Abstract
Atypical hemolytic uremic syndrome (aHUS) treatment consists of eculizumab. Severe acute respiratory syndrome coronavirus 2 causes severe pneumonia and endothelial injury that leads to a prothrombotic state that may be complicated by macrovascular and microvascular thrombosis. Complement activation is thought to contribute to endothelial injury and there are at least seven ongoing clinical trials testing six different anti-complement strategies for coronavirus disease 2019 (COVID-19), including eculizumab. We herein report on a kidney transplant patient with aHUS on chronic eculizumab therapy that developed severe COVID-19 despite eculizumab administration early in the course of the disease. Although eculizumab was unable to prevent the development of severe endothelial cell injury, as assessed by increasing D-dimer levels from 292 to 10 586 ng/mL, the patient eventually recovered following dexamethasone and convalescent plasma administration.
Keywords: aHUS, complement, eculizumab, kidney transplant, SARS-CoV-2
BACKGROUND
Since December 2019, the rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), has affected >12 377 000 people and killed >557 000 in >150 countries as of 10 July 2020 [1].
The complement pathway may be activated in COVID-19 through mannose-binding lectin (MBL) binding to viral S glycoprotein and activation of mannose-associated serine protease 2 (MASP2) [2]. Thereafter, MBL–MASP2 complexes activate the lectin pathway and a positive feedback loop, leading to sustained alternative complement pathway amplification, inflammation, endothelial injury and concurrent activation of the coagulation cascade and systemic microangiopathy [2, 3]. Once complement is activated, C5a triggers the inflammatory cascade, contributing to the cytokine storm that is thought to be key to severe COVID-19.
Atypical hemolytic uremic syndrome (aHUS) patients are at potential risk to suffer COVID-19 complications. While eculizumab protects against the viral damage on endothelial cells due to alternative complement activation, it impairs a complete response of this pathway to infections. This situation is complicated in kidney transplant patients, in whom the risk of infections increases if baseline immunosuppression is maintained or the risk of acute allograft rejection increases when immunosuppressants are discontinued or decreased to unlock the immunologic system.
CASE REPORT
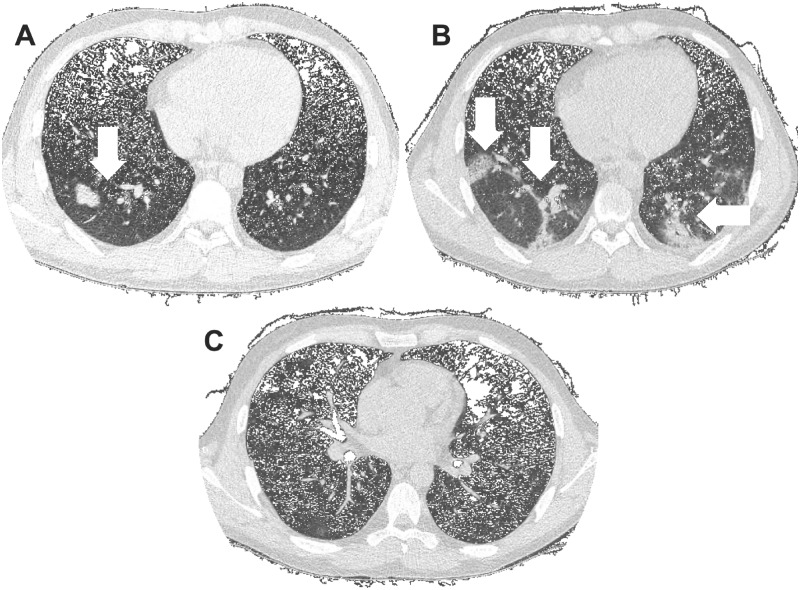
A 24-year-old male with a 6-year history of kidney transplant with aHUS (no complement molecule–associated mutation was identified) on eculizumab 900 mg every fortnight, meprednisone 4 mg/day, sodium mycophenolate 720 mg/day and belatacept 500 mg/monthly was admitted due to anosmia and fever (Table 1). A chest cmputed tomography (CT) scan disclosed patchy bilateral pneumonia (Figure 1A). A nasopharyngeal swab polymerase chain reaction revealed SARS-CoV-2. Antibiotic therapy with ceftriaxone–clarithromycin was started, plus intravenous (IV) hydrocortisone 100 twice a day. Mycophenolate was discontinued and the scheduled eculizumab infusion was administered. Microthrombotic biomarkers were absent. One week later he developed respiratory failure, fever and radiological progression of the CT scan infiltrates (Figure 1B), associated with elevated inflammatory biomarkers. In the intensive care unit (ICU), the antibiotic regime was switched to vancomycin and cefepime and hydrocortisone was changed to IV dexamethasone 6 mg/day. A 200-mL convalescent plasma infusion was prescribed. He received oxygen via mask reservoir (5 L/min) and vigil pronation cycles and showed a sustained improvement without the need for mechanical ventilation (Figure 1C). Two weeks after admission, the scheduled eculizumab and belatacept infusions were administered and the patient was discharged.
Table 1.
Laboratory results, clinical course and interventions
| Variables | Day 0 | Day 3 | Day 7 | Day 9 | Day 16 |
|---|---|---|---|---|---|
| Clinical evolution | Admission to hospital | Admission to ICU | Radiological improvement | ||
| Pharmacologic therapy | Clarithromycin + ceftriaxone | Ecululizumab 900 mg | Vancomycin + cefepime | Dexamethasone, convalescent plasma infusion | End of antibiotics |
| D-dimer (ng/mL) | 292 | 10586 | 454 | ||
| Fibrinogen (mg/dL) | 411 | 494 | 227 | ||
| Ferritin (µg/L) | 2390 | 2223 | 2287 | ||
| Procalcitonin (ng/mL) | 0.26 | 15.55 | |||
| Hematocrit (%) | 35 | 30 | 31 | 34 | 36 |
| Platelets/µL | 141 000 | 132 000 | 121 000 | 130 000 | 208 000 |
| Leukocytes/mm³ | 4500 | 10 000 | 7200 | 14 200 | 14 100 |
| Lymphocytes/mm³ | 648 | 470 | – | 213 | 2044 |
| LDH (U/L) | 226 | 323 | 513 | ||
| Haptoglobin (mg/dL) | 197 | ||||
| C3 (mg/dL) | 100 | 98 | 96 | 99 | |
| C4 (mg/dL) | 29 | 23 | 22 | 14 | |
| Creatinine (mg/dL) | 3.54 | 3.06 | 2.79 | 2.8 | 2.67 |
| Peripheral oxygen saturation (O2IF), n (%) | 98 (0.21) | 98 (0.21) | 97 (0.21) | 98 (0.36) | 96 (0.21) |
LDH: lactate dehydrogenase; O2IF: oxygen inspiration fraction.
FIGURE 1:
Chest CT scan images. (A) Bilateral and diffuse lower lung with predominant ground-glass opacities and consolidations at admission. (B) Progression of ground-glass opacities and consolidations 1 week after admission. (C) Resolution of bilateral opacities 2 weeks after admission.
DISCUSSION
To our knowledge, this is the first report of a kidney transplant recipient with aHUS on eculizumab therapy who developed SARS-CoV-2 infection. In this novel pandemic, no robust evidence-based approaches are available, thus empirical decisions were made, aimed at maintaining a fine line between patient and graft survival and control of the alternative complement pathway and a potentially fatal infection.
Many factors take place in this particular scene: the immunosuppressed state of a transplant patient, aHUS that predisposes to alternative complement activation and thrombotic microangiopathy and the SARS-CoV-2 capacity to activate the complement system, a procoagulant state with microthrombi generation and systemic inflammation. Graft loss was a concern at admission, but patient death was seriously considered 1 week after admission.
To avoid aHUS relapse, eculizumab was continued based on a normal biomarkers profile and considerably elevated D-dimer and fibrinogen concentrations, suggesting a procoagulant state and probable microthrombosis, as described in autopsies of subjects with COVID-19 infections [4].
Mycophenolate was withheld to improve T-cell response. As in preliminary data, dexamethasone appears to be beneficial in COVID-19 and it replaced hydrocortisone [5]. Finally, 1 U of convalescent plasma was given to passively contribute to the specific immunological response and also as a potential antithrombotic and immunomodulatory tool [6]. The maintenance of a partial immunosuppressant regime may have contributed to limit a systemic cytokine storm. As of 12 July 2020, there were seven ongoing clinical trials exploring six different anti-complement drugs for COVID-19 (Table 2). This is a complex case involving aHUS-associated predisposition to microvascular injury and immune suppression to preserve a kidney graft. However, eculizumab administration, both chronic and early (within 3 days of diagnosis and admission) in the course of COVID-19, was unable to prevent the development of severe pneumonia and severe endothelial cell injury as assessed by a 36-fold increase in D-dimer. Despite the limitations of this complex case, it does not appear to support the efficacy of complement targeting in COVID-19.
Table 2.
Ongoing clinical trials targeting complement in COVID-19, according to ClinicalTrials.gov
| Drug | Drug target | Phase | NCT number |
|---|---|---|---|
| Conestat alfa | Recombinant C1 esterase inhibitor | 2 | NCT04414631 |
| AMY-101 | C3 inhibitor | 2 | NCT04395456 |
| APL-9 | C3 inhibitor | 1/2 | NCT04402060 |
| Zilucoplan | C5 inhibitor | 2 | NCT04382755 |
| Eculizumab | Anti-C5 mAb | 2 | NCT04346797 |
| Ravulizumab | Anti-C5 mAb | 4 | NCT04390464 |
| Ravulizumab | Anti-C5 mAb | 3 | NCT04369469 |
mAb: monoclonal antibody.
PATIENT CONSENT
The patient gave informed consent to publish this case.
ACKNOWLEDGEMENTS
We thank María Laura Ares for her professional assistance.
CONFLICT OF INTEREST STATEMENT
H.T. was a consultant for Alexion for the drug eculizumab.
REFERENCES
- 1. Zhou P, Yang XL, Wang XG. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Magro C, Mulvey JJ, Berlin D. et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020; 220: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao T, Hu M, Zhang X. et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv 2020; doi: 10.1101/2020.03.29.20041962 [Google Scholar]
- 4. Zhou F, Yu T, Du R. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horby P, Lim W, Emberson J. et al. Effect of dexamethasone in hospitalized patients with COVID-19: Preliminary Report Recovery Collaborative Group. medRxiv 2020; doi: 10.1101/2020.06.22.20137273 [Google Scholar]
- 6. Campbell CM, Kahwash R.. Will complement inhibition be the new target in treating COVID-19 related systemic thrombosis? Circulation 2020; 141: 1739–1738 [DOI] [PubMed] [Google Scholar]