google Scholar]
- 25. Smith LA, Cornelius VR, Plummer CJ et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta‐analysis of randomised controlled trials. BMC Cancer 2010;10:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gianni L, Salvatorelli E, Minotti G. Anthracycline cardiotoxicity in breast cancer patients: synergism with trastuzumab and taxanes. Cardiovasc Toxicol 2007;7:67–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1: Supplementary Information


