Abstract
Lessons Learned
Patient compliance with the oral dosage treatment was good, with no need for hospitalization.
Patients with tracheal and esophageal fistulas can take crushed apatinib by nutrient tube, with the same bioavailability and efficacy.
Apatinib may be an effective and safe second‐ or further‐line treatment for advanced esophageal cancer.
Background
Apatinib is an inhibitor of vascular endothelial growth factor receptor‐2 (VEGFR2), which is thought to play a role in esophageal cancer progression. Our goal was to evaluate the efficacy and safety of apatinib in patients with unresectable esophageal cancer and to examine whether VEGFR2 expression influenced the clinical response.
Methods
This single‐arm, open‐label, investigator‐initiated phase II study enrolled patients with advanced squamous cell carcinoma (SCC) or adenocarcinoma of the esophagus or esophagogastric junction who were admitted to Tianjin Medical University Cancer Institute and Hospital between August 2017 and January 2019. Apatinib monotherapy (500 mg/day) was given orally or via an enteral tube until disease progression, unacceptable toxicity, withdrawal, or death. Patients were followed until treatment was discontinued or death. The main endpoints were tumor response, progression‐free survival (PFS), overall survival (OS), and adverse events (AEs).
Results
Among 32 patients screened for inclusion, 30 were included in the safety and survival analyses (i.e., received apatinib), and 26 were included in the efficacy analysis (at least one imaging follow‐up). Median follow‐up time and exposure to apatinib were 5.34 months and 72 days, respectively. Among 26 patients included in the efficacy analysis, 2 had a partial response (PR; 7.7%) and 14 had stable disease (SD; 53.8%). The overall response rate (ORR) was 7.7%, and the disease control rate (DCR) was 61.5%. Median PFS and OS were 4.63 months (95% confidence interval, 2.11–7.16 months) and 6.57 months (4.90 months to not estimable), respectively. Fifteen patients (50.0%) experienced treatment‐related AEs, most commonly hypertension (26.7%), diarrhea (20.0%), and hand‐foot‐skin reaction (10.0%). No patients had grade ≥4 treatment‐related AEs.
Conclusion
Apatinib was effective as second‐ or further‐line treatment for advanced esophageal cancer.
Discussion
Apatinib is an anticancer agent with oral bioavailability that selectively targets VEGFR2 to exert antiangiogenic and antiproliferative effects. Few previous studies have explored the use of apatinib monotherapy in the setting of advanced cancer of the esophagus or gastroesophageal junction. This study assessed the efficacy and safety of apatinib as a single agent in patients with metastatic, unresectable cancer of the esophagus or esophagogastric junction for whom standard second‐line therapy had failed or was not suitable and to examine whether the clinical response to apatinib was related to VEGFR2 expression.
This phase II trial enrolled 30 patients with advanced SCC or adenocarcinoma of the esophagus or esophagogastric junction (Fig. 1). Apatinib monotherapy (500 mg/day) was given orally or via an enteral tube until disease progression, unacceptable toxicity, withdrawal, or death. The main endpoints were tumor response, PFS, OS, and AEs.
Figure 1.
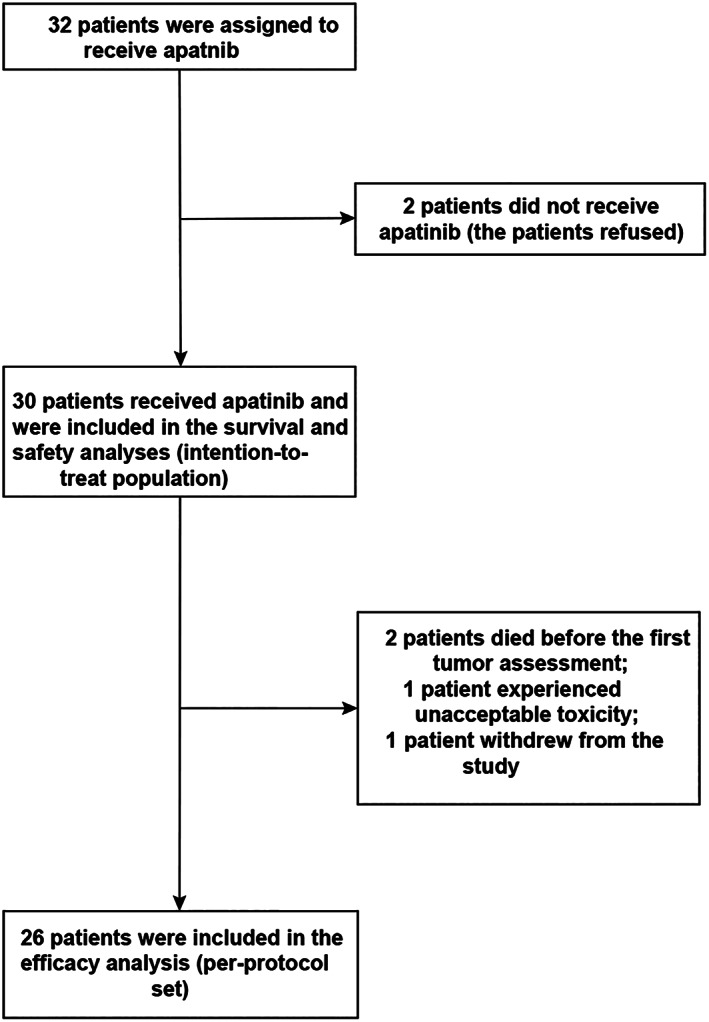
Patient disposition.
Figure 2.
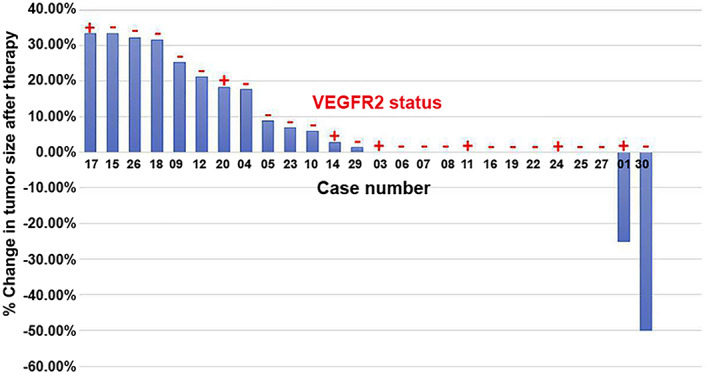
Waterfall plot illustrating the response to treatment in the 26 patients included in the efficacy analysis. The response to treatment was measured radiographically as the percentage change in tumor size after therapy, evaluated as recommended by RECIST version 1.1. Tumor expression of VEGFR2 (positive or negative) is shown in red.
Abbreviation: VEGFR2, vascular endothelial growth factor receptor‐2.
Results showed that 7.7% of patients had a PR and 53.8% had SD after apatinib monotherapy, with an ORR of 7.7% and a DCR of 61.5%. Median PFS and OS were 4.63 months and 6.57 months, respectively. Although half the patients experienced treatment‐related AEs, all were grade ≤3. Thus, the findings of this study are broadly consistent with those of previous clinical investigations, indicating that apatinib monotherapy (at a daily dose of 500 mg) may be an effective second‐ or further‐line treatment for advanced esophageal cancer.
Treatment‐related AEs were observed in 50% of patients, and these were most commonly hypertension (26.7%), diarrhea (20.0%), and hand‐foot‐skin reaction (10.0%). Most AEs were grade 1 in severity; only one patient experienced a grade 3 AE (hypertension), and no patients had grade ≥4 treatment‐related AEs. The AEs observed in this study are comparable to those observed in previous research, indicating that apatinib has an acceptable toxicity profile when used as a single agent for the management of advanced esophageal cancer.
In conclusion, apatinib monotherapy appears to be an effective and safe second‐ or further‐line treatment for patients with metastatic, unresectable cancer of the esophagus or esophagogastric junction.
Trial Information
| Disease | Esophageal cancer |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | More than 2 prior regimens |
| Type of Study | Phase II, single arm |
| Primary Endpoint | Overall survival |
| Secondary Endpoint | Progression‐free survival |
| Additional Details of Endpoints or Study Design | |
| The main inclusion criterion was histologically confirmed stage III/IV SCC/adenocarcinoma of the esophagus or SCC/adenocarcinoma of the gastroesophageal junction accompanied by high expression of VEGFR2. The inclusion criteria were (a) able to understand the nature of the study and provided informed written consent; (b) histologically confirmed stage III/IV SCC/adenocarcinoma of the esophagus or SCC/adenocarcinoma of the gastroesophageal junction accompanied by high expression of VEGFR2; (c) did not respond to or relapsed after treatment with platinum‐containing or 5‐fluorouracil‐containing chemotherapy or unable to tolerate previous chemotherapy; (d) aged 18–80 years and with an expected survival time of at least 12 weeks; (e) Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2; (f) at least one measurable lesion according to RECIST version 1.1 19, which had not been treated previously by radiotherapy; (g) bone marrow, hepatic, and renal functions measured by the Central Laboratory within 14 days after inclusion met the following criteria: white blood cell count ≥3.5 × 109/L (3,000/mm3), hemoglobin ≥10 g/dL, absolute neutrophil count ≥1.0 × 109/L (1,500/mm3), platelet count ≥100 × 109/L; v) total bilirubin ≤2 × upper limit of normal (ULN), alanine transaminase and aspartate transaminase ≤100 IU/L, serum creatinine ≤1.5 × ULN, and creatinine clearance ≥60 mL/minute according to the Cockroft‐Gault equation (other methods such as the ethylenediaminetetraacetic acid method, inulin clearance method, or 24‐hour urine analysis were used for patients with low body weight or when use of another equation yielded a substantially different result to the Cockroft‐Gault equation); (g) for women of childbearing age, a serum pregnancy test performed within 7 days before treatment was negative (all participants were required to use adequate methods of barrier contraception throughout the treatment period and for 4 weeks after treatment); and (h) high adherence to therapy and able to be followed up. The exclusion criteria were (a) other targeted antiangiogenic agents, such as bevacizumab or endostar, had been used as the first‐line therapy; (b) concurrent administration of other antitumor therapies, including hormone therapy (oral contraceptives and physiologic replacement of hormones was permitted), immunotherapy, and targeted therapy; (c) cerebral or meningeal metastases (excluding cerebral parenchymal metastases that had been controlled after local therapy and did not require hormone maintenance therapy); (d) history of interstitial lung disease, drug‐induced interstitial diseases, or radiation pneumonitis requiring hormone therapy; (e) clinical evidence suggestive of active interstitial lung disease; (f) baseline computed tomography (CT) scanning suggested idiopathic pulmonary fibrosis; (g) uncontrollable, high‐volume pleural effusion or pericardial effusion; (h) in the investigator's opinion, evidence suggesting severe or uncontrollable systemic disease (such as unstable or noncompensatable respiratory, cardiac, hepatic, or renal disease); (i) diagnosed with another malignant tumor within the last 5 years (except for completely cured cervical carcinoma in situ or basal cell carcinoma/squamous cell carcinoma of the skin); (j) clear history of psychologic or mental disorders, including epilepsy and dementia; (k) history of allograft transplantation; (l) major surgery or severe trauma within the 3 weeks before the first day of drug therapy; (m) severe allergy to any of the study drugs or vehicles; (n) also treated with phenytoin sodium, carbamazepine, rifampicin, barbital, or St John's Wort; (o) treated with an unapproved drug or another trial drug within 30 days before the first day of drug therapy; (p) pregnant or breastfeeding; (q) history of drug abuse or with medical, psychologic, or social conditions that might influence participation or outcome assessment; and (r) any condition that could endanger the safety of the patient or the adherence of the patient to the study protocol. Patients were followed up until death or patient withdrawal or May 14, 2019 (whichever occurred first). Tumor response was assessed using enhanced CT or magnetic resonance imaging at baseline, 4 weeks after the initiation of apatinib therapy and then every 6–8 weeks. The tumor response was defined as complete response (CR), PR, SD, or progressive disease (PD) based on RECIST version 1.1 19. In addition, patients were followed up every month in the clinic or by telephone for the assessment of survival status, physical status (clinical examination), ECOG performance status, and use of any further anticancer treatment. The primary endpoint was OPR (defined as CR rate + RR rate). The secondary endpoints included DCR (defined as the percentage of patients with a complete response, partial response, or stable disease), PFS (defined as the time between enrollment of the patient and any recorded tumor progression or death from any cause), and OS (defined as the time between enrollment of the patient and death from any cause). Safety was assessed based on the occurrence of any AEs, which were classified and graded according to Common Terminology Criteria for Adverse Events version 4.0 20. | |
| Investigator's Analysis | Active and should be pursued further |
Drug Information
| Drug 1 | |
| Generic/Working Name | New drug |
| Trade Name | Apatinib |
| Company Name | Hengrui company |
| Drug Type | Small molecule |
| Drug Class | VEGF |
| Dose | 500 mg per flat dose |
| Route | p.o. |
| Schedule of Administration | Daily |
Patient Characteristics
| Number of Patients, Male | 28 |
| Number of Patients, Female | 4 |
| Stage | Histologically confirmed stage III/IV SCC/adenocarcinoma of the esophagus or SCC/adenocarcinoma of the gastroesophageal junction accompanied by high expression of VEGFR2 |
| Age | Median (range): 60 (23–72) years |
Primary Assessment Method
| Title | New assessment |
| Number of Patients Screened | 32 |
| Number of Patients Enrolled | 30 |
| Number of Patients Evaluable for Toxicity | 30 |
| Number of Patients Evaluated for Efficacy | 26 |
| Evaluation Method | RECIST 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 2 (7.7%) |
| Response Assessment SD | n = 14 (53.8%) |
| Response Assessment PD | n = 8 (30.8%) |
| Response Assessment OTHER | n = 2 (7.7%) |
| (Median) Duration Assessments PFS | 4.63 months |
| (Median) Duration Assessments OS | 6.57 months |
| (Median) Duration Assessments Response Duration | 4.76 months |
| (Median) Duration Assessments Duration of Treatment | 12 months |
| Outcome Notes | |
| Among 32 patients screened for inclusion, 30 were included in the safety and survival analyses (i.e., received apatinib), and 26 were included in the efficacy analysis (at least one imaging follow‐up). Median follow‐up time and exposure to apatinib were 5.34 months and 72 days, respectively. Among 26 patients included in the efficacy analysis, 2 showed partial remission (7.7%) and 14 had stable disease (53.8%). The overall response rate was 7.7%, and the disease control rate was 61.5%. Median PFS and OS were 4.63 months (95% confidence interval, 2.11–7.16 months) and 6.57 months (4.90 months to not estimable), respectively. Fifteen patients (50.0%) experienced treatment‐related AEs, most commonly hypertension (26.7%), diarrhea (20.0%) and hand‐foot‐skin reaction (10.0%). No patients had grade ≥4 treatment‐related AEs. | |
Adverse Events
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
| Proteinuria | 97% | 3% | 0% | 0% | 0% | 0% | 3% |
| Fatigue | 93% | 7% | 0% | 0% | 0% | 0% | 7% |
| Hypertension | 74% | 20% | 3% | 3% | 0% | 0% | 26% |
| Mucositis oral | 97% | 3% | 0% | 0% | 0% | 0% | 3% |
| Nausea | 93% | 7% | 0% | 0% | 0% | 0% | 7% |
| Anemia | 93% | 7% | 0% | 0% | 0% | 0% | 7% |
| Noncardiac chest pain | 97% | 3% | 0% | 0% | 0% | 0% | 3% |
| Back pain | 93% | 7% | 0% | 0% | 0% | 0% | 7% |
| Diarrhea | 80% | 13% | 7% | 0% | 0% | 0% | 20% |
| Skin and subcutaneous tissue disorders—hand‐foot‐skin reaction | 90% | 7% | 3% | 0% | 0% | 0% | 10% |
| Investigations—esophagostoma | 97% | 0% | 3% | 0% | 0% | 0% | 3% |
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Active and should be pursued further |
Esophageal cancer is the ninth most common cancer worldwide and one of the leading causes of cancer‐related mortality 1, 2. China has a higher incidence of esophageal cancer than Western countries, and more than 50% of all new cases are diagnosed in China 3. Esophageal squamous cell carcinoma (ESCC) accounts for most cases 2, 4. Various risk factors for ESCC have been proposed, including advanced age, smoking, alcohol consumption, and caustic injury to the esophagus 5, 6. At the time of diagnosis, approximately half of patients with esophageal cancer present with metastatic disease, which is associated with a poor prognosis. Indeed, the 5‐year overall survival (OS) is 15%–25% in all patients with esophageal cancer and less than 4% in those with metastatic disease 1, 2, 4, 5.
Radiotherapy and chemotherapy are the main treatment methods for patients with advanced esophageal cancer or postoperative recurrence and metastasis. Concurrent chemoradiation therapy (CCRT) has been shown to prolong survival and reduce disease persistence and recurrence 7, 8, but the long‐term result remains unsatisfactory 9. Thus, there has been great interest in the development of novel agents with limited toxicity that target the mechanisms of tumor growth and metastasis. Several targeted therapies are now available that inhibit molecules that contribute to carcinogenesis, such as human epidermal growth receptor 2, vascular endothelial growth factor receptor‐2 (VEGFR2), programmed cell death‐1 receptor, and endothelial growth factor receptor 10.
The VEGFR2 is commonly expressed in esophageal cancer tissue and is thought to contribute to tumor angiogenesis and dissemination 11. Apatinib is a novel inhibitor of angiogenesis that targets the intracellular adenosine triphosphate–binding site of VEGFR2 12. Apatinib has shown positive results as a second‐ or further‐line treatment in patients with ESCC 13, 14, 15, 16. The overexpression of VEGF in esophageal cancer may be associated with a poor prognosis 17, 18. However, no studies published to date have examined whether tumor expression of VEGFR2 affects the clinical response of esophageal cancer to apatinib.
The aims of this study were to assess the efficacy and safety of apatinib as a single agent in patients with metastatic, unresectable cancer of the esophagus or esophagogastric junction for whom standard second‐line therapy had failed or was not suitable and to examine whether the clinical response to apatinib was related to VEGFR2 expression.
This prospective, single‐arm, open‐label, investigator‐initiated phase II study included patients with advanced cancer of the esophagus or esophagogastric junction admitted to Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) between August 2017 and January 2019.
The study was approved by the institutional ethics committee of Tianjin Medical University Cancer Institute and Hospital (E2017107). The trial was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients before the initiation of any study related procedure. This study is registered at ClinicalTrials.gov (NCT03285906).
The objective of the study was to evaluate whether apatinib monotherapy might be an effective and safe second‐ or further‐line treatment in patients with metastatic, unresectable cancer of the esophagus or esophagogastric junction. The main findings were that 7.7% of patients showed partial response (PR) and 53.8% had stable disease (SD) after apatinib monotherapy, with an overall response rate (ORR) of 7.7% and a disease control rate (DCR) of 61.5%. Median progression‐free survival (PFS) and overall survival (OS) were 4.63 months and 6.57 months, respectively. Although half the patients experienced treatment‐related adverse events (AEs), all were grade ≤3. Taken together, our findings suggest that apatinib may be effective and safe as a second‐ or further‐line treatment for advanced esophageal cancer.
Apatinib is an anticancer agent with oral bioavailability that selectively targets VEGFR2 to exert antiangiogenic and antiproliferative effects 12, 21. Apatinib has been demonstrated to have promising activity against gastric, breast, and lung tumors 12. Moreover, a retrospective analysis concluded that apatinib (500 mg daily) combined with docetaxel may be effective as a second‐line treatment for advanced esophageal cancer, with a median PFS of nearly 6 months, an ORR of 88.9%, and a DCR of 93.3% 14. However, very few previous studies have explored the use of apatinib monotherapy in the setting of advanced cancer of the esophagus or gastroesophageal junction. In a multicenter, phase III trial of patients in China with advanced gastric or gastroesophageal junction adenocarcinoma, administration of apatinib monotherapy (850 mg daily) was associated with significant improvements in OS (6.5 vs. 4.7 months) and PFS (2.6 vs. 1.8 months) when compared with placebo 13. In another study, the use of apatinib monotherapy (500 mg daily) as a second‐ or further‐line treatment for advanced ESCC resulted in a PR rate of 24.2%, SD rate of 50.0%, progressive disease (PD) rate of 25.8%, ORR of 24.2%, DCR of 74.2%, median PFS of 115 days, and median OS of 209 days 16. The current phase II trial, which also used a daily apatinib dose of 500 mg, reported a PR rate of 7.7%, SD rate of 53.8%, PD rate of 30.8%, clinical progression rate of 7.7%, ORR of 7.7%, DCR of 61.5%, median PFS of 4.63 months, and median OS of 6.57 months. Thus, the findings of our study are broadly consistent with those of previous clinical investigations, indicating that apatinib monotherapy (at a daily dose of 500 mg) may be an effective second‐ or further‐line treatment for advanced esophageal cancer.
In the present study, treatment‐related AEs were observed in 50.0% of patients, and these were most commonly hypertension (26.7%), diarrhea (20.0%), and hand‐foot‐skin reaction (10.0%). The majority of AEs were grade 1 in severity; only one patient experienced a grade 3 AE (hypertension), and no patients had grade ≥4 treatment‐related AEs. A previous study of apatinib monotherapy for advanced ESCC described hand‐foot‐skin reaction (51.6%), proteinuria (24.2%), and hypertension (21.0%) as the most common AEs, with acceptable grade 3/4 toxicities in 59.7% of patients 16. Another study of apatinib monotherapy also described proteinuria (47.7%), hypertension (35.2%) and hand‐foot‐skin reaction (27.8%) as the most common nonhematologic AEs, and the toxicity profile was deemed to be acceptable 13. The AEs observed in this study are comparable to those observed in previous research, indicating that apatinib has an acceptable toxicity profile when used as a single agent for the management of advanced esophageal cancer. Nevertheless, two cases of death due to massive hemoptysis have been reported after therapy with apatinib, which was speculated to have resulted from bronchial artery erosion by tumor 22. This would suggest that apatinib should be used with caution in patients with large vessels or airways eroded by tumor 1.
Several clinical studies have investigated the use of antiangiogenic agents in advanced gastric/gastroesophageal junction cancer 9, 23, 24, 25. The expression of VEGFR2 in esophageal cancer tissue is thought to contribute to tumor angiogenesis and dissemination 11. Furthermore, VEGF overexpression in esophageal cancer appears to be associated with a poor prognosis 17, 18. Because apatinib selectively targets VEGFR2, the present study investigated whether tumor expression of VEGFR2 might be associated with a longer PFS or OS in patients treated with apatinib. Although we found no significant association of VEGFR2 expression with PFS or OS, there appeared to be a trend toward a longer PFS in patients with VEGFR2‐positive tumor (p = .097; Table 2). Because of the small sample size, it cannot be excluded that our study was underpowered to detect a real association between VEGFR2 expression and PFS, particularly as all the patients in our study had a poor clinical status due to advanced disease that had failed to respond to previous therapy. We also observed no significant association of Eastern Cooperative Oncology Group performance status score with PFS or OS, in contrast to a previous investigation 16.
Table 2.
Univariate analyses of PFS and OS based on different subgroups
| Subgroup | PFS, median (95% CI), months | p value | OS, median (95% CI), months | p value |
|---|---|---|---|---|
| Sex | .593 | .803 | ||
| Male | 3.32 (2.23–4.63) | 7.69 (4.63–NE) | ||
| Female | 2.50 (1.87–4.76) | 7.33 (3.09–15.38) | ||
| Age, years | .849 | .690 | ||
| <65 | 2.94 (2.20–3.91) | 7.69 (5.36–NE) | ||
| ≥65 | 3.22 (1.28–5.39) | 8.84 (2.50–NE) | ||
| ECOG PS | .633 | .180 | ||
| 1 | 3.53 (2.14–4.63) | 9.26 (6.31–15.38) | ||
| 2 | 2.79 (1.31–3.71) | 4.76 (2.50–NE) | ||
| Driver gene mutation | .556 | .135 | ||
| ≥1 mutation | 2.83 (2.20–4.75) | 5.36 (2.92–NE) | ||
| None/unknown | 3.14 (2.10–4.17) | 9.26 (6.31–NE) | ||
| Mutation status | .097 | .702 | ||
| Mutation | 3.91 (2.37–6.05) | 7.69 (2.92–NE) | ||
| Wild‐type | 2.83 (2.14–4.14) | 7.33 (5.36–15.38) |
Abbreviations: 95% CI, 95% confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; NE, not estimable; OS, overall survival; PFS, progression‐free survival.
This study has several limitations. First, this was a single‐center study, so the generalizability of the results is not known. Second, the sample size was small, so the analysis may have been underpowered to detect some real effects. Third, this was an open‐label study, which may have introduced bias into the estimates of treatment effect. Fourth, we did not include a comparator group (e.g., placebo). Large‐scale, multicenter, randomized controlled trials are needed to confirm and extend our findings.
In conclusion, the results of this prospective, single‐arm, open‐label, investigator‐initiated phase II study indicate that apatinib may be effective and safe as a second‐ or further‐line treatment for advanced esophageal cancer.
Disclosures
The authors indicated no financial relationships.
Tables and Figures
Table 1.
Demographic and clinical characteristics of the 30 study participants
| Characteristic | Value |
|---|---|
| Age, median (range), years | 60 (23–72) |
| Sex | |
| Male | 26 (87) |
| Female | 4 (13) |
| Tumor location | |
| Cervical | 1 (3) |
| Upper thoracic | 5 (17) |
| Mid‐thoracic | 15 (50) |
| Mid‐to‐lower thoracic | 2 (7) |
| Lower thoracic | 7 (23) |
| Tumor histology | |
| Squamous cell carcinoma | 2 (7) |
| Adenocarcinoma | 28 (93) |
| Tumor differentiation | |
| Well differentiated | 0 (0) |
| Moderately differentiated | 16 (53) |
| Poorly differentiated | 13 (43) |
| Not differentiated | 1 (3) |
| Disease status | |
| Initially metastatic | 30 (100) |
| Recurrence after potentially curative therapy | 17 (57) |
| Prior chemotherapy | 30 (100) |
| Yes | 29 (97) |
| No | 1 (3) |
Data are presented as n () unless otherwise stated.
Figure 3.
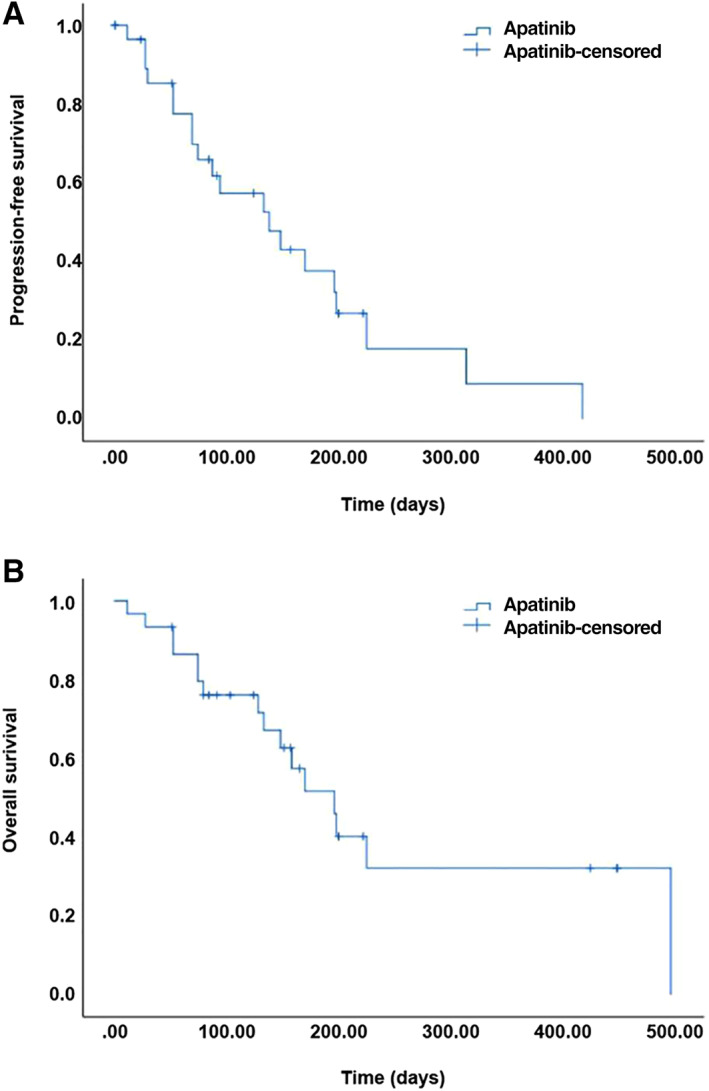
Survival analysis for patients with esophageal cancer treated with apatinib. (A): Kaplan‐Meier curve showing progression‐free survival. (B): Kaplan‐Meier curve showing overall survival.
Figure 4.
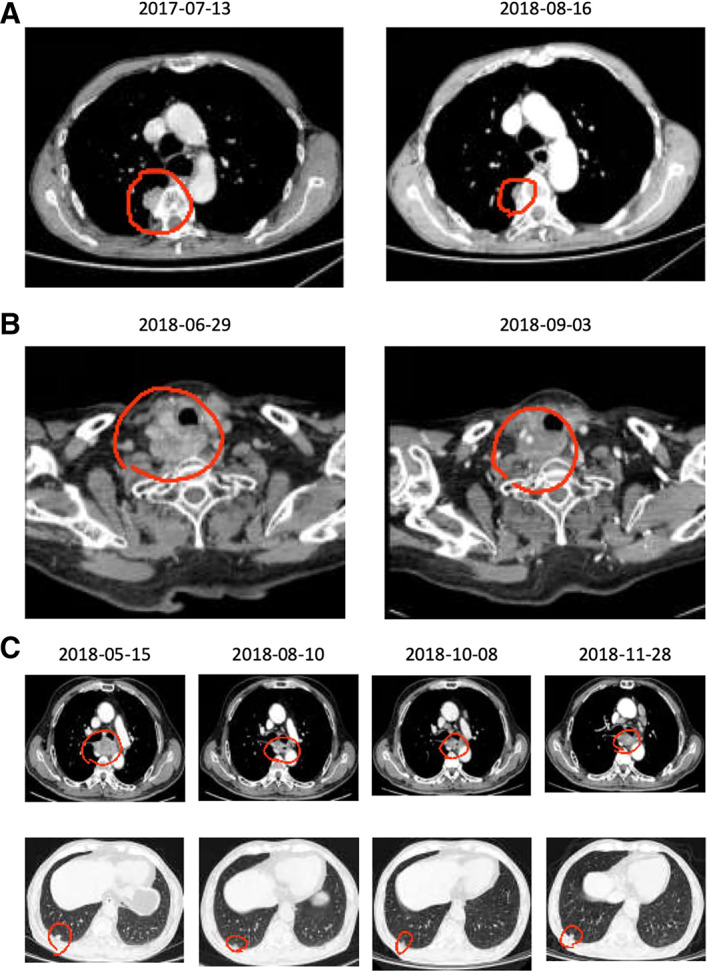
Representative imaging data showing the tumor response to therapy in three patients. (A): This 59‐year‐old male patient had previously received radical treatment for esophageal cancer, but a mass subsequently became evident under the right pulmonary pleura. Pathologic examination of a surgical specimen indicated squamous cell carcinoma, and metastasis was taken into consideration. The patient was enrolled into the study after failure of chemotherapy. The computed tomography (CT) images show a notable reduction in the size of the target lesion after treatment with apatinib. The progression‐free survival (PFS) of this patient was >1 year. (B): In this 62‐year‐old male patient, tracheal and esophageal fistulae had formed after radical treatment of esophageal cancer, and metastasis had occurred to the neck. The patient was unable to tolerate chemotherapy. After enrollment, gastrostomy was performed, and apatinib was administered via the gastrostomy tube. The CT images show a substantial reduction in the size of the target lesion after treatment with apatinib. The PFS of the patient was >1 year. (C): In this 64‐year‐old male patient, disease recurrence and lung metastasis had occurred after radical treatment of esophageal cancer. Chemotherapy had failed, and the patient was unable to eat. The administration of apatinib via a gastrostomy tube led to a notable decrease in the size of the target lesions. The PFS of this patient was >6 months.
Acknowledgments
This work was financially supported by the Chinese Society of Gynecological Oncology, Chinese Anti‐Cancer Association (CACA) Hengrui Cancer Research Fund (AHEAD‐HBE001), and the National Natural Science Foundation of China (81503622). The funders had no role in the study design, data acquisition, analysis or interpretation, decision to publish, or preparation of the manuscript.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT03285906
- Sponsors: Chinese Society of Gynecological Oncology and Chinese Anti‐Cancer Association (CACA) Hengrui Cancer Research Fund (AHEAD‐HBE001)
- Principal Investigators: Yu Zhen Tao, Zhanyu Pan
- IRB Approved: Yes
Contributor Information
Li Yanwei, Email: liyanwei127@hotmail.com.
Zhanyu Pan, Email: 32759625@qq.com.
Yu Zhen Tao, Email: panzhanyu@tjmuch.com.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Di Pardo BJ, Bronson NW, Diggs BS et al. The global burden of esophageal cancer: A disability‐adjusted life‐year approach. World J Surg 2016;40:395–401. [DOI] [PubMed] [Google Scholar]
- 3. Chen W. Cancer statistics: Updated cancer burden in China. Chin J Cancer Res 2015;27:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pennathur A, Gibson MK, Jobe BA et al. Oesophageal carcinoma. Lancet 2013;381:400–412. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mao WM, Zheng WH, Ling ZQ. Epidemiologic risk factors for esophageal cancer development. Asian Pac J Cancer Prev 2011;12:2461–2466. [PubMed] [Google Scholar]
- 7. Cooper JS, Guo MD, Herskovic A et al. Chemoradiotherapy of locally advanced esophageal cancer: Long‐term follow‐up of a prospective randomized trial (RTOG 85‐01). Radiation Therapy Oncology Group. JAMA 1999;281:1623–1627. [DOI] [PubMed] [Google Scholar]
- 8. Zhu LL, Yuan L, Wang H et al. A meta‐analysis of concurrent chemoradiotherapy for advanced esophageal cancer. PLoS One 2015;10:e0128616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Catenacci DVT, Tebbutt NC, Davidenko I et al. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first‐line therapy in advanced met‐positive gastric or gastro‐oesophageal junction cancer (RILOMET‐1): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2017;18:1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belkhiri A, El‐Rifai W. Advances in targeted therapies and new promising targets in esophageal cancer. Oncotarget 2015;6:1348–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gockel I, Moehler M, Frerichs K et al. Co‐expression of receptor tyrosine kinases in esophageal adenocarcinoma and squamous cell cancer. Oncol Rep 2008;20:845–850. [PubMed] [Google Scholar]
- 12. Zhao D, Hou H, Zhang X. Progress in the treatment of solid tumors with apatinib: A systematic review. Onco Targets Ther 2018;11:4137–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li J, Qin S, Xu J et al. Randomized, double‐blind, placebo‐controlled phase III trial of apatinib in patients with chemotherapy‐refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–1454. [DOI] [PubMed] [Google Scholar]
- 14. Li J, Jia Y, Gao Y et al. Clinical efficacy and survival analysis of apatinib combined with docetaxel in advanced esophageal cancer. Onco Targets Ther 2019;12:2577–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang LJ, Wen YX, Xia YY et al. Apatinib combined with docetaxel as a salvage treatment for metastatic esophageal squamous cancer: A case report. Onco Targets Ther 2018;11:5821–5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J, Wang L. Efficacy and safety of apatinib treatment for advanced esophageal squamous cell carcinoma. Onco Targets Ther 2017;10:3965–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kashyap MK, Abdel‐Rahman O. Expression, regulation and targeting of receptor tyrosine kinases in esophageal squamous cell carcinoma. Mol Cancer 2018;17:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang C, Wang J, Chen Z et al. Immunohistochemical prognostic markers of esophageal squamous cell carcinoma: A systematic review. Chin J Cancer 2017;36:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 20.Common terminology criteria for adverse events v4.0 (CTCAE). Available at https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Conversion_Amendment_Request.pdf. Accessed May 14, 2020.
- 21. Roviello G, Ravelli A, Polom K et al. Apatinib: A novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett 2016;372:187–191. [DOI] [PubMed] [Google Scholar]
- 22. Wang W, Zhang L, Xie Y et al. Fatal hemoptysis in patients with advanced esophageal cancer treated with apatinib. Onco Targets Ther 2018;11:2565–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Cutsem E, de Haas S, Kang YK et al. Bevacizumab in combination with chemotherapy as first‐line therapy in advanced gastric cancer: A biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012;30:2119–2127. [DOI] [PubMed] [Google Scholar]
- 24. Wilke H, Muro K, Van Cutsem E et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro‐oesophageal junction adenocarcinoma (RAINBOW): A double‐blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–1235. [DOI] [PubMed] [Google Scholar]
- 25. Fuchs CS, Tomasek J, Yong CJ et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro‐oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo‐controlled, phase 3 trial. Lancet 2014;383:31–39. [DOI] [PubMed] [Google Scholar]


