Abstract
Background
As neoadjuvant therapy of borderline resectable pancreatic cancer (BRPC) is becoming more widely used, better indicators of progression are needed to help guide therapeutic decisions.
Materials and Methods
A retrospective review was performed on all patients with BRPC who received 24 weeks of neoadjuvant chemotherapy. Patients with chemotoxicity or medical comorbidities limiting treatment completion and nonexpressors of carbohydrate antigen 19‐9 (CA19‐9) were excluded. Serum CA19‐9 response was analyzed as a predictor of disease progression, recurrence, and survival.
Results
One hundred four patients were included; 39 (37%) progressed on treatment (18 local and 21 distant) and 65 (63%) were resected (68% R0). Multivariate logistic regression analysis determined that the percent decrease in CA19‐9 from baseline to minimum value (odds ratio [OR] 0.947, p ≤ .0001) and the percent increase from minimum value to final restaging CA19‐9 (OR 1.030, p ≤ .0001) were predictive of progression. A receiver operating characteristics curve analysis determined cutoff values predictive of progression, which were used to create four prognostic groups. CA19‐9 responses were categorized as follows: (1) always normal (n = 6); (2) poor response (n = 31); (3) unsustained response (n = 19); and (4) sustained response (n = 48). Median overall survival for Groups 1–4 was 58, 16, 20, and 38 months, respectively (p ≤ .0001).
Conclusion
Patients with initially elevated CA19‐9 levels who do not have a decline to a sustained low level are at risk for progression, recurrence, and poor survival. Alternative treatment strategies prior to an attempt at curative resection should be considered in this cohort.
Implications for Practice
This study identified percent changes in carbohydrate antigen 19‐9 blood levels while on chemotherapy that predict tumor growth in patients with advanced pancreas cancer. These changes could be used to better select patients who would benefit from surgical removal of their tumors and improve survival.
Keywords: Pancreas cancer, Carbohydrate antigen 19‐9, Neoadjuvant, Biomarker, Borderline resectable
Short abstract
Treatment of patients with borderline resectable pancreas cancer is a topic of great debate. This article analyzes serum CA19‐9 response as a predictor of disease progression, recurrence, and survival.
Introduction
Adenocarcinoma of the pancreas is the fourth leading cause of cancer‐related death in the U.S. 1. Surgical resection offers the best opportunity for cure. However, less than 20% of patients are considered to be upfront resectable, and more than 50% of patients have metastatic disease at presentation 2. The other 30% of patients have either unresectable (locally advanced) or borderline resectable pancreas cancer (BRPC) by various consensus criteria 3, 4. Treatment of patients with BRPC is a topic of great debate with only a few phase III randomized trials to guide treatment type or duration 5, 6. Many centers use neoadjuvant chemoradiotherapy or chemotherapy alone in this subpopulation and are reporting a median overall survival approaching 3 years 7, 8, 9. However, up to 40% of patients will progress on neoadjuvant treatment 10. It is unknown if this represents treatment failure or the natural progression of a systemic disease.
Previous studies have attempted to identify predictors of progression before or during treatment. Tumor characteristics such as size and response by radiographic criteria have been evaluated and not shown to be predictive 11, 12. Tumor response to treatment as measured by changes in F‐fluorodeoxyglucose positron emission tomography/computed tomography (CT) scan avidity have shown some promise but are expensive and can be confounded by pancreatitis or biliary endoprostheses 13. Serum carbohydrate antigen 19‐9 (CA19‐9) is a sialylated Lewis antigen generated by exocrine epithelial cells 14. It has been used to track response to treatment, guide the use of diagnostic laparoscopy, and monitor for tumor recurrence 15, 16, 17, 18. CA19‐9 can be elevated in many types of gastrointestinal malignancies as well as with benign biliary obstructions 19, 20. Up to 10% of the population may not express CA19‐9 owing to a lack of fucosyltransferase, an enzyme required for its production 21, 22. It has been shown that serum CA19‐9 levels over 1,000 U/mL are associated with worse overall survival, but this has not correlated with response to neoadjuvant treatment 23. In patients undergoing systemic treatment, a 50% reduction in pretreatment CA19‐9 levels or a normalization of CA19‐9 have been associated with resectability and survival 24, 25. A recent study suggested that any elevation in CA19‐9, regardless of tumor stage, may predict poor survival 26. For patients on treatment for metastatic disease, any decrease in CA19‐9 at 8 weeks has been associated with an increased overall survival 27. Some authors have combined radiographic response by RECIST and a 30% CA19‐9 decrease from pretreatment to postneoadjuvant levels and found an additive benefit in predicting resection and survival 28.
Although there have been many retrospective reports proposing prognostic biomarkers in pancreas cancer, none of these biologic surrogates are able to accurately predict progression of disease while treatment is ongoing. Using 50% reduction in pretreatment CA19‐9 is only useful at the completion of treatment to help identify patients most likely to benefit from resection. Basing resectability on normalization of CA19‐9 does not account for degree of response to treatment and may have reduced sensitivity. We hypothesized that more frequent measurement of CA19‐9 response in patients undergoing neoadjuvant treatment for BRPC would identify more dynamic changes than just measuring at the initiation and completion of treatment, thereby allowing for better risk stratification of patients at risk for disease progression.
Materials and Methods
Patient Selection Criteria
Consecutive patients evaluated from August 2009 to February 2017 with AHPBA/SSO consensus criteria–defined borderline resectable pancreatic head cancer were reviewed by a multidisciplinary tumor board and offered 24 weeks (eight cycles) of gemcitabine/docetaxel‐based neoadjuvant chemotherapy 3. Radiation was considered if local progression occurred. Patients were tracked in a prospectively maintained database with institutional review board approval. Clinical variables were collected by retrospective review of the electronic medical record and billing data. Initial staging included a thin slice pancreatic protocol CT scan, chest x‐ray or chest CT, and a diagnostic laparoscopy with peritoneal washings prior to starting therapy. Patients were excluded from analysis if they were found to have metastatic disease at initial staging, received any element of neoadjuvant therapy at an outside institution, discontinued neoadjuvant treatment because of intercurrent illness or treatment toxicity precluding resection, or never had a measurable serum CA19‐9 level. Patients experiencing significant toxicity or progression with gemcitabine or docetaxel were switched to second‐line therapy. Patients were restaged at our institution by computed tomography and serum tumor markers every 2 months while on treatment. After completing the neoadjuvant chemotherapy regimen, patients were restaged and considered for surgical resection if their disease had not progressed by RECIST 1.1 criteria and had reconstructable vascular involvement and if they were medically fit for surgery 29.
Surgical Resection
Patients were offered either standard or pylorus‐preserving pancreatoduodenectomy based on surgeon preference. A single patient had a total pancreatectomy. Venous resection and reconstruction was performed whenever portomesenteric venous involvement was suspected intraoperatively. Drains were placed at the discretion of the surgeon. Surgical resection margins were inked by the pathologist in the presence of the surgeon and evaluated according to the Leeds protocol. Margins were considered positive if microscopic tumor was present within 1.0 mm of any inked section 30.
Serum Carbohydrate Antigen 19‐9 Evaluation
All serum CA19‐9 levels were collected from the medical record from the date of neoadjuvant chemotherapy initiation through the date of final restaging. Serum CA19‐9 levels were typically drawn prior to each chemotherapy infusion. Values of CA19‐9 with concomitant total serum bilirubin greater than 1.2 mg/dL were excluded from analysis. Serum CA19‐9 levels were determined by the Abbott i2000 Architect, CA 19‐9XR2 assay (Abbott Diagnostics, Abbott Park, IL) with normal values being ≤37.5 U/mL. Patients with all recorded CA19‐9 values <3 U/mL (undetectable) were considered nonexpressors.
Definition of Progression
Progression was defined as meeting RECIST 1.1 criteria or one of the following: (a) development of cytology‐positive ascites, (b) radiographic evidence of local tumor progression precluding resection as determined by responsible surgeon, or (c) attempted resection with intraoperative findings of local unresectability or metastatic disease.
Statistical Analysis
Comparison of categorical variables was performed by a Fisher's exact test and continuous variables by Mann‐Whitney U test. Univariate and multivariable logistic regression were used to determine influence of clinicopathologic parameters on progression. Kaplan‐Meier curves were generated to determine survival with the date of neoadjuvant therapy initiation as the starting point. Date of imaging or biopsy proving recurrence was used as the endpoint for disease‐free survival (DFS) calculations. Statistical significance was defined as having a p value of <.05. Statistical analysis was performed with MedCalc 18.6 software (Mariakerke, Belgium).
Results
Patient Selection
One hundred twenty‐six patients with borderline resectable pancreatic head cancer were started on neoadjuvant treatment during the study period (Fig. 1). Fifteen patients were excluded secondary to intercurrent illness (combination of disease related and unrelated) or treatment‐related toxicity that prevented completion of full‐course neoadjuvant chemotherapy. Seven patients never had a serum CA19‐9 >3 U/mL (undetectable). One hundred four patients completed a median of 178 days (25 weeks) of neoadjuvant therapy; 28 patients (26%) were determined to be unresectable at final restaging and 76 (74%) underwent abdominal exploration with an attempt at resection. Eleven of the 76 patients (14.5%) who underwent exploration were found to be unresectable (6 local progression and 5 distant disease). Hence, a total of 39 patients (37%) were found to progress on neoadjuvant chemotherapy and 65 (63%) were resected with curative intent. Three patients who were judged to be unresectable after completing all neoadjuvant chemotherapy underwent neoadjuvant chemoradiation and then were resected for cure. Of the 28 patients who progressed prior to attempted resection, 16 had distant disease, 7 had local progression by radiographic imaging criteria, and 5 had local progression preventing resection based on anatomy. Of the 65 patients who underwent resection, 53 (82%) had adjuvant treatment (24 chemotherapy only and 29 chemoradiotherapy).
Figure 1.
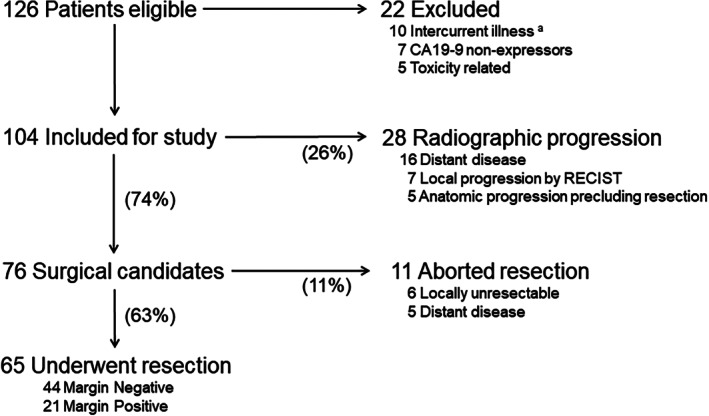
Consort diagram depicting patient treatment schema. aIncludes both disease‐related (e.g., pulmonary embolism) and unrelated (e.g., myocardial infarction) illnesses.
Abbreviation: CA19‐9, carbohydrate antigen 19‐9.
Demographics
Patient demographics and neoadjuvant treatment information are shown in Table 1. Basic demographics and chemotherapy toxicity requiring change in agents was similar between those who progressed and those who did not. Patients who progressed were less likely to have completed all intended chemotherapy (74% vs. 94%; p = .007).
Table 1.
General demographics
| Characteristic | All patients | Progressed | No progression | p value |
|---|---|---|---|---|
| No. of patients | 104 | 39 | 65 | |
| Age, years | 66 (59–73) | 66 (60–74) | 65 (59–72) | .566 |
| Male, n (%) | 53 (51) | 23 (59) | 30 (46) | .229 |
| Changed agents, n (%) | 10 (10) | 2 (5) | 8(12) | .314 |
| Completed intended chemotherapy, n (%) | 90 (87) | 29 (74) | 61 (94) | .007 |
| Duration of neoadjuvant, days | 178 (168–194) | 182 (156–203) | 178 (172–191) | .963 |
| Had neoadjuvant radiation | 3 (3) | 0 (0) | 3 (5) | — |
| Adjuvant therapy, n (%resected) | 53 (51) | — | 53 (82) | — |
Data are presented as the median (interquartile range) unless otherwise indicated.
Abbreviation: —, no data.
CA19‐9 Response
Serum CA19‐9 response characteristics are reported in Table 2. Patients had a median of 12 laboratory values on treatment. There was no difference between baseline and maximum CA19‐9 levels in patients who progressed and those that did not. An equal percentage of patients had a normal CA19‐9 throughout treatment in each cohort. Patients with progression had higher CA19‐9 levels at nadir (47 vs. 17; p = .0007) and final restaging (191 vs. 36; p ≤ .0001). Patients with progression had less of a decrease of CA19‐9 levels at nadir and final restaging and had a larger increase from nadir to final restaging. Patients who did not progress were more likely to have a normal CA19‐9 at any time and at final restaging.
Table 2.
Response to treatment
| Characteristic | All patients | Progressed | No progression | p value |
|---|---|---|---|---|
| No. of patients | 104 | 39 | 65 | |
| No. of CA19‐9 levels reported | 12 (10–14) | 12 (9–14) | 13 (11–14) | .502 |
| Initial CA19‐9, U/mL | 364 (80–1,514) | 342 (52–1,391) | 563 (114–1,655) | .334 |
| Maximum CA19‐9, U/mL | 520 (122–1,586) | 453 (106–1,517) | 562 (125–1,661) | .690 |
| Minimum (nadir) CA19‐9, U/mL | 25 (12–105) | 47 (20–165) | 17 (10–56) | .0007 |
| Final restaging CA19‐9, U/mL | 60 (21–263) | 191 (50–659) | 36 (15–145) | <.0001 |
| CA19‐9 % decrease (baseline to minimum) | 90 (70–97) | 70 (58–85) | 94 (91–96) | <.0001 |
| CA19‐9 % increase (minimum to final) | 43 (22–71) | 64 (36–80) | 33 (12–59) | .001 |
| CA19‐9 % increase (penultimate to final) | 11.5 (–10 to 34) | 16 (3–38) | 6 (–15 to 29) | .096 |
| CA19‐9 % decrease (baseline to final) | 76 (39–94) | 37 (8–53) | 90 (81–94) | <.0001 |
| Always normal, n (%) | 6 (6) | 2 (5) | 4 (6) | >.999 |
| CA19‐9 ever normal, n (%) | 64 (62) | 17 (44) | 47 (73) | .006 |
| Final CA19 normal, n (%) | 41 (40) | 6 (15) | 35 (54) | .0001 |
| Days to normal | 53 (21–91) | 28 (11–56) | 63 (21–91) | .046 |
Data are presented as the median (interquartile range) unless otherwise indicated.
Abbreviation: CA19‐9, carbohydrate antigen 19‐9.
Regression Analysis
Logistic regression was performed to determine correlation of CA19‐9 response to progression (Table 3) using absolute values of response (baseline, maximum, minimum, and final restaging values) as well as relative response (percent change from baseline to minimum, minimum to final, and baseline to final). None of the absolute values correlated with progression. However, the percent change from baseline CA19‐9 to minimum value (odds ratio [OR] 0.947 [0.919–0.976]; p ≤ .0001) and minimum value to final CA19‐9 value (OR 1.030 [1.011–1.049]; p ≤ .0001) did correlate with progression on both univariable and multivariable analyses.
Table 3.
Logistic regression of CA19‐9‐related variables associated with progression
| Characteristic | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| CA19‐9 decrease baseline to minimum, % | 0.958 | 0.937–0.979 | <.0001 | 0.947 | 0.919–0.976 | <.0001 |
| CA19‐9 increase minimum to final, % | 1.027 | 1.009–1.039 | .001 | 1.030 | 1.011–1.049 | <.0001 |
| CA19‐9 decrease baseline to final, % | 0.999 | 0.999–1.000 | .230 | |||
| Baseline CA19‐9, U/mL | 1.000 | 1.000–1.000 | .895 | |||
| Maximum CA19‐9, U/mL | 1.000 | 1.000–1.000 | .964 | |||
| Minimum CA19‐9, U/mL | 1.000 | 1.000–1.003 | .076 | |||
| Final CA19‐9, U/mL | 1.000 | 1.000–1.000 | .714 | |||
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CI, confidence interval.
Receiver Operating Characteristics Curve Analysis
A receiver operating characteristics (ROC) curve was created to determine the optimal predictive cutoff value for progression of the two variables found correlative by multivariable regression (supplemental online Fig. 1; supplemental online Table 1). The percent change from baseline to minimum CA19‐9 value had an area under the curve (AUC) of 0.757 ± 0.05 with an optimal cutoff of ≤78% based on Youden's method (p ≤ .0001). The minimum to final restaging CA19‐9 had an AUC of 0.688 ± 0.5 with an optimal cutoff of >66% (p = .0004).
Survival Analysis
Median overall survival (OS) was 27, 15, and 38 months for all patients, those that progressed on treatment, and those that were resected, respectively. Disease‐free survival for resected patients was 24.6 months. A Cox proportional hazards regression was performed for the same absolute and relative CA19‐9 variables used in the logistic regression (Table 4). Mirroring our findings with preoperative progression, we found the percent change from baseline CA19‐9 to minimum value (hazard ratio [HR] 0.975 [0.963–0.987]; p ≤ .0001) and minimum value to final CA19‐9 value (HR 1.017 [1.007–1.027]; p ≤ .0001) to be strongly correlated with overall survival. Using the cutoff values determined by ROC analysis, four predictive Groups were created: (1) patients with an “always normal” CA19‐9, (2) those that fail to have a 78% CA19‐9 decrease during treatment (poor response), (3) those who had a 78% decrease but a subsequent rise from nadir to final restaging of >66% (unsustained response), and (4) those with a >78% decrease and a “sustained response.” Representative patient CA19‐9 curves are depicted in Figure 2 with a flowchart defining these response groups. Median OS for response Groups 1–4 was 58, 16, 20, and 38 months, respectively (p ≤ .0001), whereas median DFS for response Groups 2–4 was 16, 14, and 31 months, respectively (p = .0004), with Group 1 not reaching median (Fig. 3). Subgroup analysis found no difference in OS (34 vs. 41 months; p = .633) between patients in Group 4 who had normalization of CA19 levels (n = 31) and those did not (n = 17). Adjuvant treatment was varied across response groups, and no conclusions regarding differences between cohorts could be determined (supplemental online Table 2).
Table 4.
Cox proportional hazards regression of continuous variables related to CA19‐9 response
| Characteristic | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value | |
| CA19‐9 decrease baseline to minimum, % | 0.981 | 0.972–0.990 | .0002 | 0.975 | 0.963–0.987 | <.0001 |
| CA19‐9 increase minimum to final, % | 1.015 | 1.006–1.023 | .001 | 1.017 | 1.007–1.027 | <.0001 |
| CA19‐9 decrease baseline to final, % | 1.000 | 0.999–1.000 | .095 | |||
| Baseline CA19‐9, U/mL | 1.000 | 1.000–1.000 | .431 | |||
| Maximum CA19‐9, U/mL | 1.000 | 1.000–1.000 | .232 | |||
| Minimum CA19‐9, U/mL | 1.001 | 1.000–1.002 | .014 | |||
| Final CA19‐9, U/mL | 1.000 | 1.000–1.000 | .013 | |||
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CI, confidence interval.
Figure 2.
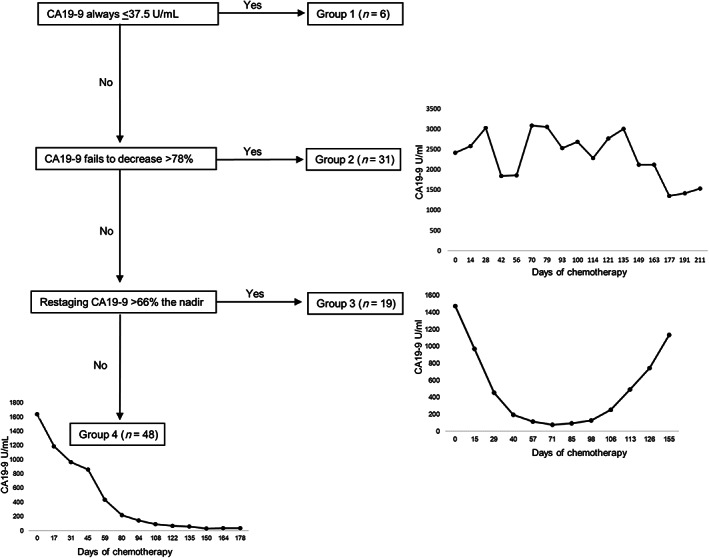
Flow diagram with representative graphs of each response Group. Always normal (Group 1 not shown), poor response (Group 2), unsustained response (Group 3), sustained response (Group 4).
Abbreviation: CA19‐9, carbohydrate antigen 19‐9.
Figure 3.
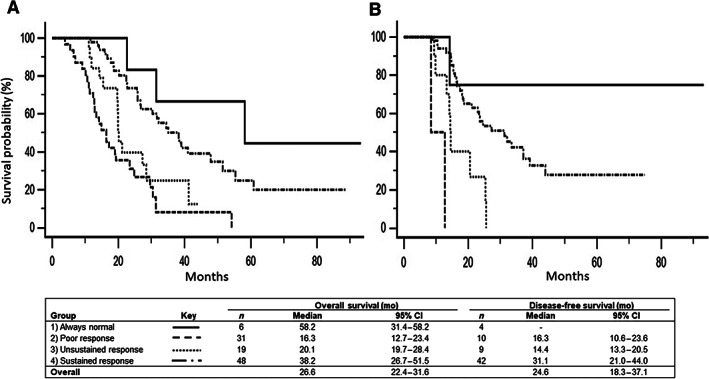
Overall and disease‐free survivals. (A): Overall survival for response Groups 1–4 with associated medians of 58, 16, 20, and 38 months, respectively. (B): Disease‐free survival for response Groups 1–4 with associated medians of no recurrence, 16, 14, and 31 months, respectively.
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CI, confidence interval.
Discussion
More than one third of patients treated with neoadjuvant therapy for BRPC will progress on treatment. There is currently no reliable tumor marker or radiographic method to predict progression before or during treatment. Radiographic response to treatment has not been accurate in the neoadjuvant setting for pancreatic cancer 11. High initial CA19‐9 values were originally thought to be predictive of poor outcomes, but contemporary data suggest no correlation 23, 31. More recent data suggest that, even in early‐stage resectable disease, any elevation of serum CA19‐9 may be associated with worse outcomes compared with patients with normal or unmeasurable levels 26. Various relative or absolute decreases in CA19‐9 after neoadjuvant treatment have shown promise in guiding resectability or predicting survival 15, 24, 28. However, these metrics are based on two snapshots in time and this linear interpretation may not fully represent the entire spectrum of an individual's tumor biology.
To investigate whether serum CA19‐9 response to neoadjuvant treatment could be predictive of tumor progression, we analyzed consecutive patients from 2009 to 2017 with borderline resectable tumors that initiated eight cycles of extended neoadjuvant chemotherapy at our institution. We excluded patients with intercurrent illness and toxicity precluding resection to focus our investigation on the prognostic ability of CA19‐9 independently of medical fitness. One hundred four patients met inclusion criteria, 87% completed all eight cycles of chemotherapy, and 63% underwent resection for cure. Multivariable logistic regression and Cox proportional hazards analysis determined percent change from baseline to final restage and minimum to final restage CA19‐9 correlated with both progression and survival. Care should be taken in interpreting these odds ratios as they correlate with changes in continuous variables; therefore, every percent change in CA19‐9 response compounds the risk. For example, a 78% decrease in CA19‐9 from baseline represents a fourfold risk reduction in progression. ROC analysis was used to provide optimal cutoffs to create informed prognostic response groups.
Patients in response Group 1 had consistently normal serum CA19‐9 values and a very favorable median overall survival of 58 months. These patients represented 6% of our cohort. Four patients in response Group 1 were resected, whereas two had local progression that prevented resection. The 31 patients in response Group 2 had a poor response to treatment with CA19‐9 declines of ≤78%. Twenty‐one of the 31 (68%) progressed on treatment. This response was associated with a median overall survival (16 months) that was equivalent to unresected patients (15 months). Interestingly, Group 3 patients, who initially had a good response to treatment but had a subsequent >66% rise from their nadir to final CA19‐9 value, had an OS of 20 months, which was not statistically different from Group 2. Of the 19 patients in Group 3, 10 progressed on treatment (5 distant disease, 5 local) and all had disease recurrence. Of the 48 patients in Group 4, only 6 progressed on treatment (3 distant, and 3 local only). These patients with sustained CA19‐9 responses to treatment did very favorably with an OS of 38 months and a DFS of 31. A subgroup analysis of patients in Group 4 that had normalization of final CA19‐9 (n = 31) versus those that did not (n = 17) found equivalent OS of 34 versus 41 months (p = .633).
Our observations are contrary to previous studies that reported normalization of CA19‐9 is a strong predictor of improved survival. Our study is unique in that the relative response in CA19‐9 over time, even when it does not normalize, is a better predictor of progression and overall survival than absolute response 25. A recent study looked at both normalization of CA19‐9 and relative change between baseline and final values and found that normalization was a better predictor of OS 32. Our data support this finding in that the change between baseline CA19‐9 and final value is not predictive of survival (HR 1); however, we propose that a change from baseline to minimum is more predictive than an absolute value (e.g., normalization), with every 1% decrease representing a 0.025% reduction in risk of death. This illustrates how important it is to follow CA19‐9 values more frequently to accurately determine a true nadir. A normalized value in our study was not as specific for progression on neoadjuvant treatment or overall survival as the relative drop from baseline to nadir. Relying solely on normalization would have falsely predicted a poor response in nearly a third of patients in Group 4, all of whom who had similar OS on subgroup analysis.
As neoadjuvant treatment of borderline resectable pancreatic cancer becomes more common, many questions regarding best practices remain. Current regimens are typically gemcitabine or 5‐flurouracil based, may include radiation, are given over 3–6 months, and have median survivals approaching 3 years 7, 8. There are no published clinical trials establishing superiority of one neoadjuvant approach over another for patients with borderline resectable pancreatic cancer. However, the current study suggests that CA19‐9 response over time may be a way to individually tailor neoadjuvant treatment for patients with variable prognosis. It is unclear if the unsustained response noted in response Group 3 is a result of biologically more aggressive disease, the development of resistance to specific chemotherapy, or emergence of a new tumor clonal population or if it represents a missed opportunity for resection during an extended course of neoadjuvant treatment. We cannot comment on how the length of treatment may have affected the survival outcomes of patients in Group 3 or whether earlier resection may have prevented progression. However, analysis of this group does provide a natural‐history observation of CA19‐9 kinetic response during systemic treatment and identifies a threshold of elevation from nadir that may be considered prognostic of progression. Further study will be necessary to better understand this particular group of patients.
This study is limited by its retrospective nature. Response Groups 1 and 2 were somewhat underrepresented, although we feel that good outcomes in patients with always normal (Group 1) or poor outcomes in patients with limited or no biochemical response to treatment (Group 2) is not an unexpected finding and consistent with prior reports. Determining progression is difficult in this population, and surgeon bias is also introduced by their judgment of resectability. However, this is unavoidable given the multiple anatomic considerations required for a successful resection. The retrospective nature of the study makes it hard to determine if chemotherapeutic agents were changed in response to CA19‐9 trends, and this could introduce a confounder that was not controlled for. However, this occurred in only 10% of patients. Adjuvant treatment was not standardized and may confound the results; however, this variability is present across all response groups. Differences in commercially available CA19‐9 assays may also change cutoff values used to define high‐risk response groups, so it would be worthwhile investigating whether or not similar results are found across the spectrum of different assays prior to adoption 33. Patients who did not express CA19‐9 were excluded from analysis as the focus of this study was on using CA19‐9 response during treatment prognostically, and this could not be done if a serum level was unmeasurable.
Conclusion
A decline in CA19‐9 that is sufficient and sustained in patients with BRPC receiving neoadjuvant chemotherapy is predictive of better outcomes than patients without this biochemical response. These findings should be validated in a larger patient cohort before widespread clinical application. If confirmed, alternative strategies should be considered prior to an attempt at resection in this cohort.
Author Contributions
Conception/design: J. Bart Rose, Alicia M. Edwards, Flavio G. Rocha, W. Scott Helton
Provision of study material or patients: J. Bart Rose, Alicia M. Edwards, Flavio G. Rocha, Carolyn Clark, Adnan A. Alseidi, Thomas R. Biehl, Bruce S. Lin, Vincent J. Picozzi, W. Scott Helton
Collection and/or assembly of data: J. Bart Rose, Alicia M. Edwards, Carolyn Clark
Data analysis and interpretation: J. Bart Rose, Alicia M. Edwards, W. Scott Helton
Manuscript writing: J. Bart Rose, Alicia M. Edwards, Flavio G. Rocha, Carolyn Clark, Adnan A. Alseidi, Thomas R. Biehl, Bruce S. Lin, Vincent J. Picozzi, W. Scott Helton
Final approval of manuscript: J. Bart Rose, Alicia M. Edwards, Flavio G. Rocha, Carolyn Clark, Adnan A. Alseidi, Thomas R. Biehl, Bruce S. Lin, Vincent J. Picozzi, W. Scott Helton
Disclosures
Carolyn Clark: Crowd Sourced Assessment of Technical Skills (C‐SATS) (IP [patent holder]); Bruce S. Lin: Exelixis, QED Therapeutics (C/A), Sillajen, Exelixis, Merck, Bristol‐Myers Squibb, Boston Biomedical, Five Prime Therapeutics, Incyte, Zymeworks (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables
Supplemental Figure
Acknowledgments
We would like to thank the Digestive Disease Institute at Virginia Mason Medical Center for their administrative support. This work was presented at the Society of Surgical Oncology 2016 Annual Meeting.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019 (US statistics). CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 2. Gillen S, Schuster T, Meyer Zum Büschenfelde C et al. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta‐analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Callery MP, Chang KJ, Fishman EK et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: Expert consensus statement. Ann Surg Oncol 2009;16:1727–1733. [DOI] [PubMed] [Google Scholar]
- 4. Isaji S, Mizuno S, Windsor JA et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2–11. [DOI] [PubMed] [Google Scholar]
- 5. Jang JY, Han Y, Lee H et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer. Ann Surg 2018;268:215–222. [DOI] [PubMed] [Google Scholar]
- 6. Van Tienhoven G, Versteijne E, Suker M et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC‐1): A randomized, controlled, multicenter phase III trial. J Clin Oncol 2018;36(suppl 18):LBA4002a. [Google Scholar]
- 7. Katz MH, Pisters PW, Evans DB et al. Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. J Am Coll Surg 2008;206:833–846; discussion 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rose JB, Rocha FG, Alseidi A et al. Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Ann Surg Oncol 2014;21:1530–1537. [DOI] [PubMed] [Google Scholar]
- 9. Tsai S, Christians KK, George B et al. A phase II clinical trial of molecular profiled neoadjuvant therapy for localized pancreatic ductal adenocarcinoma. Ann Surg 2018;268:610–619. [DOI] [PubMed] [Google Scholar]
- 10. Tang K, Lu W, Qin W et al. Neoadjuvant therapy for patients with borderline resectable pancreatic cancer: A systematic review and meta‐analysis of response and resection percentages. Pancreatology 2015;16:28–37. [DOI] [PubMed] [Google Scholar]
- 11. Ferrone CR, Marchegiani G, Hong TS et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marchegiani G, Andrianello S, Malleo G et al. Does size matter in pancreatic cancer?: Reappraisal of tumour dimension as a predictor of outcome beyond the TNM. Ann Surg 2017;266:142–148. [DOI] [PubMed] [Google Scholar]
- 13. Akita H, Takahashi H, Ohigashi H et al. FDG‐PET predicts treatment efficacy and surgical outcome of pre‐operative chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Eur J Surg Oncol 2017;43:1061–1067. [DOI] [PubMed] [Google Scholar]
- 14. Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol 2013;107:15–22. [DOI] [PubMed] [Google Scholar]
- 15. Tzeng CW, Balachandran A, Ahmad M et al. Serum carbohydrate antigen 19‐9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2014;16:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maithel SK, Maloney S, Winston C et al. Preoperative CA 19‐9 and the yield of staging laparoscopy in patients with radiographically resectable pancreatic adenocarcinoma. Ann Surg Oncol 2008;15:3512–3520. [DOI] [PubMed] [Google Scholar]
- 17. Strobel O, Hartwig W, Hackert T et al. Re‐resection for isolated local recurrence of pancreatic cancer is feasible, safe, and associated with encouraging survival. Ann Surg Oncol 2013;20:964–972. [DOI] [PubMed] [Google Scholar]
- 18. Satoi S, Yanagimoto H, Toyokawa H et al. Selective use of staging laparoscopy based on carbohydrate antigen 19‐9 level and tumor size in patients with radiographically defined potentially or borderline resectable pancreatic cancer. Pancreas 2011;40:426–432. [DOI] [PubMed] [Google Scholar]
- 19. Koprowski H, Herlyn M, Steplewski Z et al. Specific antigen in serum of patients with colon carcinoma. Science 1981;212:53–55. [DOI] [PubMed] [Google Scholar]
- 20. Koprowski H, Steplewski Z, Mitchell K et al. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet 1979;5:957–971. [DOI] [PubMed] [Google Scholar]
- 21. Orntoft TF, Vestergaard EM, Holmes E et al. Influence of Lewis alpha1‐3/4‐L‐fucosyltransferase (FUT3) gene mutations on enzyme activity, erythrocyte phenotyping, and circulating tumor marker sialyl‐Lewis a levels. J Biol Chem 1996;271:32260–32268. [DOI] [PubMed] [Google Scholar]
- 22. Yu LC, Yang YH, Broadberry RE et al. Correlation of a missense mutation in the human Secretor alpha 1,2‐fucosyltransferase gene with the Lewis(a+b+) phenotype: A potential molecular basis for the weak Secretor allele (Sew). Biochem J 1995;312:329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aldakkak M, Christians KK, Krepline AN et al. Pre‐treatment carbohydrate antigen 19‐9 does not predict the response to neoadjuvant therapy in patients with localized pancreatic cancer. HPB (Oxford) 2015;17:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boone BA, Steve J, Zenati MS et al. Serum CA 19‐9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann Surg Oncol 2014;21:4351–4358. [DOI] [PubMed] [Google Scholar]
- 25. Williams JL, Kadera BE, Nguyen AH et al. CA19‐9 normalization during pre‐operative treatment predicts longer survival for patients with locally progressed pancreatic cancer. J Gastrointest Surg 2016;20:1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bergquist JR, Puig CA, Shubert CR et al. Carbohydrate antigen 19‐9 elevation in anatomically resectable, early‐stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: A National Cancer Database Study. J Am Coll Surg 2016;223:52–65. [DOI] [PubMed] [Google Scholar]
- 27. Chiorean EG, Von Hoff DD, Reni M et al. CA19‐9 decrease at 8 weeks as a predictor of overall survival in a randomized phase III trial (MPACT) of weekly nab‐paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol 2016;27:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Veldhuisen E, Vogel JA, Klompmaker S et al. Added value of CA19‐9 response in predicting resectability of locally advanced pancreatic cancer following induction chemotherapy. HPB (Oxford) 2018;20:605–611. [DOI] [PubMed] [Google Scholar]
- 29. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 30. Verbeke CS, Leitch D, Menon KV et al. Redefining the R1 resection in pancreatic cancer. Br J Surg 2006;93:1232–1237. [DOI] [PubMed] [Google Scholar]
- 31. Hartwig W, Strobel O, Hinz U et al. CA19‐9 in potentially resectable pancreatic cancer: Perspective to adjust surgical and perioperative therapy. Ann Surg Oncol 2013;20:2188–2196. [DOI] [PubMed] [Google Scholar]
- 32. Tsai S, George B, Wittmann D et al. Importance of normalization of CA19‐9 levels following neoadjuvant therapy in patients with localized pancreatic cancer. Ann Surg 2020;271:740–747. [DOI] [PubMed] [Google Scholar]
- 33. La'ulu SL, Roberts WL. Performance characteristics of five automated CA 19‐9 assays. Am J Clin Pathol 2007;127:436–440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Tables
Supplemental Figure


