Abstract
Background
Survival after liver resection of hepatocellular carcinoma (HCC) remains poor because of a high incidence of recurrence. We sought to investigate risk factors, patterns, and long‐term prognosis among patients with early and late recurrence after liver resection for hepatitis B virus (HBV)–associated HCC.
Methods
Data of consecutive patients undergoing curative resection for HBV‐associated HCC were analyzed. According to the time to recurrence after surgery, recurrence was divided into early (≤2 years) and late recurrence (>2 years). Characteristics, patterns of initial recurrence, and postrecurrence survival (PRS) were compared between patients with early and late recurrence. Risk factors of early and late recurrence and predictors of PRS were identified by univariable and multivariable Cox regression analyses.
Results
Among 894 patients, 322 (36.0%) and 282 (31.5%) developed early and late recurrence, respectively. On multivariable analyses, preoperative HBV‐DNA >104 copies/mL was associated with both early and late recurrence, whereas postoperative no/irregular antiviral therapy was associated with late recurrence. Compared with patients with late recurrence, patients with early recurrence had a lower proportion of intrahepatic‐only recurrence (72.0% vs. 91.1%, p < .001), as well as a lower chance of receiving potentially curative treatments for recurrence (33.9% vs. 50.7%, p < .001) and a worse median PRS (19.1 vs. 37.5 months, p < .001). Multivariable analysis demonstrated that early recurrence was independently associated with worse PRS (hazard ratio, 1.361; 95% confidence interval, 1.094–1.692; p = .006).
Conclusion
Although risk factors associated with early recurrence and late recurrence were different, a high preoperative HBV‐DNA load was an independent hepatitis‐related risk for both early and late recurrence. Early recurrence was associated with worse postrecurrence survival among patients with recurrence.
Implications for Practice
Liver resection is the main curative treatment for hepatocellular carcinoma (HCC), but postoperative survival remains poor because of high recurrence rates. This study investigated the risk factors and patterns of early and late recurrence and found that a high preoperative hepatitis B virus (HBV) DNA load was an independent hepatitis‐related risk factor for both. Early recurrence was also independently associated with worse postrecurrence survival. These data may provide insights into different biological origin and behavior of early versus late recurrence after resection for HBV‐associated HCC, which could be helpful to make individualized treatment decision for recurrent HCC, as well as strategies for surveillance recurrence after resection.
Keywords: Hepatocellular carcinoma, Resection, Hepatitis B, Survival, Recurrence
Short abstract
Worldwide, hepatitis B virus (HBV) infection is the main etiology of hepatocellular carcinoma (HCC). This article defines risk factors and patterns of recurrence after curative resection of HBV‐associated HCC, aiming to provide evidence for appropriate selection of treatment options for recurrent HCC.
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent malignancies and a leading cause of cancer‐related death globally 1. Hepatitis B virus (HBV) infection is the main etiology of HCC worldwide, especially in sub‐Saharan Africa and East Asia 2. China alone accounts for more than half of patients with HCC worldwide. Although liver resection is the mainstay of curative therapy for HCC, the high incidence of recurrence remains the major obstacle to improving long‐term survival, with almost 70% of patients developing recurrence within 5 years of resection 3, 4. Thus, investigation of risk factors and patterns of recurrence after curative resection for HCC is important to better define treatment modalities and surveillance, as well as characterize long‐term prognosis among patients who develop recurrence.
Relative to the time from surgery to initial recurrence, HCC recurrence is typically divided into early and late recurrence, which has generally been defined using 2 years as the cutoff value 5, 6, 7, 8, 9, 10. Early recurrence most likely originates from occult micrometastasis derived from the initial tumor and is commonly associated with aggressive tumor characteristics, such as multinodularity, large tumor size, macrovascular and microvascular invasion, poor tumor differentiation, and satellite nodules 7, 8, 10, 11, 12, 13. In contrast, late recurrence has a clonal origin that is distinct from the original tumor, suggesting a de novo tumor in the remnant liver with different clonal origins. As such, late recurrence, though sometimes associated with aggressive initial HCC characteristics, is often characterized by several host and viral factors, such as sex, cirrhosis, hepatitis activity, and high viral loads 8, 9, 10, 14, 15, 16, 17. Until recently, there have been very few published studies on early and late recurrence of HBV‐associated HCC, and most had a limited sample size 8, 16, 17. For example, in one study of 193 patients with HBV‐associated HCC, 134 (69.4%) patients had HCC recurrence after resection 8. In this study, Wu et al. investigated independent risk factors of early and late recurrence for HBV‐associated HCC and concluded that tumor‐related factors were associated with early recurrence, whereas viral‐related factors were associated with late recurrence 8. Most past studies did not, however, elucidate details regarding the patterns of recurrence, treatment of recurrence, postrecurrence survival, or the relevant predictors associated with recurrence 8, 16, 17.
Therefore, the objective of the present study was to define risk factors and patterns of early and late recurrence after curative resection of HBV‐associated HCC using a large prospectively collected database. In addition, treatment and postrecurrence survival of patients with HCC recurrence were assessed with the aim of providing clinicians with more evidence on appropriate selection of treatment options for recurrent HCC, as well as to inform rational strategies of surveillance after liver resection for HBV‐associated HCC.
Materials, Subjects, and Methods
Study Population
Using a prospectively collected database, patients with chronic HBV infection who underwent open curative‐intent liver resection for an index HCC at the Eastern Hepatobiliary Surgery Hospital of Shanghai between January 2002 and June 2016 were identified. The diagnosis of HCC was confirmed by postoperative histopathological examination. Curative liver resection was defined as complete removal of all tumor with a microscopically clear margin (R0 resection). Chronic HBV infection was defined as positive serum HBV surface antigen (HBsAg). The exclusion criteria were patients who (a) had recurrent HCC; (b) had concurrent etiologies of liver disease other than HBV infection, such as hepatitis C infection or alcoholic hepatitis; (c) underwent palliative liver resection with microscopically or grossly positive margins (R1 or R2 resection); (d) received preoperative antitumor treatments; (e) had postoperative mortality within 30 days after surgery; (f) were lost to follow‐up within 2 years after surgery; and (g) had missing data on essential prognostic variables. The data were censored on December 31, 2018. Written informed consent was obtained from all enrolled patients for their data to be analyzed in clinical researches. This study was approved by the Institutional Review Board and Ethics Committee of the Eastern Hepatobiliary Surgery Hospital of Shanghai, China.
Clinicopathological and Operative Variables
Potential risk factors contributing to recurrence and survival after HCC resection were classified as factors associated with the host, hepatitis, initial tumor, and the operation. Host‐related factors included sex, age, American Society of Anesthesiologists score, obesity (body mass index >30), diabetes mellitus, cirrhosis, portal hypertension, and Child‐Pugh grading; hepatitis‐related factors included positivity of HBV envelope antigen (HBeAg), preoperative HBV‐DNA load, history of preoperative anti‐HBV therapy, raised preoperative alanine transaminase and aspartate aminotransferase (AST) levels, and postoperative anti‐HBV therapy and HBV reactivation until recurrence or the last follow‐up. Initial tumor‐related factors included raised preoperative serum alpha‐fetoprotein (AFP) level, largest tumor size, tumor number, macrovascular and microvascular invasion, satellite nodules, tumor differentiation, tumor encapsulation, and tumor stage according to the Barcelona Clinic Liver Cancer (BCLC) staging; operation‐related factors included intraoperative blood loss, intraoperative blood transfusion, extent of hepatectomy (minor or major), type of liver resection (anatomical or nonanatomical), and the width of resection margin. Portal hypertension was diagnosed by the presence of either esophageal varices or splenomegaly with a decreased platelet count (≤100 × 109/L). Minor hepatectomy was defined as resection of fewer than three Couinaud's segments, whereas major hepatectomy was defined as resection of three or more segments. Nonanatomical liver resection included a limited resection or wedge resection; anatomical resections were defined by the Brisbane 2000 system.
Postoperative Follow‐Up
After hospital discharge, patients were prospectively followed up with physical examination, serum AFP, ultrasonography or contrast‐enhanced computed tomography (CT) scan or magnetic resonance imaging (MRI) of the abdomen, and chest x‐ray once every 2 months for the first 6 months and then every 3 months for the next 1.5 years. For patients who were free of HCC recurrence 2 years after surgery, recurrence surveillance was performed at a 6‐month interval thereafter. Regular surveillance included routine abdominal imaging and/or AFP monitoring every 6 months or less before HCC recurrence was diagnosed; irregular surveillance was defined as long surveillance intervals of more than 6 months or diagnosis of HCC recurrence because of symptoms or other unrelated reasons. Oral antiviral therapy with 100 mg lamivudine, 10 mg adefovir dipivoxil, or 0.5 mg entecavir was administrated daily to patients with chronic HBV infection once the preoperative HBV‐DNA content was 1,000 copies/mL or greater.
Tumor recurrence was suspected based on appearance of new intra‐ or extrahepatic tumor lesion(s) that possessed typical imaging characteristics consistent with HCC on contrast‐enhanced CT or MRI, with or without an elevation of serum AFP level. Further examinations including positron emission tomography–CT, full‐body bone scan or angiography were carried out when there were clinical suspicions of HCC recurrence or distant metastases after surgery. The diagnosis of HCC recurrence was made based on the results of clinical investigations or was confirmed by histological biopsy of re‐resected tumor samples. Tumor recurrence was categorized into early and late recurrence using a cutoff value of 2 years. Patients who developed recurrence were treated with re‐resection if feasible, liver transplantation, local ablation, transcatheter arterial chemoembolization, radiotherapy, oral sorafenib, or supportive care, depending on the pattern of recurrence, liver functional reserve, and patient general conditions. Re‐resection, liver transplantation, and local ablation therapy were defined as potentially curative treatments, whereas other treatments were considered as noncurative treatments. To investigate the predictors of postrecurrence survival (PRS) among patients who developed recurrence, data on diagnosis of recurrence, interval to recurrence (early or late recurrence), positivity of HBeAg, HBV‐DNA load, cirrhosis, Child‐Pugh grading, AFP level, patterns of recurrence (sole intrahepatic, sole extrahepatic, or intrahepatic and extrahepatic), extent of recurrence (within or beyond Milan criteria), treatment modality for recurrence (potentially curative vs. noncurative), and postrecurrence anti‐HBV therapy and HBV reactivation were assessed. Overall survival (OS) was calculated from the date of initial liver resection to either the date of death or the date of the last follow‐up, whereas PRS was calculated from the date of the diagnosis of initial recurrence to the date of death or the date of the last follow‐up.
Statistical Analysis
Continuous variables were expressed as mean ± SD or median (range). Categorical variables were reported as number (n) or proportion (%). Student's t test was used for comparisons of continuous variables when applicable; otherwise, the Mann‐Whitney U test was applied. Categorical variables were compared with the χ2 test with the Yates correction or the Fisher's exact test, as appropriate. The Kaplan‐Meier method was used to compare the OS and PRS rates. Univariable and multivariable Cox proportional hazard regression analyses were performed to identify risk factors contributing to early and late recurrence, as well as to evaluate predictors associated with PRS among patients who developed recurrence. The statistical analyses were performed using the SPSS software version 25.0 (SPSS, Chicago, IL). A two‐tailed p value <.05 was considered statistically significant.
Results
HCC Recurrence During Follow‐Up
Overall 2,046 patients who underwent curative‐intent liver resection for HCC were screened for inclusion (supplemental online Fig. 1). The final analytic cohort included 894 patients with HBV‐associated HCC. With a median follow‐up period of 53.9 months, 604 (67.6%) of 894 patients developed recurrence. Among the 604 patients with recurrence, 322 (53.3%) and 282 (46.7%) had early and late recurrence, respectively. Among the remaining 290 patients who did not develop recurrence before their death or the last follow‐up, 68 experienced a non–cancer‐specific death (14 within 2 years of resection and 54 after 2 years of resection), and 222 were alive and recurrence‐free at the last follow‐up.
The estimated overall recurrence curve after HCC resection is depicted in Figure 1. Of note, there was an initial peak of recurrence that occurred at 1–2 years after surgery (approximately 23% per year). The incidence of recurrence gradually decreased until 2–3 years postoperative. Interestingly, the rate then increased again with a second peak at 4–5 years after surgery (approximately 35% per year).
Figure 1.
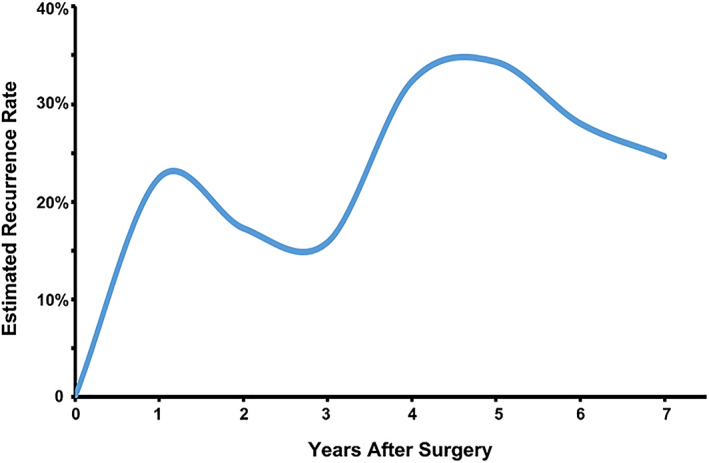
The estimated rate of recurrence (per year) over time after curative liver resection of hepatitis B virus–associated hepatocellular carcinoma.
Clinicopathological and Operative Variables
Comparisons of clinicopathological and operative variables among patients with early and late recurrence, as well as patients without recurrence, are demonstrated in Table 1. Of note, there were differences among the three groups related to host‐, hepatitis‐, and operation‐specific factors, as well as almost all tumor‐related factors. Compared with patients who had late recurrence, patients with early recurrence more often had Child‐Pugh grade B, a higher preoperative HBV‐DNA load, AST level and AFP level, larger tumor size, multiple tumors, microvascular invasion, satellite nodules, poor tumor differentiation, incomplete tumor encapsulation, more intraoperative blood loss, more blood transfusions, a higher proportion of major hepatectomy, and a smaller width of resection margin. Related to the initial tumor staging of HCC, the proportion of intermediate or advanced HCC (BCLC stage B or C) among patients with early recurrence was higher than among patients who had late recurrence (p < .001).
Table 1.
Comparisons of clinicopathological and operative variables among patients without recurrence, and with early and late recurrence
| Variables | Total (n = 894) | Without recurrence (n = 290) | Early recurrence (n = 322) | Late recurrence (n = 282) | p valuea | p valueb |
|---|---|---|---|---|---|---|
| Sex, male | 805 (90.0) | 259 (89.3) | 292 (90.7) | 254 (90.1) | .852 | .890 |
| Age >60 years | 156 (17.4) | 50 (17.2) | 59 (18.3) | 47 (16.7) | .861 | .668 |
| ASA score >2 | 100 (11.2) | 36 (12.4) | 32 (9.9) | 32 (11.3) | .621 | .598 |
| Obesity | 45 (5.0) | 6 (2.1) | 25 (7.8) | 14 (5.0) | .006 | .186 |
| Diabetes mellitus | 47 (5.3) | 11 (3.8) | 19 (5.9) | 17 (6.0) | .396 | .999 |
| Cirrhosis | 642 (71.3) | 200 (69.0) | 241 (74.8) | 201 (71.3) | .264 | .357 |
| Portal hypertension | 273 (30.5) | 94 (32.4) | 98 (30.4) | 81 (28.7) | .631 | .656 |
| Child‐Pugh grade B | 83 (9.3) | 25 (8.6) | 39 (12.1) | 19 (6.7) | .068 | .027 |
| HBeAg (+) | 194 (21.7) | 55 (19.0) | 76 (23.6) | 63 (22.3) | .362 | .771 |
| Preoperative HBV‐DNA load >104 copies/mL | 486 (54.4) | 129 (44.5) | 204 (63.4) | 153 (54.3) | <.001 | .025 |
| Preoperative anti‐HBV therapy | 189 (21.1) | 50 (17.2) | 74 (23.0) | 65 (23.0) | .216 | .999 |
| Preoperative ALT >80 U/L | 145 (16.2) | 42 (14.5) | 59 (18.3) | 44 (15.6) | .412 | .388 |
| Preoperative AST >80 U/L | 110 (12.3) | 27 (9.3) | 57 (17.7) | 26 (9.2) | .001 | .003 |
| Preoperative AFP level >400 μg/L | 330 (36.9) | 97 (33.4) | 147 (45.7) | 86 (30.5) | <.001 | <.001 |
| Tumor size >5.0 cm | 379 (42.4) | 88 (30.3) | 185 (57.5) | 106 (37.6) | <.001 | <.001 |
| Multiple tumors | 188 (21.0) | 42 (14.5) | 100 (31.1) | 46 (16.3) | <.001 | <.001 |
| Macrovascular invasion | 33 (3.7) | 1 (0.3) | 20 (6.2) | 12 (4.3) | .001 | .363 |
| Microvascular invasion | 426 (47.7) | 116 (40.0) | 185 (57.5) | 125 (44.3) | <.001 | .001 |
| Satellite nodules | 195 (21.8) | 38 (13.1) | 108 (33.5) | 49 (17.4) | <.001 | <.001 |
| Poor tumor differentiation | 714 (79.9) | 211 (72.8) | 280 (87.0) | 223 (79.1) | <.001 | .012 |
| Incomplete tumor encapsulation | 524 (58.6) | 135 (46.6) | 246 (76.4) | 143 (50.7) | <.001 | <.001 |
| BCLC tumor stage of initial tumor | ||||||
| BCLC stage A | 329 (36.8) | 138 (47.6) | 66 (20.5) | 125 (44.3) | <.001 | <.001 |
| BCLC stage B | 239 (26.7) | 66 (22.8) | 105 (32.6) | 68 (24.1) | ||
| BCLC stage C | 326 (36.5) | 86 (29.7) | 151 (46.9) | 89 (31.6) | ||
| Intraoperative blood loss >400 mL | 352 (39.4) | 99 (34.1) | 161 (50.0) | 92 (32.6) | <.001 | <.001 |
| Intraoperative blood transfusion | 171 (19.1) | 38 (13.1) | 84 (26.1) | 49 (17.4) | <.001 | .011 |
| Major hepatectomy | 192 (21.5) | 38 (13.1) | 105 (32.6) | 49 (17.40 | <.001 | <.001 |
| Anatomical liver resection | 630 (70.5) | 198 (68.3) | 236 (73.3) | 196 (69.5) | .362 | .321 |
| Resection margin <1 cm | 300 (33.6) | 50 (17.2) | 159 (49.4) | 91 (32.3) | <.001 | <.001 |
Comparison among patients without recurrence, with early recurrence, and with late recurrence.
Comparison between patients with early and late recurrence.
Abbreviations: AFP, alpha‐fetoprotein; ALT, alanine aminotransferase; ASA, American Society of Anesthesiologists; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; HBeAg, hepatitis B virus envelope antigen.
Long‐Term Outcomes and Treatment Modality for Recurrence
Comparison of long‐term outcomes, treatment modality for recurrence, and other relevant prognostic variables during follow‐up among patients with early versus late recurrence are noted in Table 2. With regard to hepatitis‐related factors, although there was no difference in the proportion of patients who received postoperative regular anti‐HBV therapy until recurrence between the two groups (83.2% vs. 79.4%, p = .249), the incidence of postoperative HBV reactivation among patients with early recurrence was lower than among patients who had late recurrence (7.8% vs. 16.0%, p < .001). Among 282 patients with late recurrence, there were 257 (91.1%) patients who developed intrahepatic‐only recurrence, which was higher than the incidence among patients with early recurrence (72.0%, 232/322, p < .001). Moreover, the incidence of initial recurrence within Milan criteria among patients with late recurrence was also higher than that noted in patients with early recurrence (51.1% vs. 41.1%, p = .014). Similar results were noted among patients undergoing potentially curative treatment for initial recurrence (patients with late recurrence: 50.7% vs. patients with early recurrence: 33.9%, p < .001). In addition, the proportion of patients with overall mortality and cancer‐specific mortality among patients with early recurrence was higher than among patients with late recurrence (81.7% and 69.9% vs. 59.2% and 48.6%, both p < .001).
Table 2.
Comparisons of long‐term outcomes and treatment modality for recurrence among patients with early and late recurrence
| Outcomes and treatment modality | Overall recurrence (n = 604), n (%) | Early recurrence (n = 322), n (%) | Late recurrence (n = 282), n (%) | p value |
|---|---|---|---|---|
| Period of follow‐up after surgery, median (range), months | 53.9 (2.3–192.0) | 30.5 (2.0–156.5) | 88.9 (28.6–192.0) | <.001 |
| Postoperative anti‐HBV therapy until recurrence | ||||
| Regular | 492 (81.5) | 268 (83.2) | 224 (79.4) | .249 |
| Irregular | 112 (18.5) | 54 (16.8) | 58 (20.6) | |
| Postoperative HBV reactivation until recurrence | 70 (11.6) | 25 (7.8) | 45 (16.0) | .002 |
| Postoperative recurrence surveillance | ||||
| Regular | 403 (66.7) | 268 (83.2) | 135 (47.9) | <.001 |
| Irregular | 201 (33.3) | 54 (16.8) | 147 (52.1) | |
| Patterns of initial recurrence | ||||
| Intrahepatic only | 489 (81.0) | 232 (72.0) | 257 (91.1) | <.001 |
| Extrahepatic only | 28 (4.6) | 28 (8.7) | 0 (0) | |
| Intrahepatic and extrahepatic | 87 (14.4) | 62 (19.3) | 25 (8.9) | |
| Extent of initial recurrence | ||||
| Within Milan criteria | 276 (45.7) | 132 (41.0) | 144 (51.1) | .014 |
| Beyond Milan criteria | 328 (54.3) | 190 (59.0) | 138 (48.9) | |
| Treatment modality for initial recurrence | ||||
| Potentially curative treatment | 248 (41.1) | 109 (33.9) | 143 (50.7) | <.001 |
| Noncurative treatment | 356 (58.9) | 213 (66.1) | 139 (49.3) | |
| Mortality during the follow‐up | 430 (71.2) | 263 (81.7) | 167 (59.2) | <.001 |
| Cancer‐specific mortality | 362 (59.9) | 225 (69.9) | 137 (48.6) | <.001 |
| Non–cancer‐specific mortality | 68 (12.5) | 38 (11.8) | 30 (13.5) | .598 |
| Median OS from surgery (95% CI), months | 63.1 (57.4–68.8) | 30.6 (27.2–33.9) | 106.8 (98.3–115.4) | <.001 |
| 1‐year OS rate, % | 92.9 | 86.6 | 100.0 | |
| 3‐year OS rate, % | 68.6 | 64.5 | 97.2 | |
| 5‐year OS rate, % | 51.2 | 43.5 | 81.0 | |
| Median PRS from recurrence diagnosis (95% CI), months | 26.7 (23.2–30.1) | 19.1 (15.3–22.8) | 37.5 (32.2–42.8) | <.001 |
| 1‐year PRS rate, % | 72.5 | 67.0 | 79.1 | |
| 3‐year PRS rate, % | 41.4 | 33.2 | 51.8 | |
| 5‐year PRS rate, % | 27.6 | 20.1 | 37.4 |
Abbreviations: CI, confidence interval; HBV, hepatitis B virus; OS, overall survival; PRS, postrecurrence survival.
OS for patients without recurrence, as well as for patients with early and late recurrence, are demonstrated in Figure 2A. As expected, among the three groups, OS was highest in patients without recurrence, followed by patients with late recurrence; the worst OS was noted among patients with early recurrence (all p < .001). As noted in Table 2, the 1‐, 3‐ and 5‐year OS for patients with early recurrence was 86.6%, 64.5%, and 43.5%, which was lower than among patients with late recurrence (100%, 97.2%, and 81.0%, p < .001).
Figure 2.
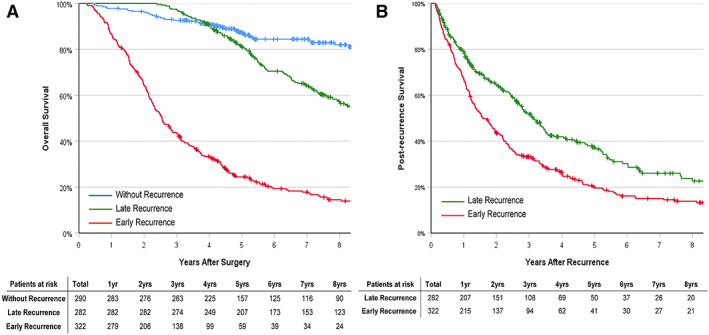
Kaplan‐Meier analysis of overall survival and postrecurrence survival. (A): Kaplan‐Meier analysis of overall survival in patients without recurrence, patients with early recurrence, and patients with late recurrence after curative liver resection of hepatitis B virus–associated hepatocellular carcinoma: p < .001 (without recurrence vs. early recurrence), p < .001 (without recurrence vs. late recurrence), and p < .001 (early recurrence vs. late recurrence) (log‐rank test). (B): Kaplan‐Meier analysis of postrecurrence survival in patients with early and late recurrence after curative liver resection of hepatitis B virus–associated hepatocellular carcinoma: p < .001 (log‐rank test).
Comparison of PRS among patients with early and late recurrence are noted in Table 2 and Figure 2B. The median PRS of patients with early recurrence was worse than patients with late recurrence (19.1 vs. 37.5 months, p < .001). Meanwhile, 1‐, 3‐, and 5‐year PRS among patients with early recurrence was 67.0%, 33.2%, and 20.1%, which was lower than the corresponding PRS in patients with late recurrence (79.1%, 51.8%, and 37.4%, p < .001).
Risk Factors of Early and Late Recurrence
Table 3 and Table 4 summarize the results of univariable and multivariable Cox regression analyses predicting early and late recurrence after curative liver resection for HBV‐associated HCC, respectively. On multivariable Cox regression analyses, independent risk factors associated with early recurrence included preoperative HBV‐DNA load >104 copies/mL, preoperative AFP level >400 μg/L, tumor size >5 cm, multiple tumors, macrovascular and microvascular invasion, satellite nodules, incomplete tumor encapsulation, and resection margin <1 cm. Independent risk factors associated with late recurrence included male sex, cirrhosis, preoperative HBV‐DNA load >104 copies/mL, tumor size >5 cm, macrovascular and microvascular invasion, satellite nodules, and irregular postoperative anti‐HBV therapy.
Table 3.
Univariate and multivariate Cox regression analyses predicting early recurrence in 894 patients who underwent liver resection of hepatitis B virus–associated hepatocellular carcinoma
| Variables | HR comparison | UV HR (95% CI) | UV p value | MV HR (95% CI) | MV p valuea |
|---|---|---|---|---|---|
| Age | >60 vs. ≤60 years | 1.055 (0.796–1.400) | .708 | ||
| Sex | Male vs. female | 1.100 (0.755–1.602) | .619 | ||
| ASA score | >2 vs. ≤2 | 0.806 (0.560–1.162) | .248 | ||
| Obesity | Yes vs. no | 1.824 (1.213–2.744) | .004 | NS | NS |
| Diabetes mellitus | Yes vs. no | 1.194 (0.751–1.899) | .453 | ||
| Cirrhosis | Yes vs. no | 1.207 (0.938–1.552) | .143 | ||
| Portal hypertension | Yes vs. no | 0.983 (0.775–1.246) | .885 | ||
| Child‐Pugh grade | B vs. A | 1.397 (0.999–1.952) | .051 | NS | NS |
| Preoperative ALT | >80 vs. ≤80 U/L | 1.197 (0.903–1.588) | .212 | ||
| Preoperative AST | >80 vs. ≤80 U/L | 1.804 (1.355–2.402) | <.001 | NS | NS |
| Preoperative HBV‐DNA load | >104 vs. ≤104 copies/mL | 1.549 (1.235–1.944) | <.001 | 1.557 (1.239–1.956) | <.001 |
| HBeAg (+) | Yes vs. no | 1.171 (0.905–1.515) | .229 | ||
| Preoperative anti‐HBV therapy | No vs. yes | 1.278 (0.714–1.987) | .578 | ||
| Preoperative AFP level | >400 vs. ≤400 μg/L | 1.664 (1.336–2.073) | <.001 | 1.419 (1.133–1.777) | .002 |
| Tumor size | >5.0 cm vs. ≤5.0 cm | 2.188 (1.754–2.730) | <.001 | 1.512 (1.196–1.910) | .001 |
| Multiple tumors | Yes vs. no | 2.012 (1.589–2.549) | <.001 | 1.323 (1.024–1.709) | .032 |
| Macrovascular invasion | Yes vs. no | 2.253 (1.433–3.543) | <.001 | 1.904 (1.522–2.381) | <.001 |
| Microvascular invasion | Yes vs. no | 1.645 (1.319–2.051) | <.001 | 1.394 (1.113–1.745) | .004 |
| Satellite nodules | Yes vs. no | 2.283 (1.810–2.878) | <.001 | 1.736 (1.368–2.204) | <.001 |
| Poor tumor differentiation | Yes vs. no | 1.897 (1.372–2.624) | <.001 | NS | NS |
| Incomplete tumor encapsulation | Yes vs. no | 2.716 (2.099–3.513) | <.001 | 2.057 (1.579–2.679) | <.001 |
| Resection margin | <1 cm vs. ≥1 cm | 2.383 (1.915–2.966) | <.001 | 1.942 (1.553–2.430) | <.001 |
| Intraoperative blood loss | >400 vs. ≤400 mL | 1.730 (1.391–2.153) | <.001 | NS | NS |
| Intraoperative blood transfusion | Yes vs. no | 1.695 (1.322–2.174) | <.001 | NS | NS |
| Extent of hepatectomy | Major vs. minor | 2.214 (1.753–2.796) | <.001 | NS | NS |
| Type of resection | Anatomical vs. nonanatomical | 0.870 (0.680–1.114) | .269 | ||
| Postoperative anti‐HBV therapy until recurrence or last follow‐up | No/irregular vs. regular | 0.593 (0.221–1.592) | .299 | ||
| Postoperative HBV reactivation until recurrence or last follow‐up | Yes vs. no | 0.918 (0.707–1.191) | .520 |
Those variables found significant at p < .1 in univariable analyses were entered into multivariable Cox regression analyses.
Abbreviations: AFP, alpha‐fetoprotein; ALT, alanine aminotransferase; ASA, American Society of Anesthesiologists; AST, aspartate aminotransferase; CI, confidence interval; HBeAg, hepatitis B virus envelope antigen; HBV, hepatitis B virus; HR, hazard ratio; MV, multivariate; NS, not significant; UV, univariate.
Table 4.
Univariate and multivariate Cox regression analyses predicting late recurrence in 558 patients who were free of early recurrence at 2 years after liver resection of hepatitis B virus–associated hepatocellular carcinoma
| Variables | HR comparison | UV HR (95% CI) | UV p value | MV HR (95% CI) | MV p valuea |
|---|---|---|---|---|---|
| Age | >60 vs. ≤60 years | 1.036 (0.757–1.417) | .826 | ||
| Sex | Male vs. female | 1.309 (1.056–1.998) | .040 | 1.750 (1.017–3.011) | .043 |
| ASA score | >2 vs. ≤2 | 0.967 (0.669–1.398) | .860 | ||
| Obesity | Yes vs. no | 1.227 (0.969–1.554) | .090 | NS | NS |
| Diabetes mellitus | Yes vs. no | 1.447 (0.886–2.363) | .140 | ||
| Cirrhosis | Yes vs. no | 2.537 (1.252–5.142) | .010 | 1.302 (1.022–1.658) | .032 |
| Portal hypertension | Yes vs. no | 0.914 (0.706–1.184) | .497 | ||
| Child‐Pugh grade | B vs. A | 1.103 (0.852–1.428) | .456 | ||
| Preoperative ALT | >80 vs. ≤80 U/L | 0.953 (0.691–1.315) | .769 | ||
| Preoperative AST | >80 vs. ≤80 U/L | 0.946 (0.632–1.417) | .789 | ||
| Preoperative HBV‐DNA load | >104 vs. ≤104 copies/mL | 1.345 (1.064–1.701) | .013 | 1.463 (1.095–1.955) | .010 |
| HBeAg (+) | Yes vs. no | 1.143 (0.863–1.514) | .350 | ||
| Preoperative anti‐HBV therapy | No vs. yes | 1.262 (0.941–1.691) | .120 | ||
| Preoperative AFP level | >400 vs. ≤400 μg/L | 0.937 (0.727–1.208) | .616 | ||
| Tumor size | >5.0 cm vs. ≤5.0 cm | 1.288 (1.012–1.640) | .039 | 1.295 (1.017–1.650) | .036 |
| Multiple tumors | Yes vs. no | 1.365 (0.995–1.874) | .054 | NS | NS |
| Macrovascular invasion | Yes vs. no | 4.504 (2.516–8.065) | <.001 | 5.480 (3.012–9.973) | <.001 |
| Microvascular invasion | Yes vs. no | 2.113 (1.643–2.718) | <.001 | 1.554 (1.135–2.129) | .006 |
| Satellite nodules | Yes vs. no | 1.499 (1.101–2.042) | .010 | 1.347 (1.007–1.800) | .044 |
| Poor tumor differentiation | Yes vs. no | 1.341 (1.006–1.787) | .045 | NS | NS |
| Incomplete tumor encapsulation | Yes vs. no | 1.113 (0.881–1.407) | .368 | ||
| Resection margin | <1 cm vs. ≥1 cm | 1.191 (0.940–1.508) | .147 | ||
| Intraoperative blood loss | >400 vs. ≤400 mL | 1.002 (0.781–1.286) | .987 | ||
| Intraoperative blood transfusion | Yes vs. no | 1.257 (0.924–1.712) | .146 | ||
| Extent of hepatectomy | Major vs. minor | 1.292 (0.949–1.759) | .104 | ||
| Type of resection | Anatomical vs. nonanatomical | 0.957 (0.743–1.234) | .735 | ||
| Postoperative anti‐HBV therapy until recurrence or last follow‐up | No/irregular vs. regular | 1.872 (1.092–3.207) | .023 | 1.704 (1.036–2.802) | .036 |
| Postoperative HBV reactivation until recurrence or last follow‐up | Yes vs. no | 1.296 (0.909–1.847) | .152 |
Those variables found significant at p < .1 in univariable analyses were entered into multivariable Cox regression analyses.
Abbreviations: AFP, alpha‐fetoprotein; ALT, alanine aminotransferase; ASA, American Society of Anesthesiologists; AST, aspartate aminotransferase; CI, confidence interval; HBeAg, hepatitis B virus envelope antigen; HBV, hepatitis B virus; HR, hazard ratio; MV, multivariate; NS, not significant; UV, univariate.
Predictors of PRS in Patients with Recurrence
Univariable and multivariable Cox regression analyses were also performed to identify predictors associated with PRS in 604 patients who developed recurrence after curative liver resection for HBV‐associated HCC. As noted in Table 5, independent risk factors predicting worse PRS included early recurrence (≤2 years after surgery; hazard ratio [HR], 1.361; 95% confidence interval [CI], 1.094–1.692; p = .006), postoperative irregular recurrence surveillance (HR, 1.293; 95% CI, 1.059–1.578; p = .012), presence of portal hypertension at diagnosis of recurrence (HR, 1.248; 95% CI, 1.028–1.516; p = .025), AFP level >400 μg/L at diagnosis of recurrence (HR, 1.325; 95% CI, 1.086–1.615; p = .006), recurrent HCC beyond Milan criteria (HR, 1.359; 95% CI, 1.115–1.654; p = .002), and noncurative treatments for recurrence (HR, 1.517; 95% CI, 1.253–1.835; p < .001).
Table 5.
Univariate and multivariate Cox regression analyses predicting postrecurrence survival in 604 patients who developed recurrence after curative liver resection for hepatitis B virus–associated hepatocellular carcinoma
| Variables | HR comparison | UV HR (95% CI) | UV p value | MV HR (95% CI) | MV p valuea |
|---|---|---|---|---|---|
| BCLC tumor staging of the initial tumor | BCLC stage B/C vs. A | 1.238 (0.994–1.542) | .057 | NS | NS |
| Interval to recurrence | Early recurrence vs. late recurrence | 1.580 (1.301–1.919) | <.001 | 1.361 (1.094–1.692) | .006 |
| Postoperative recurrence surveillance | Irregular vs. regular | 1.371 (1.133–1.659) | .001 | 1.293 (1.059–1.578) | .012 |
| Age at diagnosis of recurrence | >60 vs. ≤60 years | 1.024 (0.806–1.299) | .847 | ||
| Sex | Male vs. female | 0.986 (0.714–1.360) | .930 | ||
| HBeAg (+) at diagnosis of recurrence | Yes vs. no | 0.891 (0.562–1.413) | .623 | ||
| HBV‐DNA load at diagnosis of recurrence | >104 vs. ≤104 copies/mL | 1.032 (0.850–1.252) | .751 | ||
| Cirrhosis at diagnosis of recurrence | Yes vs. no | 1.193 (0.956–1.488) | .118 | ||
| Portal hypertension at diagnosis of recurrence | Yes vs. no | 1.310 (1.069–1.605) | .009 | 1.248 (1.028–1.516) | .025 |
| Child‐Pugh grade at diagnosis of recurrence | B/C vs. A | 1.356 (0.924–1.578) | .229 | ||
| AFP level at diagnosis of recurrence | >400 vs. ≤400 μg/L | 1.357 (1.102–1.671) | .004 | 1.325 (1.086–1.615) | .006 |
| Patterns of recurrence | Only intrahepatic vs. extrahepatic ± intrahepatic | 1.442 (1.192–1.744) | <.001 | NS | NS |
| Extent of recurrence | Beyond vs. within Milan criteria | 1.814 (1.252–2.628) | .001 | 1.359 (1.115–1.654) | .002 |
| Treatment modalities for recurrence | Noncurative vs. potentially curative | 1.769 (1.565–1.998) | <.001 | 1.517 (1.253–1.835) | <.001 |
| Postrecurrence anti‐HBV therapy | Irregular vs. regular | 0.748 (0.600–0.933) | .010 | NS | NS |
| Postrecurrence HBV reactivation | Yes vs. no | 1.409 (0.947–2.097) | .090 | NS | NS |
Those variables found significant at p < .1 in univariable analyses were entered into multivariable Cox regression analyses.
Abbreviations: AFP, alpha‐fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; HBeAg, hepatitis B virus envelope antigen; HBV, hepatitis B virus; HR, hazard ratio; MV, multivariate; NS, not significant; UV, univariate.
Discussion
In this large study from a prospectively collected database, the risk factors, patterns, and long‐term survival outcomes of patients with both early and late recurrence after curative resection for HBV‐associated HCC were evaluated. Using multivariable Cox regression analyses, host‐, hepatitis‐, tumor‐, and operation‐related risk factors were found to be associated with either early or late recurrence. Several risk factors contributing to early recurrence were related to initial tumor characteristics (e.g., preoperative AFP level >400 μg/L, tumor size >5 cm, multiple tumors, macrovascular and microvascular invasion, satellite nodules, and incomplete tumor encapsulation), whereas fewer were related to the operation (resection margin <1 cm) or hepatitis (preoperative HBV‐DNA load >104 copies/mL). In addition, multiple factors were associated with late recurrence including initial tumor characteristics (e.g., tumor size >5 cm, macrovascular and microvascular invasion, and satellite nodules), host‐related factors (male sex and cirrhosis), and hepatitis‐related factors (preoperative HBV‐DNA load >104 copies/mL and postoperative no/irregular anti‐HBV therapy). In addition, patterns and extent of initial recurrence were different among patients with early and late recurrence, as patients with early recurrence had a lower proportion of intrahepatic‐only recurrence (72.0% vs. 91.1%, p < .001) and recurrence within Milan criteria (41.0 vs. 51.1%, p = .014), as well as a lower chance of receiving potentially curative treatments for recurrence (33.9% vs .50.7%, p < .001) and a worse median PRS (19.1 vs. 37.5 months, p < .001). Moreover, early recurrence, post operative irregular recurrence surveillance, presence of portal hypertension at diagnosis of recurrence, AFP level >400 μg/L at diagnosis of recurrence, recurrent HCC beyond Milan criteria, and noncurative treatments for recurrence were independently associated with worse PRS among patients with recurrence after curative resection for HBV‐associated HCC. In turn, data from the current study may provide insights into different biological origin and behavior of early versus late recurrence after resection for HBV‐associated HCC, which could be helpful to inform decision making about treatment options for recurrent HCC, as well as rational strategies for recurrence surveillance after HCC resection.
After the early peak of recurrence (at around 1 year postoperatively), the recurrence rate decreased over the next 2 years, followed by a second peak 4–5 years postoperatively (Fig. 1). This finding was most likely to be related to recurrence caused by occult micrometastasis of the initial HCC being mainly responsible for the early peak. In contrast, the majority of the second recurrence peak was likely to be attributable to new tumor(s) developing de novo with different clonal origins. These results were consistent with the data reported by Imamura et al. 10. In our study, we also identified independent risk factors associated with early recurrence after HCC resection to be mainly related to the initial tumor characteristics and operative variables. In contrast, cirrhosis and male sex were independent risk factors of late recurrence, which supported the hypotheses that late recurrence is more likely to be due to multicentric tumors or de novo cancer formation from the underlying liver background of hepatitis and cirrhosis, as well as the potentially tumorigenic effects of sex hormones 6, 7, 14, 18, 19, 20. Differences in risk factors for late recurrence highlight the need for close and stringent recurrence surveillance among male patients with cirrhosis in the late postoperative period of follow‐up 15. In addition, postoperative regular anti‐HBV therapy was independently associated with reduced late recurrence, which could be explained by suppression of viral replication and inflammation within the liver microenvironment 8, 17, 21, 22. In aggregate, the data emphasized the importance of regular postoperative antiviral therapy in preventing late HCC recurrence after curative resection for HBV‐associated HCC.
In contrast to some previous studies 5, 6, 7, tumor size >5 cm, multiple tumors, macro‐ and microvascular invasion, and satellite nodules were independent risk factors of late recurrence, suggesting that late recurrence was also correlated with the initial tumor. The use of 2 years as the cutoff value to differentiate metastasis from the initial tumor from de novo tumors remains controversial. Actual examination of clonal differences within the tumors is difficult from a practical point of view in the clinical setting, and there is a lack of reliable and clinically applicable markers. Further novel histopathological and genetic tests of both the initial and recurrent tumors are needed to better define whether recurrent tumors originate from the initial tumor or represent a clonally different lesion 23, 24.
Notedly, a high preoperative HBV‐DNA load was identified as an important risk factor associated with both early and late recurrence. The relationship between a high HBV‐DNA load and late recurrence has clinical “face validity” 8, 16, 17, 22, as sustained viremia and succedent active viral replication may contribute to HCC development by generating a carcinogenic microenvironment in the liver, known as the “field effect” 16. Specifically, integration of subgenomic HBV‐DNA fragments into the host hepatic cells may activate cellular genes directly to allow selective growth advantages. The production of HBV X‐protein could then act as a trans‐activator on various cellular genes for HCC development 25, 26. Continuing HBV replication could, in turn, induce chronic hepatitis inflammation and fibrosis and alter the production of alpha‐2 macroglobulin and transform the growth factor‐beta1, thereby leading to carcinogenesis 27. The upregulation of adhesion molecules on the cells lining the sinusoids may also enhance tumor development and spread 28. However, the effect of a high preoperative HBV‐DNA load on early recurrence had not been well defined previously. One study suggested a high preoperative HBV‐DNA load to be an independent risk factor of microvascular invasion of HCC, and preoperative antiviral therapy administered more than 90 days before surgery was associated with a reduced incidence of microvascular invasion and early tumor recurrence at 6 months, 1 year, and 2 years after surgery 29. Other studies have also revealed that the HBV‐initiated tumorigenic process may play a role in development of vascular invasion of HCC 30, 31. These data support an association of a high preoperative HBV‐DNA load and early recurrence of HCC. Because HBV‐mediated inflammatory response, genome integration, and mutations play central roles in HCC development, patients with a high HBV‐DNA load should be followed up closely. These patients should be given lifelong antiviral therapy to suppress HBV replication in an attempt to prevent HCC recurrence. A recent randomized controlled trial demonstrated that antiviral therapy for patients with a low preoperative HBV‐DNA load not only prevented HBV reactivation but also reduced tumor recurrence and improved postoperative survival 32. These data suggested that even for patients with a low preoperative HBV‐DNA load, early and regular antiviral treatment after HCC resection should be given.
Understanding the patterns, extents, and long‐term prognosis of early and late recurrence after HCC resection can assist in designing surveillance strategies. The liver is recognized as the predominant organ involved in initial recurrence after HCC resection 6, 12. In our study, compared with patients who had late recurrence, patients with early recurrence had a lower proportion of intrahepatic‐only recurrence (72.0% vs. 91.1%, p < .001) and recurrence within the Milan criteria (41.0 vs. 51.1%, p = .014). Moreover, among patients with late recurrence, there was no patient who had sole extrahepatic metastasis without intrahepatic recurrence. These data indicated that screening for late recurrence after 2 years of surgery should focus on intrahepatic recurrence, whereas screening for extrahepatic metastasis using skeletal emission computerized tomography and chest CT may be unnecessary for patients who have no intrahepatic recurrence. According to our data, the time to recurrence and the patterns of recurrence should be taken into account to establish an individualized and a more cost‐effective surveillance strategy for patients with HCC after surgery.
In this study, the long‐term survival of patients with early recurrence was worse than that of patients with late recurrence (median PRS, 19.1 vs. 37.5 months, p < .001). For patients with early recurrence, the higher occurrence of extrahepatic recurrence indicated a worse prognosis with no chance to undergo any curative treatment. On the other hand, patients with late recurrence had a better chance to undergo potentially curative‐intent therapy for recurrent HCC (50.7% vs. 33.9%, p < .001). Meanwhile, as most recurrent lesions that developed within a short time after the initial resection were more likely to be related to intrahepatic occult micrometastasis, even if the recurrent lesions could be resected with curative intent, the tumor re‐relapse is expected to be high because of the possibility of other undetectable micrometastases. Thus, a good selection of suitable treatment modalities for the initial recurrence and appropriate selection of patients who will benefit from treatment are of paramount clinical importance. Our study also identified irregular postoperative recurrence surveillance to be an independent risk factor of PRS for patients with recurrence, suggesting that a stringent recurrence surveillance program on follow‐up is helpful to detect and treat recurrent lesions early to improve the long‐term prognosis. In clinical practice, many patients do not take surveillance seriously, and they lose the chance to undergo curative treatment when symptoms develop.
The present study had several limitations. First, inherent biases are inevitable because of the retrospective nature of the study. Second, because of medical insurance payment restrictions, some hepatitis‐related indicators were also not routinely tested, such as HBV genotypes 33, quantitative detection of HBsAg 17, intrahepatic covalently closed circular DNA 34, and precore and basal core promoter mutations in the HBV genome 35. These indicators have previously been reported to show some association with recurrence after resection of HBV‐associated HCC. Third, this study was conducted on HBV‐related HCC. The results of this study may not be applied to other etiologies of HCC. Lastly, further novel histopathological examinations and genetic tests on both the initial and recurrent tumors are worth carrying out to find out whether recurrent tumors originate from micrometastasis of the initial tumors or de novo second primary HCCs.
Conclusion
Early and late recurrence after resection of HBV‐associated HCC were related to different risk factors. A high preoperative HBV‐DNA load was an independent hepatitis‐related risk factor of both early and late recurrence of HBV‐associated HCC, whereas regular postoperative antiviral therapy was associated with a decreased late recurrence rate. In addition, early recurrence, irregular recurrence surveillance, presence of portal hypertension, recurrent HCC beyond the Milan criteria, noncurative treatments for recurrence, a high HBV‐DNA load, and AFP level at diagnosis of recurrence were independently associated with worse PRS in patients who developed recurrence after curative resection of HBV‐associated HCC.
Author Contributions
Conception/design: Ming‐Da Wang, Feng Shen, Tian Yang
Provision of study material or patients: Ming‐Da Wang, Li‐Yang Sun, Bing Quan, Han Wu, Xin‐Fei Xu, Meng‐Chao Wu, Feng Shen, Tian Yang
Collection and/or assembly of data: Feng Shen, Tian Yang
Data analysis and interpretation: Ming‐Da Wang, Chao Li, Lei Liang, Hao Xing, Li‐Yang Sun, Bing Quan, Han Wu, Xin‐Fei Xu, Meng‐Chao Wu, Feng Shen, Tian Yang
Manuscript writing: Ming‐Da Wang, Chao Li, Meng‐Chao Wu, Timothy M. Pawlik, Wan Yee Lau, Feng Shen, Tian Yang
Final approval of manuscript: Ming‐Da Wang, Chao Li, Lei Liang, Hao Xing, Li‐Yang Sun, Bing Quan, Han Wu, Xin‐Fei Xu, Meng‐Chao Wu, Timothy M. Pawlik, Wan Yee Lau, Feng Shen, Tian Yang
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Figure S1 Flowchart of study population. HBV, hepatitis B virus; HCC, hepatocellular carcinoma.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (81702334 to M.‐D.W. and 81672699 and 81972726 to T.Y.) and the Shanghai Sailing Program (17YF1424900 to M.‐D.W.). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Villanueva A. Hepatocellular carcinoma. N Engl J Med 2019;380:1450–1462. [DOI] [PubMed] [Google Scholar]
- 2. El‐Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264–1273.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dhir M, Melin AA, Douaiher J et al. A review and update of treatment options and controversies in the management of hepatocellular carcinoma. Ann Surg 2016;263:1112–1125. [DOI] [PubMed] [Google Scholar]
- 4. Rahbari NN, Mehrabi A, Mollberg NM et al. Hepatocellular carcinoma: Current management and perspectives for the future. Ann Surg 2011;253:453–469. [DOI] [PubMed] [Google Scholar]
- 5. Poon RT, Fan ST, Ng IO et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 2000;89:500–507. [PubMed] [Google Scholar]
- 6. Portolani N, Coniglio A, Ghidoni S et al. Early and late recurrence after liver resection for hepatocellular carcinoma: Prognostic and therapeutic implications. Ann Surg 2006;243:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng Z, Yang P, Qu S et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford) 2015;17:422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu JC, Huang YH, Chau GY et al. Risk factors for early and late recurrence in hepatitis B‐related hepatocellular carcinoma. J Hepatol 2009;51:890–897. [DOI] [PubMed] [Google Scholar]
- 9. Xu XF, Xing H, Han J et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: A multicenter study from China. JAMA Surg 2019;154:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Imamura H, Matsuyama Y, Tanaka E et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200–207. [DOI] [PubMed] [Google Scholar]
- 11. Shah SA, Greig PD, Gallinger S et al. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg 2006;202:275–283. [DOI] [PubMed] [Google Scholar]
- 12. Zheng J, Chou JF, Gönen M et al. Prediction of hepatocellular carcinoma recurrence beyond Milan criteria after resection: Validation of a clinical risk score in an international cohort. Ann Surg 2017;266:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shimoda M, Tago K, Shiraki T et al. Risk Factors for early recurrence of single lesion hepatocellular carcinoma after curative resection. World J Surg 2016;40:2466–2471. [DOI] [PubMed] [Google Scholar]
- 14. Poon RT. Differentiating early and late recurrences after resection of HCC in cirrhotic patients: Implications on surveillance, prevention, and treatment strategies. Ann Surg Oncol 2009;16:792–794. [DOI] [PubMed] [Google Scholar]
- 15. Cucchetti A, Piscaglia F, Caturelli E et al. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann Surg Oncol 2009;16:413–422. [DOI] [PubMed] [Google Scholar]
- 16. Qu LS, Jin F, Huang XW et al. High hepatitis B viral load predicts recurrence of small hepatocellular carcinoma after curative resection. J Gastrointest Surg 2010;14:1111–1120. [DOI] [PubMed] [Google Scholar]
- 17. Sohn W, Paik YH, Kim JM et al. HBV DNA and HBsAg levels as risk predictors of early and late recurrence after curative resection of HBV‐related hepatocellular carcinoma. Ann Surg Oncol 2014;21:2429–2435. [DOI] [PubMed] [Google Scholar]
- 18. White DL, Thrift AP, Kanwal F et al. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology 2017;152:812–820.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee CM, Lu SN, Changchien CS et al. Age, gender, and local geographic variations of viral etiology of hepatocellular carcinoma in a hyperendemic area for hepatitis B virus infection. Cancer 1999;86:1143–1150. [DOI] [PubMed] [Google Scholar]
- 20. El‐Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557–2576. [DOI] [PubMed] [Google Scholar]
- 21. Huang G, Lau WY, Wang ZG et al. Antiviral therapy improves postoperative survival in patients with hepatocellular carcinoma: A randomized controlled trial. Ann Surg 2015;261:56–66. [DOI] [PubMed] [Google Scholar]
- 22. Yang T, Lu JH, Zhai J et al. High viral load is associated with poor overall and recurrence‐free survival of hepatitis B virus‐related hepatocellular carcinoma after curative resection: A prospective cohort study. Eur J Surg Oncol 2012;38:683–691. [DOI] [PubMed] [Google Scholar]
- 23. Pecchi A, Besutti G, De Santis M et al. Post‐transplantation hepatocellular carcinoma recurrence: Patterns and relation between vascularity and differentiation degree. World J Hepatol 2015;7:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidt C, Marsh JW. Molecular signature for HCC: Role in predicting outcomes after liver transplant and selection for potential adjuvant treatment. Curr Opin Organ Transplant 2010;15:277–282. [DOI] [PubMed] [Google Scholar]
- 25. Chan HL, Sung JJ. Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis 2006;26:153–161. [DOI] [PubMed] [Google Scholar]
- 26. Kao JH, Chen PJ, Chen DS. Recent advances in the research of hepatitis B virus‐related hepatocellular carcinoma: Epidemiologic and molecular biological aspects. Adv Cancer Res 2010;108:21–72. [DOI] [PubMed] [Google Scholar]
- 27. Bréchot C. Pathogenesis of hepatitis B virus‐related hepatocellular carcinoma: Old and new paradigms. Gastroenterology 2004;127:S56–61. [DOI] [PubMed] [Google Scholar]
- 28. Bréchot C, Gozuacik D, Murakami Y et al. Molecular bases for the development of hepatitis B virus (HBV)‐related hepatocellular carcinoma (HCC). Semin Cancer Biol 2000;10:211–231. [DOI] [PubMed] [Google Scholar]
- 29. Li Z, Lei Z, Xia Y et al. Association of preoperative antiviral treatment with incidences of microvascular invasion and early tumor recurrence in hepatitis B virus‐related hepatocellular carcinoma. JAMA Surg 2018;153:e182721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen L, Zhang Q, Chang W et al. Viral and host inflammation‐related factors that can predict the prognosis of hepatocellular carcinoma. Eur J Cancer 2012;48:1977–1987. [DOI] [PubMed] [Google Scholar]
- 31. Wei X, Li N, Li S et al. Hepatitis B virus infection and active replication promote the formation of vascular invasion in hepatocellular carcinoma. BMC Cancer 2017;17:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang G, Li PP, Lau WY et al. Antiviral therapy reduces hepatocellular carcinoma recurrence in patients with low HBV‐DNA levels: A randomized controlled trial. Ann Surg 2018;268:943–954. [DOI] [PubMed] [Google Scholar]
- 33. Chen JD, Liu CJ, Lee PH et al. Hepatitis B genotypes correlate with tumor recurrence after curative resection of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2004;2:64–71. [DOI] [PubMed] [Google Scholar]
- 34. Wong DK, Yuen MF, Poon RT et al. Quantification of hepatitis B virus covalently closed circular DNA in patients with hepatocellular carcinoma. J Hepatol 2006;45:553–559. [DOI] [PubMed] [Google Scholar]
- 35. Kao JH, Chen PJ, Lai MY et al. Basal core promoter mutations of hepatitis B virus increase the risk of hepatocellular carcinoma in hepatitis B carriers. Gastroenterology 2003;124:327–334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Figure S1 Flowchart of study population. HBV, hepatitis B virus; HCC, hepatocellular carcinoma.


