Abstract
Estrogen receptor (ER) status by immunohistochemistry (IHC) of cancer tissue is currently used to direct endocrine therapy in breast cancer. Positron emission tomography (PET) with 16α‐18F‐fluoro‐17β‐estradiol (18F‐FES) noninvasively characterizes ER ligand–binding function of breast cancer lesions. Concordance of imaging and tissue assays should be established for 18F‐FES PET to be an alternative or complement to tissue biopsy for metastatic lesions. We conducted a meta‐analysis of published results comparing 18F‐FES PET and tissue assays of ER status in patients with breast cancer. PubMed and EMBASE were searched for English‐language manuscripts with at least 10 patients and low overall risk of bias. Thresholds for imaging and tissue classification could differ between studies but had to be clearly stated. We used hierarchical summary receiver‐operating characteristic curve models for the meta‐analysis. The primary analysis included 113 nonbreast lesions from 4 studies; an expanded analysis included 327 total lesions from 11 studies. Treating IHC results as the reference standard, sensitivity was 0.78 (95% confidence region 0.65–0.88) and specificity 0.98 (0.65–1.00) for the primary analysis of nonbreast lesions. In the expanded analysis including non‐IHC tissue assays and all lesion sites, sensitivity was 0.81 (0.73–0.87) and specificity 0.86 (0.68–0.94). These results suggest that 18F‐FES PET is useful for characterization of ER status of metastatic breast cancer lesions. We also review current best practices for conducting 18F‐FES PET scans. This imaging assay has potential to improve clinically relevant outcomes for patients with (historically) ER‐positive metastatic breast cancer, including those with brain metastases and/or lobular histology.
Implications for Practice
16α‐18F‐fluoro‐17β‐estradiol positron emission tomography (18F‐FES PET) imaging assesses estrogen receptor status in breast cancer in vivo. This work reviews the sensitivity and specificity of 18F‐FES PET in a meta‐analysis with reference tissue assays and discusses best practices for use of the tracer as an imaging biomarker. 18F‐FES PET could enhance breast cancer diagnosis and staging as well as aid in therapy selection for patients with metastatic disease. Tissue sampling limitations, intrapatient heterogeneity, and temporal changes in molecular markers make it likely that 18F‐FES PET will complement existing assays when clinically available in the near future.
Keywords: Metastatic breast cancer, Positron emission tomography, Estrogen receptor, Meta‐analysis, 16α‐18F‐fluoro‐17β‐estradiol, Tissue biopsy, Immunohistochemistry
Short abstract
Positron emission tomography (PET) with 18F‐FES is being developed for the characterization of estrogen receptor (ER) status of known or suspected metastatic lesions in patients with a history of ER‐positive breast cancer. This study was conducted to assess concordance of ER by 18F‐FES PET and immunohistochemistry in non‐breast lesions and to incorporate new published data, as part of a new drug application. This report presents the results of the meta‐analysis and reviews current best practices for conducting 18F‐FES PET scans.
Introduction
More than 70% of primary breast cancers are hormone receptor positive, and their incidence is increasing in the U.S. 1. Determination of estrogen receptor (ER) status in a primary breast lesion is an integral part of initial patient workup. Breast tissue is relatively easy and safe to biopsy, and immunohistochemical (IHC) analysis is consequently standardized 2. Despite a favorable prognosis and effective therapies available for ER‐positive breast cancer, metastatic breast cancer (MBC) from an ER‐positive primary tumor is responsible for the majority of breast cancer–related deaths 3.
Patients with metastatic disease from an ER‐positive primary breast cancer (ER+ MBC) can live for many productive years with appropriate therapy. Because there are many classes of treatment options (endocrine therapy, biologics, cytotoxic chemotherapy, etc.) and a myriad of evolving disease phenotypes (loss of ER, gain of human epidermal growth receptor 2 [HER2], emergence of PI3Kinase mutations, ESR1, and others), determining a sequence of therapies is a major challenge for each individual patient 4, 5, 6. Hormone receptor–directed therapy can only be effective if there is receptor expression in metastatic lesions.
A proportion of patients with ER‐positive primary disease eventually present with ER‐negative metastases 7, 8, 9, 10. These patients will not benefit from ER‐directed therapies, and it is therefore important for ER status to be determined in all patients with historically ER‐positive disease 11, 12. However, metastatic disease is often not amenable to IHC analysis. The lesion may be located in a site that is difficult or impossible to biopsy 11, and bone lesions face the additional challenge of decalcification 13. A fine‐needle or core biopsy may not be representative of the entire lesion; tumor heterogeneity may result in phenotype variation between lesions, limiting utility of single lesion assessment 14.
Positron emission tomography (PET) with 16α‐18F‐fluoro‐17β‐estradiol (18F‐FES) assesses ER functioning in a manner analogous to in vitro ligand‐binding assays 15, 16, 17. 18F‐FES PET has been shown by several groups to be an excellent noninvasive method for determining ER status in multiple lesions throughout the body (with the exception of the liver, whence it is cleared) 18, 19, 20, 21, 22. ER positivity by 18F‐FES PET has been shown to predict, beyond clinical and pathological predictors, longer progression‐free survival (PFS) on endocrine monotherapy 23, potentially impacting therapy choices in patients with a clinical dilemma 22, 24.
IHC assays are the current gold standard for use of ER as a predictive biomarker in primary breast cancer 2. Multiple studies and meta‐analyses have demonstrated that 18F‐FES PET results are indeed concordant with ER status determined by invasive immunohistochemical methods; meta‐analyses have estimated sensitivity (probability of 18F‐FES‐positive scan for an ER‐positive lesion) of 0.82–0.84 and specificity (probability of 18F‐FES‐negative scan for an ER‐negative lesion) of 0.93–0.98 17, 21, 25. However, in all previous meta‐analyses, results for metastatic lesions have been merged with results for breast lesions.
18F‐FES PET is being developed for the characterization of ER status of known or suspected metastatic lesions in patients with a history of ER‐positive breast cancer. Breast lesions may be easier to assess by 18F‐FES PET because of low uptake in normal breast 26. In order to assess concordance of ER by 18F‐FES PET and IHC in nonbreast lesions, and to incorporate new published data, Zionexa conducted a new meta‐analysis as part of the Food and Drug Administration New Drug Application for 18F‐FES. This report presents the results of our meta‐analysis. In this context, we review current best practices for conducting 18F‐FES PET scans, from the referring oncologist's, patient's, and nuclear medicine physician's point of view.
Materials and Methods
The goal of the current meta‐analysis was to assess lesion‐level agreement between ER IHC assays and qualitative assessment by 18F‐FES PET. We considered nonbreast and breast lesions separately. Separate analysis of breast lesions also allowed for inclusion of patients with nonmetastatic disease in secondary analyses.
Search criteria, study assessment, and other meta‐analysis design factors are described in the supplemental online data. The approach for data synthesis followed the recommendations made in the Handbook for Diagnostic Test Accuracy Reviews of the Cochrane Collaboration 27. Heterogeneity across study sensitivity and specificity was summarized by the chi‐square test and the I2 statistic 28. Hierarchical summary receiver‐operating characteristic (HSROC) curves 29 were estimated to summarize test accuracy while accounting for variability due to threshold across studies. The HSROC curve was fitted only in the range of observed values for both sensitivity and specificity, so studies without estimates of specificity (without ER‐negative lesions by tissue assay) were excluded. Summary sensitivity and specificity were derived with associated 95% confidence regions 30, 31. Four separate HSROC models were estimated: (a) Primary analysis—studies that provided 18F‐FES PET lesion‐specific information for nonbreast tumors in patients with metastatic breast cancer; (b) Secondary analysis 1—studies that provided 18F‐FES PET lesion‐specific information for breast tumors; (c) Secondary analysis 2—combined analysis of all lesions with paired ER status by 18F‐FES PET and IHC, and (d) Secondary analysis 3—expanded combined analysis with all evaluable studies, which used a variety of standards for the tissue reference for 18F‐FES PET performance.
Computations were performed using R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) and the mada package 31.
Results
PubMed and EMBASE searches identified 103 breast cancer studies involving 18F‐FES PET, and 12 studies met the criteria for inclusion in our meta‐analysis (published in English; included imaging and tissue findings for at least 10 patients; identified reference standards for imaging and tissue findings; rated as low overall risk of bias. See supplemental online data for details). Definitions of ER‐positive by IHC (the reference standard) and of 18F‐FES‐positive (the test result) differed slightly between studies. These study‐specific classifications were preserved for the meta‐analysis. Data were not available for harmonization of tissue or imaging assays across studies.
Table 1 shows lesion‐level results for the 12 studies considered for the meta‐analysis. Five studies included lesion‐level results for nonbreast metastatic lesions. In the four smaller studies 32, 33, 34, 35, all 26 ER‐positive lesions by IHC were also ER positive by 18F‐FES PET, and all 6 ER‐negative lesions were ER negative by 18F‐FES PET. (Sensitivity and specificity estimates below 100% are due to continuity corrections to stabilize calculations.) In the largest study of nonbreast lesions 21, all 38 tumors that were ER negative by IHC were also ER negative by 18F‐FES PET, but 11 of 47 ER‐positive reference tumors had “false negative” 18F‐FES PET test results (18F‐FES negative by PET but ER positive by the IHC reference standard), for a sensitivity of 0.77 (95% confidence interval [CI] 0.63–0.86).
Table 1.
Diagnostic performance of 18F‐FES PET for the identification of estrogen receptor status in tumors of patients with breast cancer
| Study | TP | FP | FN | TN | Total n | Sensitivity (95% CI)a | Specificity (95% CI)a | Tissue assayb definition of ER‐positive | 18F‐FES PET definition of ER‐positive | Meta‐analysis inclusionc | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||||||||
| Chae 2019 21 | 36 | 0 | 11 | 38 | 85 | 0.77 (0.63–0.86) | 1.00 (0.90–1) | Allred ≥3 | Qualitative | X | X | X | |
|
Nonbreast |
4 | 0 | 0 | 0 | 4 | 0.98 (0.50–1) | Indeterminate | Not stated, but all ER‐positive were ≥ 15% of cells | Qualitative | —e | X | X | |
| Breast | 6 | 0 | 0 | 0 | 6 | 0.98 (0.60–1) | Indeterminate | —e | (X) | (X) | |||
| Unclear location | 0 | 0 | 0 | 2 | 2 | (X) | (X) | ||||||
|
Nonbreast |
3 | 0 | 0 | 2 | 5 | 0.97 (0.43–1) | 0.96 (0.33–1) | ≥5% of cells | SUVmean >1 | X | X | X | |
| Breast | 9 | 1 | 0 | 2 | 12 | 0.99 (0.69–1) | 0.66 (0.21–0.93) | X | (X) | (X) | |||
|
Nonbreast |
8 | 0 | 0 | 2 | 10 | 0.99 (0.67–1) | 0.96 (0.33–1) | Allred >2 | SUVmean >1.5 | X | X | X | |
| Breast | 4 | 0 | 0 | 0 | 4 | 0.98 (0.50–1) | Indeterminate | —e | (X) | (X) | |||
| Venema 2017 35 | 11 | 0 | 0 | 2 | 13 | 0.99 (0.73–1) | 0.96 (0.33–1) | ≥1% of cells | SUVmax >1.5 | X | X | X | |
| Chae 2017 38 | 24 | 0 | 2 | 0 | 26 | 0.92 (0.75–0.98) | Indeterminate | Allred ≥6 | Qualitative | —e | —e | —e | |
| Gemignani 2013 37 | 34 | 2 | 6 | 6 | 48 | 0.85 (0.71–0.93) | 0.74 (0.41–0.93) | ≥1% of cells | SUVmean >1.5 | X | X | X | |
| Yang 2013 36 | 11 | 1 | 0 | 6 | 18 | 0.99 (0.73–1) | 0.85 (0.48–0.97) | ≥1% of cells | SUVmax >1.5h | X | X | X | |
| Dehdashti 1995 39 | 11 | 0 | 5 | 20 | 36 | 0.69 (0.44–0.86) | 1 (0.83–1) | RBA, >3 fmol/mg or IHC (criteria not stated) | Qualitative | X | |||
| Mintun 1988 15 | 8 | 2 | 0 | 0 | 10 | 0.99 (0.67–1) | 0.05 (0–0.67) | RBA, >3 fmol/mg | Qualitative | X | |||
| Mortimer 1996 18 | 16 | 0 | 5 | 20 | 41 | 0.76 (0.55–0.89) | 1 (0.83–1) | RBA, >3 fmol/mg or IHC (criteria not stated) | Qualitative | X | |||
| van Kruchten 2012 24 | 22 | 0 | 1 | 10 | 33 | 0.95 (0.79–0.99) | 0.99 (0.71–1) | IHC (criteria not stated) and/or clinical outcome | SUVmax >1.5 | X | |||
Wilson confidence interval, continuity correction of 0.1 applied to all cells.
All immunohistochemistry in primary analysis and secondary analysis 1.
(1) Primary analysis: Nonbreast lesions in patients with advanced/metastatic breast cancer—includes lymph nodes, chest wall, and pleura (37 Chae 2019, 3 Gupta, 4 Peterson 2014, 4 Peterson 2008, all ER positive; 30 Chae 2019 ER negative); (2) Secondary analysis 1: Breast lesions in patients with any‐stage breast cancer; (3) Secondary analysis 2: Combined breast and nonbreast lesions; (4) Secondary analysis 3: Additional breast and nonbreast lesions, in patients with any‐stage breast cancer, non‐IHC tissue assay.
As determined from patient‐level data provided in Table 4 of the publication; location not provided for 2 TN lesions.
Excluded from hierarchical summary receiver‐operating characteristic analysis (meta‐analysis summary sensitivity and specificity)—no ER‐negative lesions in study.
Unpublished patient‐level lesion location data provided by the authors.
As determined from patient‐level data provided in Appendix Table 2 of the publication.
No 18F‐FES positive/negative determination in manuscript, but data values available for use of SUVmax cutoff.
Abbreviations: CI, confidence interval; ER, estrogen receptor; 18F‐FES, 16α‐18F‐fluoro‐17β‐estradiol; FN, false negative; FP, false positive; IHC, immunohistochemistry; PET, positron emission tomography; RBA, relative binding affinity; SUV, standardized uptake value; TN, true negative; TP, true positive.
For additional studies with IHC reference (three additional studies of breast lesions, and breast or unspecified lesions in three of the five primary studies) 32, 33, 34, 36, 37, 38, sensitivity ranged from 0.85 to 0.99, and specificity ranged from 0.66 to 0.85 (Table 1). Finally, for four studies with non‐IHC reference 15, 18, 24, 39, sensitivity ranged from 0.69 to 0.99, and specificity ranged from 0.05 to 1.00. Reported sensitivity and specificity and 95% confidence intervals are plotted in receiver‐operating characteristic space in Figure 1, for each study in which both sensitivity and specificity could be estimated (that is, excluding studies with no ER‐negative reference tumors).
Figure 1.
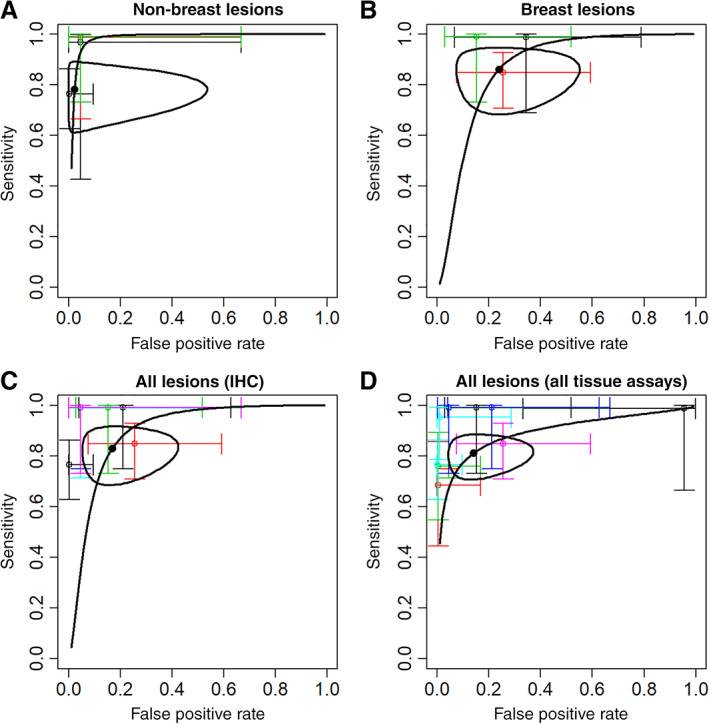
Meta‐analysis summary sensitivity and specificity (•) with 95% confidence region (— closed curve) and hierarchical summary receiver‐operating characteristic curve (— curve), and sensitivity and specificity (95% confidence interval) for individual studies (thin colored lines). (A): Nonbreast lesions (4 studies with both sensitivity and specificity estimates). (B): Breast lesions (3 studies). (C): All lesions, tissue estrogen receptor (ER) by IHC (7 studies). (D): All lesions, tissue ER by IHC or other assay (11 studies).
Abbreviation: IHC, immunohistochemistry.
Meta‐analysis summary sensitivity and specificity estimates are superimposed on the plots in Figure 1 and summarized in Table 2. For the primary analysis of nonbreast lesions, overall sensitivity estimated by HSROC was 0.78 (95% confidence region 0.65–0.88); overall specificity was 0.98 (0.65–1.00; Fig. 1A). Secondary analysis 1 analyzed breast lesions for three studies, in patients with any‐stage breast cancer. The overall sensitivity estimated by HSROC was 0.86 (0.73–0.94), and specificity was 0.76 (0.52–0.90; Fig. 1B). Secondary analysis 2 combined analysis of all lesions with paired ER status by IHC, finding an overall sensitivity of 0.83 (0.72–0.90) and overall specificity of 0.83 (0.64–0.93; Fig. 1C). Finally, secondary analysis 3 expanded the combined analysis of 18F‐FES PET performance to include tissue standards other than IHC. Overall sensitivity was 0.81 (0.73–0.87), and overall specificity was 0.86 (0.66–0.94; Fig. 1D).
Table 2.
Hierarchical summary receiver‐operating characteristic summaries of 18F‐FES PET test accuracya
| Hierarchical summary ROC model | No. of studiesb | Pooled no. of ER‐positive lesions | Sensitivity (95% confidence region) | Variation in sensitivity attributed to study heterogeneity (test of homogeneity) | Pooled no. of ER‐negative lesions | Specificity (95% confidence region) | Variation in specificity attributed to study heterogeneity (test of homogeneity) | Model area under the ROC curve |
|---|---|---|---|---|---|---|---|---|
| Metastatic lesions, IHC (primary analysis) | 4 | 69 | 0.78 (0.65–0.88) | I2 = 46% | 44 | 0.98 (0.65–1) | I2 = 0% | 0.98 |
| Breast lesions, IHC (secondary analysis 1) | 3 | 60 | 0.86 (0.73–0.94) | I2 = 32% | 18 | 0.76 (0.52–0.90) | I2 = 0% | 0.87 |
| Combined, IHC (secondary analysis 2) | 7 | 143 | 0.83 (0.72–0.90) | I2 = 53% | 64 | 0.83 (0.64–0.93) | I2 = 35% | 0.89 |
| Combined, all reference standards (secondary analysis 3) | 11 | 211 | 0.81 (0.73–0.87) | I2 = 52% | 116 | 0.86 (0.68–0.94) | I2 = 78% | 0.89 |
A continuity correction of 0.1 was applied to all cells; random effects not fitted when sample size inadequate.
Excludes studies with no ER‐negative lesions (“Indeterminate” specificity in Table 1, although only one study is excluded from secondary analyses 2 and 3 for this reason—see Table 1 footnotes).
Abbreviations: ER, estrogen receptor; IHC, immunohistochemistry; ROC, receiver operating characteristic.
Figure 1 demonstrates the uniformly high specificity for studies of nonbreast lesions (Fig. 1A), and the low sensitivity and/or specificity of some older studies (Fig. 1D). Tests of homogeneity did not find differences in sensitivity or specificity among the studies in the primary analysis or other analyses with IHC as the tissue reference standard (Table 2). In contrast, heterogeneity among the 11 studies in secondary analysis 3 (Fig. 1D) was found for both sensitivity and specificity (Table 2). The finding of “substantial” 27 heterogeneity among 11 studies in secondary analysis 3 (which included non‐IHC tissue reference standards) but not among 7 studies in secondary analysis 2 is reassuring for the validity of the smaller primary analysis of nonbreast lesions.
Discussion
Our meta‐analysis built upon prior 18F‐FES PET meta‐analyses by including recent studies and focusing on metastatic disease. Meta‐analysis of metastatic lesions found sensitivity of 0.78 (95% CI 0.65–0.88) and specificity of 0.98 (0.65–1; Table 2). Sensitivity decrease in our analysis was driven by 11 “false‐negative” lesions in the Chae 2019 study 21 (lesions that were not included in a pooled analysis of 39 ER‐positive metastatic lesions from three studies, reported in the appendix of that study) and illustrates that small lesions in the chest wall and lymph nodes are difficult to assess with 18F‐FES PET.
Examining all tumor sites and all breast cancer stages, our results are consistent with other published meta‐analyses. Overall sensitivity was estimated as 0.82–0.84 in four meta‐analyses, including ours (Table 2; Fig. 1C) 17, 21, 25. Our overall specificity estimate of 0.83 (95% CI 0.64–0.93) was lower than the specificities from other meta‐analyses (0.93–0.98), despite that those studies also included the studies with false positives 15, 33, 36, 37 (Table 1; supplemental online Table 4).
Summarizing meta‐analysis results, agreement between ER status determined by IHC of tissue from a single lesion and contemporaneous 18F‐FES PET results endorses the validity of 18F‐FES PET assessment of metastatic ER status. Study sample sizes were modest, reporting of inclusion/exclusion criteria for both patients and lesions was not always clear, and qualitative and quantitative thresholds for both 18F‐FES positivity and ER status were not uniform across studies. Another limitation is that presence of the estrogen receptor (as measured by IHC) is arguably less relevant than functional ligand‐binding (by 18F‐FES PET) to endocrine therapy prediction, and neither is a perfect predictor of endocrine therapy benefit. Our 18F‐FES PET sensitivity and specificity estimates treat IHC as the reference standard because it is the current clinical standard for ER assessment. Regardless of these limitations, high specificity (ER‐negative lesions by IHC mostly had 18F‐FES at or near background) in metastatic lesions supports the ability of 18F‐FES PET to detect loss of ER expression in those lesions.
18F‐FES PET is a noninvasive method to determine the presence and ligand‐binding function of the estrogen receptor in metastatic breast cancer lesions throughout the body. A positive 18F‐FES PET scan is associated with benefit from endocrine therapy, in both first‐line and the salvage setting 18, 23, 40. Current options for salvage ER+ MBC therapy are extremely broad 12, 41, including chemotherapy, synergistic molecularly targeted agents, and potentially reusing drugs of a similar class, such as aromatase inhibitors or selective estrogen receptor down‐regulators with or without molecularly targeted agents (e.g., cyclin‐dependent kinase (CDK)4/6 inhibitors, everolimus, alpelisib). Therefore, ER status by 18F‐FES PET has potential for assisting with therapy selection. Additional potential applications for 18F‐FES PET include selecting sites for (re‐)biopsy, and assistance with diagnosis, staging, and restaging 17, 42, 43. The following sections discuss considerations for use of 18F‐FES PET from the referring oncologist's, patient's, and nuclear medicine physician's point of view.
Clinical Applications for 18F‐FES PET
A growing literature has demonstrated that 18F‐FES PET offers complementary information beyond that provided by tissue sampling, especially in metastatic lesions. There are advantages to using 18F‐FES PET to determine ER status of metastatic breast cancer: the technique is noninvasive compared with biopsy, and more importantly, receptor status can be evaluated in all lesions throughout the body, in contrast to the limited sampling provided by biopsy. The ER status of a patient's metastatic breast cancer is usually determined by IHC analysis of biopsy tissue (or from archival primary breast tissue). There may be considerable phenotypic heterogeneity both within a given tumor and across multiple metastases in the same patient 14, 20. Whole‐body 18F‐FES PET will provide the ability to determine ER status in all known or suspected lesions (except for liver metastases). For example, in preclinical and clinical drug development, 18F‐FES PET is used as an in vivo pharmacodynamic marker to confirm ER blockade and help determine dosing 44, 45, 46. Thus 18F‐FES PET can assist with diagnosis (identification of lesion to sample), staging, restaging, and resolution of clinical dilemmas 17, 42, 43.
Two representative examples (from a study undertaken to assess the accuracy of 18F‐FES PET in identifying ER‐positive disease) illustrate the potential for 18F‐FES PET to determine ER status of metastatic lesions in patients with ER+ MBC. Figure 2 demonstrates FES uptake in a brain metastasis (arrow), an area not easily amenable to biopsy. Figure 3 illustrates the utility of 18F‐FES PET in a patient with invasive lobular carcinoma (ILC) of the breast. ILC is characterized by a disseminated growth pattern, low proliferation, and lower tumor glycolysis than invasive ductal carcinomas, while carrying a poor prognosis considering its relative indolence 47, 48. In addition to creating unique challenges for diagnosis and treatment, ILC features such as dissemination and indolence cast doubt on the utility of 18F‐FDG PET for staging 48. For the patient with ILC shown, disease burden was estimated by 18F‐FDG PET (left) followed the next day by 18F‐FES PET. The far greater disease burden detected by 18F‐FES reflects its ability to identify ILC lesions based on the function and density of the estrogen receptor, independent of the Warburg effect (which drives tumor 18F‐FDG avidity) and despite low resolution of PET compared with structural imaging such as computed tomography (CT). These representative examples highlight the ability of this noninvasive imaging technique to assess ER status of lesions in hard‐to‐biopsy locations (the brain, in Fig. 2) as well as provide a characterization of whole‐body assessment of ER status in patients with ER+ MBC (Fig. 3).
Figure 2.
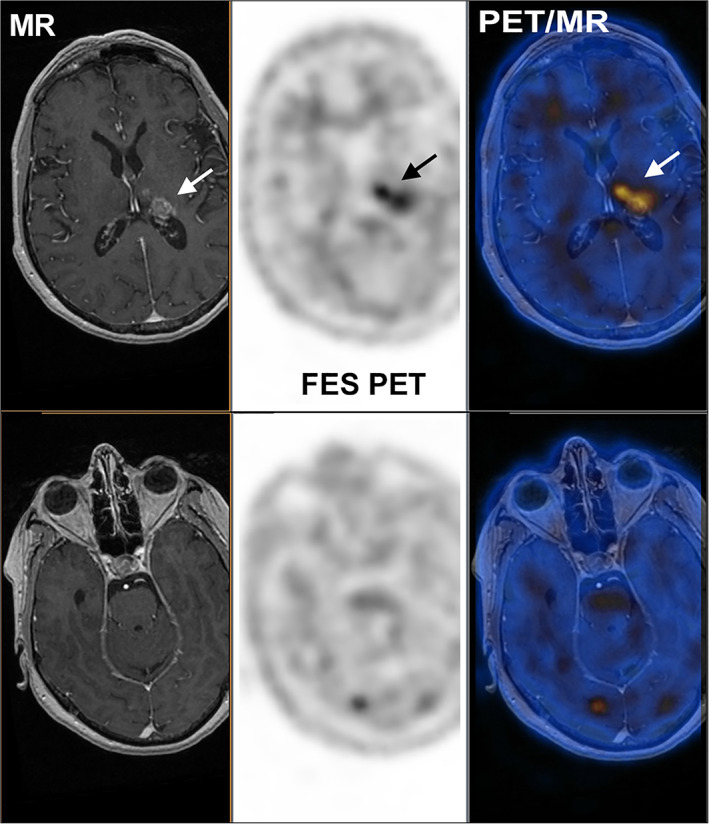
MR (left), 18F‐FES PET (center), and fused PET/MR (right) of a patient with brain metastases from a historically ER‐positive breast cancer. The upper panel illustrates a lesion evident on MR, whereas the lower panel illustrates an 18F‐FES‐positive lesion that was not readily apparent on MR.
Abbreviations: 18F‐FES, 16α‐18F‐fluoro‐17β‐estradiol; MR, magnetic resonance; PET, postitron emission tomography.
Figure 3.
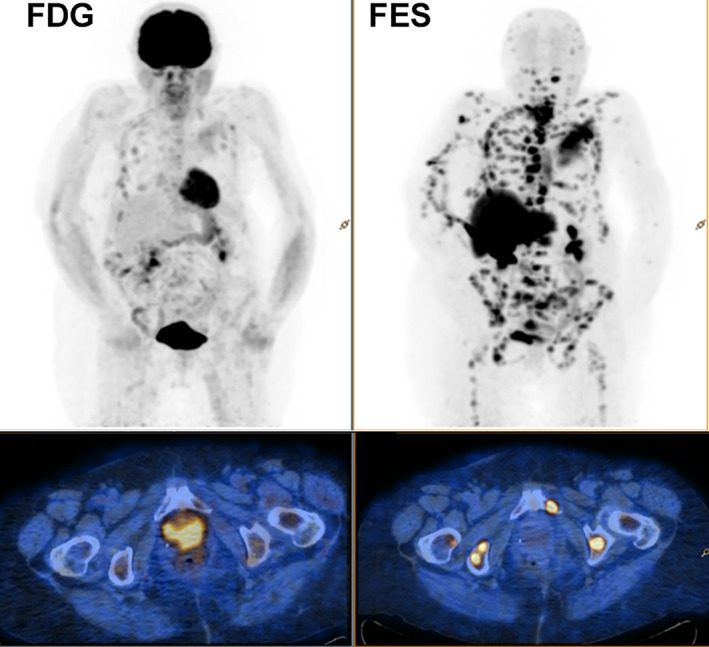
Transaxial pelvic (at the level of the pubic symphyses) positron emission tomography (PET)/computed tomography fusion images (18F‐FDG [left] and 18F‐FES [right]). The insets represent the whole‐body PET image, of a patient with metastatic lobular ductal carcinoma of the breast. Note that 18F‐FDG uptake in many lesions is less than corresponding 18F‐FES uptake.
Abbreviations: 18F‐FDG, 18F‐fluorodeoxyglucose; 18F‐FES, 16α‐18F‐fluoro‐17β‐estradiol.
Several studies have examined 18F‐FES PET as a predictor of response to endocrine monotherapy 18, 23, 38, 40, 44, 45, 49, 50 and in combination with other agents, including the CDK inhibitor palbociclib 51, 52, 53. Therefore, ER status by 18F‐FES PET has potential for assisting with therapy selection decisions for patients with ER+ MBC. Additional genomic and histologic assays of biopsy material or liquid biopsy may be a complement to 18F‐FES to address HER2, PI3K mutational status, or ESR1 mutations 54, 55; 18F‐FES may also help identify a site of interest for biopsy. 18F‐FDG PET assessment of a metabolic flare reaction to estradiol challenge has also been proposed for prediction of response to endocrine therapy 56. Ongoing multicenter trials with 18F‐FES PET and liquid and tissue biopsy correlates (NCT01957332; NCT02398773) will help refine therapy selection for future patients and help to identify targetable changes in tumor characteristics 10.
Suggestions for 18F‐FES PET Imaging Scanning Protocols
From the patient's and technologist's point of view, 18F‐FES PET has few restrictions. Fasting prior to injection and restricted physical activity following injection are not required. The recommended 18F‐FES dose is 111–222 MBq (3.0–6.0 mCi). Radiation exposure is similar to 18F‐FDG PET, for an effective dose of approximately 4.9 mSv (0.49 rem) after intravenous injection of 222 MBq in an adult weighing 70 kg. The highest absorbed dose is in the liver, where 18F‐FES is metabolized. However, mild hepatic dysfunction is unlikely to impact patient safety or tumor 18F‐FES uptake elsewhere 57. Other organs with relatively high radiation absorption in dosimetry studies in patients with breast cancer were the gallbladder and urinary bladder 26.
Tumor uptake will vary over time as the tracer is distributed and excreted 58. Sixty minutes after injection is accepted as a time of uptake equilibrium, with an acceptable range of 50–70 minutes 57. A scan from knee to skull vertex takes about 25 minutes (20–30 minutes) 57. The lack of 18F‐FES uptake by normal intracranial structures also enables determination of ER status in brain metastases, a difficult site for biopsy (Fig. 2). Acquisition in the caudocephalad direction is recommended 59 but is not essential.
Most patients receiving 18F‐FES PET will be women who are postmenopausal owing to age or prior therapy. Accumulated evidence also supports the use of 18F‐FES PET in premenopausal women 17, 60, as well as in men with ER‐positive breast cancer 61.
The primary special consideration for 18F‐FES PET imaging is washout of prior ER antagonists. Because 18F‐FES PET measures regional binding of estrogens to the estrogen receptor, therapies like tamoxifen 57 and fulvestrant 17, 62 that block ER will prevent uptake of 18F‐FES by estrogen receptors. An ER‐positive lesion subjected to successful blocking therapy will appear to be 18F‐FES negative. A washout period is indicated for tamoxifen and fulvestrant, to prevent false 18F‐FES‐negative imaging due to residual presence of these antagonist agents. Aromatase inhibitors block estrogen synthesis rather than estrogen receptors and do not interfere with 18F‐FES uptake 63. Thus, 18F‐FES PET may be performed to assess ER status during aromatase inhibitor therapy.
Image Analysis
Clinicians and nuclear medicine physicians will need to consolidate information from an 18F‐FES PET scan in order to use the scan to make clinical decisions. Primarily, the nuclear medicine physician will want to identify lesions and assess their ER uptake. Lesion identification can be driven by conventional imaging such as CT, magnetic resonance imaging, and bone scan. A qualitative assessment will declare identified lesions to be 18F‐FES positive if 18F‐FES uptake is above background. Minimal extratumoral uptake enables considerable confidence in classifying lesions as 18F‐FES positive or ‐negative.
Hepatobiliary clearance of 18F‐FES precludes assessment of liver lesions and makes interpretation of abdominal nodal involvement (particularly in nodal basins adjacent to the bowel) difficult. Nonetheless, careful image review particularly of the PET/CT may improve confidence (for example, by distinguishing uptake in a nodal basin evident on CT). Preliminary reader studies in multiple tumor sites have suggested very good agreement among nuclear medicine physician readers 34.
Intratumoral heterogeneity is of great research interest and is more readily assessed by molecular imaging than by tissue or blood assays. However, heterogeneity measures remain exploratory, because treatment decisions are at the patient level and unlike other tumors 64 there is no evidence that intratumoral heterogeneity has prognostic or predictive significance in metastatic breast cancer 65.
For patients with multiple lesions, a patient‐level summary of ER status is needed to assist treatment decisions, which will also incorporate prior treatment history, patient preferences, and other assays (such as HER2, PR, ESR1, or PIK3CA mutations). Although it seems sensible to assess a scan with any negative lesions as 18F‐FES negative, the recommendation by the Groningen University Medical Center group is to “describe the overall ER status of the metastases” 57. This recommendation is supported by observations of within‐patient variability and by use of overall ER status in the prediction of endocrine therapy success 19, 23. Additionally, partial volume effects may result in a false 18F‐FES‐negative assessment for a small or irregularly shaped lesion. In comparison with tissue assays, 18F‐FES has potential to overcome tissue sampling error and can better demonstrate the ER status of the body burden of disease; thus, it may provide a better prediction of clinical benefit 18, 23.
Although qualitative assessment is reliable for overall image interpretation, quantitative FES PET uptake measures may have benefit, especially in equivocal lesions or in determining change in ER status during or after therapy. These measures are highly dependent on scanning instrumentation and protocol and have not yet been subjected to protocol standardization 57, 59 or harmonization 66. There are several semiquantitative measures of tumor 18F‐FES uptake. The two most commonly reported are SUVmax—the standardized uptake value (SUV) for the hottest (highest uptake) pixel in the tumor—and SUVmean—the average SUV for all pixels in a “region of interest,” which could incorporate the entire tumor or be a standard size. A cutoff value of 1.5 as 18F‐FES positive has been suggested to apply to the SUVmean 30–60 minutes after acquisition 40, to SUVmax (60 minutes acquisition time) 36, to the geometric mean of SUVmax lesions as well as individual SUVmax 20, and to SUVmax with background correction 44. Validation using predefined semiquantitative measures of tumor 18F‐FES uptake is ongoing and requires replication of measures in independent cohorts.
In summary, a negative 18F‐FES PET is a compelling reason to move away from ER‐directed therapy in patients with ER+ MBC. If most lesions are found to be 18F‐FES positive, then ER‐directed therapy (with or without CDK inhibitors) is associated with clinical benefit, such as 6 months on therapy without progressive disease. On the other hand, if most lesions in overall patient disease burden are 18F‐FES negative by qualitative assessment, then ER‐directed therapy is likely to be ineffective.
Conclusion
This meta‐analysis demonstrates accuracy of 18F‐FES PET in the characterization of tumor ER status in patients with metastatic breast cancer from an ER‐positive primary breast cancer, validating the results of prior reports and extending the analysis to metastatic lesions. Tissue sampling limitations, intrapatient heterogeneity, and temporal changes in molecular markers make it likely that 18F‐FES PET will complement existing assays when clinically available in the near future. If most lesions are 18F‐FES negative in qualitative assessment, then ER‐directed therapy is likely to be ineffective. This manuscript reviews scanning protocol and image analysis considerations and describes clinical scenarios in which 18F‐FES PET may play a role in guiding therapy selection.
Author Contributions
Conception/design: Brenda F. Kurland, Jay R. Wiggins, Amandine Coche, Charlotte Fontan, Yann Bouvet, Peter Webner, Chaitanya Divgi, Hannah M. Linden
Collection and/or assembly of data: Brenda F. Kurland, Jay R. Wiggins, Amandine Coche, Charlotte Fontan, Chaitanya Divgi, Hannah M. Linden
Data analysis and interpretation: Brenda F. Kurland, Jay R. Wiggins, Amandine Coche, Charlotte Fontan, Yann Bouvet, Peter Webner, Chaitanya Divgi, Hannah M. Linden
Manuscript writing: Brenda F. Kurland, Jay R. Wiggins, Amandine Coche, Charlotte Fontan, Yann Bouvet, Peter Webner, Chaitanya Divgi, Hannah M. Linden
Final approval of manuscript: Brenda F. Kurland, Jay R. Wiggins, Amandine Coche, Charlotte Fontan, Yann Bouvet, Peter Webner, Chaitanya Divgi, Hannah M. Linden
Disclosures
Brenda F. Kurland: Zionexa (RF), Telix (C/A), ERT (E); Jay R. Wiggins: Zionexa, Pharmtrace, Medtrace (C/A); Amandine Coche: Cyclopharma/Zionexa (E); Charlotte Fontan: Cyclopharma/Zionexa US Corporation (E); Yann Bouvet: Cyclopharma/Zionexa (E); Peter Webner: Zionexa (OI); Chaitanya Divgi: Zionexa, Telix, Tomopath, Janssen, Curium, RefleXion (C/A); Hannah M. Linden: Zionexa (RF).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. DeSantis CE, Ma J, Gaudet MM et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019;69:438–451. [DOI] [PubMed] [Google Scholar]
- 2. Hammond ME, Hayes DF, Dowsett M et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Ommen‐Nijhof A, Konings IR, van Zeijl CJJ et al. Selecting the optimal position of CDK4/6 inhibitors in hormone receptor‐positive advanced breast cancer ‐ The SONIA study: Study protocol for a randomized controlled trial. BMC Cancer 2018;18:1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bardia A, Iafrate JA, Sundaresan T et al. Metastatic breast cancer with ESR1 mutation: Clinical management considerations from the molecular and precision medicine (MAP) tumor board at Massachusetts General Hospital. The Oncologist 2016;21:1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaklamani VG, Gradishar WJ. Endocrine therapy in the current management of postmenopausal estrogen receptor‐positive metastatic breast cancer. The Oncologist 2017;22:507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brufsky AM, Dickler MN. Estrogen receptor‐positive breast cancer: Exploiting signaling pathways implicated in endocrine resistance. The Oncologist 2018;23:528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoefnagel LD, van de Vijver MJ, van Slooten HJ et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res 2010;12:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aurilio G, Disalvatore D, Pruneri G et al. A meta‐analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer 2014;50:277–289. [DOI] [PubMed] [Google Scholar]
- 9. Sighoko D, Liu J, Hou N et al. Discordance in hormone receptor status among primary, metastatic, and second primary breast cancers: Biological difference or misclassification? The Oncologist 2014;19:592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kimbung S, Kovacs A, Danielsson A et al. Contrasting breast cancer molecular subtypes across serial tumor progression stages: Biological and prognostic implications. Oncotarget 2015;6:33306–33318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amir E, Clemons M, Purdie CA et al. Tissue confirmation of disease recurrence in breast cancer patients: Pooled analysis of multi‐centre, multi‐disciplinary prospective studies. Cancer Treat Rev 2012;38:708–714. [DOI] [PubMed] [Google Scholar]
- 12. Cardoso F, Costa A, Senkus E et al. 3rd ESO‐ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol 2017;28:16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Es SC, van der Vegt B, Bensch F et al. Decalcification of breast cancer bone metastases with EDTA does not affect ER, PR, and HER2 results. Am J Surg Pathol 2019;43:1355–1360. [DOI] [PubMed] [Google Scholar]
- 14. Lindstrom LS, Yau C, Czene K et al. Intratumor heterogeneity of the estrogen receptor and the long‐term risk of fatal breast cancer. J Natl Cancer Inst 2018;110:726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mintun MA, Welch MJ, Siegel BA et al. Breast cancer: PET imaging of estrogen receptors. Radiology 1988;169:45–48. [DOI] [PubMed] [Google Scholar]
- 16. Kelloff GJ, Krohn KA, Larson SM et al. The progress and promise of molecular imaging probes in oncologic drug development. Clin Cancer Res 2005;11:7967–7985. [DOI] [PubMed] [Google Scholar]
- 17. van Kruchten M, de Vries EGE, Brown M et al. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol 2013;14:e465–e475. [DOI] [PubMed] [Google Scholar]
- 18. Mortimer JE, Dehdashti F, Siegel BA et al. Positron emission tomography with 2‐[18F]Fluoro‐2‐deoxy‐D‐glucose and 16alpha‐[18F]fluoro‐17beta‐estradiol in breast cancer: Correlation with estrogen receptor status and response to systemic therapy. Clin Cancer Res 1996;2:933–939. [PubMed] [Google Scholar]
- 19. Kurland BF, Peterson LM, Lee JH et al. Between‐patient and within‐patient (site‐to‐site) variability in estrogen receptor binding, measured in vivo by 18F‐Fluoroestradiol PET. J Nucl Med 2011;52:1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nienhuis HH, van Kruchten M, Elias SG et al. (18)F‐Fluoroestradiol tumor uptake is heterogeneous and influenced by site of metastasis in breast cancer patients. J Nucl Med 2018;59:1212–1218. [DOI] [PubMed] [Google Scholar]
- 21. Chae SY, Ahn SH, Kim SB et al. Diagnostic accuracy and safety of 16alpha‐[(18)F]fluoro‐17beta‐oestradiol PET‐CT for the assessment of oestrogen receptor status in recurrent or metastatic lesions in patients with breast cancer: A prospective cohort study. Lancet Oncol 2019;20:546–555. [DOI] [PubMed] [Google Scholar]
- 22. Liu C, Gong C, Liu S et al. (18)F‐FES PET/CT influences the staging and management of patients with newly diagnosed estrogen receptor‐positive breast cancer: A retrospective comparative study with (18)F‐FDG PET/CT. The Oncologist 2019;24:e1277–e1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kurland BF, Peterson LM, Lee JH et al. Estrogen receptor binding (18F‐FES PET) and glycolytic activity (18F‐FDG PET) predict progression‐free survival on endocrine therapy in patients with ER+ breast cancer. Clin Cancer Res 2017;23:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Kruchten M, Glaudemans AW, de Vries EF et al. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med 2012;53:182–190. [DOI] [PubMed] [Google Scholar]
- 25. Evangelista L, Guarneri V, Conte PF. 18F‐Fluoroestradiol positron emission tomography in breast cancer patients: Systematic review of the literature & meta‐analysis. Curr Radiopharm 2016;9:244–257. [DOI] [PubMed] [Google Scholar]
- 26. Mankoff DA, Peterson LM, Tewson TJ et al. [18F]fluoroestradiol radiation dosimetry in human PET studies. J Nucl Med 2001;42:679–684. [PubMed] [Google Scholar]
- 27. Macaskill P, Gatsonis C, Deeks JJ et al. Chapter 10: Analysing and presenting results In: Deeks JJ, Bossuyt PM, Gatsonis C, eds. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2010. Available at http://srdta.cochrane.org/ [Google Scholar]
- 28. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 29. Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Stat Med 2001;20:2865–2884. [DOI] [PubMed] [Google Scholar]
- 30. Reitsma JB, Glas AS, Rutjes AW et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982–990. [DOI] [PubMed] [Google Scholar]
- 31. Doebler P, Holling H, Bohning D. A mixed model approach to meta‐analysis of diagnostic studies with binary test outcome. Psychol Methods 2012;17:418–436. [DOI] [PubMed] [Google Scholar]
- 32. Gupta M, Datta A, Choudhury PS et al. Can (18)F‐Fluoroestradiol positron emission tomography become a new imaging standard in the estrogen receptor‐positive breast cancer patient: A prospective comparative study with (18)F‐Fluorodeoxyglucose positron emission tomography? World J Nucl Med 2017;16:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peterson LM, Mankoff DA, Lawton T et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F‐fluoroestradiol. J Nucl Med 2008;49:367–374. [DOI] [PubMed] [Google Scholar]
- 34. Peterson LM, Kurland BF, Schubert EK et al. A phase 2 study of 16alpha‐[18F]‐fluoro‐17beta‐estradiol positron emission tomography (FES‐PET) as a marker of hormone sensitivity in metastatic breast cancer (MBC). Mol Imaging Biol 2014;16:431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Venema CM, Mammatas LH, Schroder CP et al. Androgen and estrogen receptor imaging in metastatic breast cancer patients as a surrogate for tissue biopsies. J Nucl Med 2017;58:1906–1912. [DOI] [PubMed] [Google Scholar]
- 36. Yang Z, Sun Y, Xue J et al. Can positron emission tomography/computed tomography with the dual tracers fluorine‐18 fluoroestradiol and fluorodeoxyglucose predict neoadjuvant chemotherapy response of breast cancer?–A pilot study. PLoS One 2013;8:e78192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gemignani ML, Patil S, Seshan VE et al. Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer. J Nucl Med 2013;54:1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chae SY, Kim SB, Ahn SH et al. A randomized feasibility study of (18)F‐Fluoroestradiol PET to predict pathologic response to neoadjuvant therapy in estrogen receptor‐rich postmenopausal breast cancer. J Nucl Med 2017;58:563–568. [DOI] [PubMed] [Google Scholar]
- 39. Dehdashti F, Mortimer JE, Siegel BA et al. Positron tomographic assessment of estrogen receptors in breast cancer: Comparison with FDG‐PET and in vitro receptor assays. J Nucl Med 1995;36:1766–1774. [PubMed] [Google Scholar]
- 40. Linden HM, Stekhova SA, Link JM et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol 2006;24:2793–2799. [DOI] [PubMed] [Google Scholar]
- 41. Goetz MP, Gradishar WJ, Anderson BO et al. NCCN guidelines insights: Breast cancer, version 3.2018. J Natl Compr Canc Netw 2019;17:118–126. [DOI] [PubMed] [Google Scholar]
- 42. Linden HM, Peterson LM, Fowler AM. Clinical potential of estrogen and progesterone receptor imaging. PET Clin 2018;13:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liao GJ, Clark AS, Schubert EK et al. 18F‐Fluoroestradiol PET: Current status and potential future clinical applications. J Nucl Med 2016;57:1269–1275. [DOI] [PubMed] [Google Scholar]
- 44. Wang Y, Ayres KL, Goldman DA et al. (18)F‐Fluoroestradiol PET/CT measurement of estrogen receptor suppression during a Phase I trial of the novel estrogen receptor‐targeted therapeutic GDC‐0810: Using an imaging biomarker to guide drug dosage in subsequent trials. Clin Cancer Res 2017;23:3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin FI, Gonzalez EM, Kummar S et al. Utility of (18)F‐fluoroestradiol ((18)F‐FES) PET/CT imaging as a pharmacodynamic marker in patients with refractory estrogen receptor‐positive solid tumors receiving Z‐endoxifen therapy. Eur J Nucl Med Mol Imaging 2017;44:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jia X, Li C, Li L et al. Neddylation inactivation facilitates FOXO3a nuclear export to suppress estrogen receptor transcription and improve fulvestrant sensitivity. Clin Cancer Res 2019;25:3658–3672. [DOI] [PubMed] [Google Scholar]
- 47. Jacobs C, Clemons M, Addison C et al. Issues affecting the loco‐regional and systemic management of patients with invasive lobular carcinoma of the breast. Breast J 2016;22:45–53. [DOI] [PubMed] [Google Scholar]
- 48. Hogan MP, Goldman DA, Dashevsky B et al. Comparison of 18F‐FDG PET/CT for systemic staging of newly diagnosed invasive lobular carcinoma versus invasive ductal carcinoma. J Nucl Med 2015;56:1674–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mortimer JE, Dehdashti F, Siegel BA et al. Metabolic flare: Indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol 2001;19:2797–2803. [DOI] [PubMed] [Google Scholar]
- 50. van Kruchten M, Glaudemans A, de Vries EFJ et al. Positron emission tomography of tumour [(18)F]fluoroestradiol uptake in patients with acquired hormone‐resistant metastatic breast cancer prior to oestradiol therapy. Eur J Nucl Med Mol Imaging 2015;42:1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Park JH, Kang MJ, Ahn JH et al. Phase II trial of neoadjuvant letrozole and lapatinib in Asian postmenopausal women with estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2)‐positive breast cancer [Neo‐ALL‐IN]: Highlighting the TILs, ER expressional change after neoadjuvant treatment, and FES‐PET as potential significant biomarkers. Cancer Chemother Pharmacol 2016;78:685–695. [DOI] [PubMed] [Google Scholar]
- 52. Gong C, Yang Z, Sun Y et al. A preliminary study of (18)F‐FES PET/CT in predicting metastatic breast cancer in patients receiving docetaxel or fulvestrant with docetaxel. Sci Rep 2017;7:6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boers J, Venema CM, de Vries EFJ et al. Molecular imaging to identify patients with metastatic breast cancer who benefit from endocrine treatment combined with cyclin‐dependent kinase inhibition. Eur J Cancer 2019;126:11–20. [DOI] [PubMed] [Google Scholar]
- 54. Coombes RC, Page K, Salari R et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res 2019;25:4255–4263. [DOI] [PubMed] [Google Scholar]
- 55. Wang P, Bahreini A, Gyanchandani R et al. Sensitive detection of mono‐ and polyclonal ESR1 mutations in primary tumors, metastatic lesions, and cell‐free DNA of breast cancer patients. Clin Cancer Res 2016;22:1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dehdashti F, Mortimer JE, Trinkaus K et al. PET‐based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen‐receptor‐positive breast cancer. Breast Cancer Res Treat 2009;113:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Venema CM, Apollonio G, Hospers GA et al. Recommendations and technical aspects of 16alpha‐[18F]fluoro‐17beta‐estradiol PET to image the estrogen receptor in vivo: The Groningen experience. Clin Nucl Med 2016;41:844–851. [DOI] [PubMed] [Google Scholar]
- 58. Mankoff DA, Tewson TJ, Eary JF. Analysis of blood clearance and labeled metabolites for the estrogen receptor tracer [F‐18]‐16 alpha‐fluoroestradiol (FES). Nucl Med Biol 1997;24:341–348. [DOI] [PubMed] [Google Scholar]
- 59. Graham MM, Wahl RL, Hoffman JM et al. Summary of the UPICT protocol for 18F‐FDG PET/CT imaging in oncology clinical trials. J Nucl Med 2015;56:955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Peterson LM, Kurland BF, Link JM et al. Factors influencing the uptake of 18F‐fluoroestradiol in patients with estrogen receptor positive breast cancer. Nucl Med Biol 2011;38:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peterson L, Manohar P, Wu V et al. F‐18‐Fluoroestradiol (FES) and F‐18‐Fluorodeoxyglucose (FDG) PET imaging in male breast cancer. J Nucl Med 2018;59:54. [Google Scholar]
- 62. Robertson JF, Odling‐Smee W, Holcombe C et al. Pharmacokinetics of a single dose of fulvestrant prolonged‐release intramuscular injection in postmenopausal women awaiting surgery for primary breast cancer. Clin Ther 2003;25:1440–1452. [DOI] [PubMed] [Google Scholar]
- 63. Linden HM, Kurland BF, Peterson LM et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res 2011;17:4799–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eary JF, O'Sullivan F, O'Sullivan J et al. Spatial heterogeneity in sarcoma 18F‐FDG uptake as a predictor of patient outcome. J Nucl Med 2008;49:1973–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang Z, Sun Y, Xu X et al. The assessment of estrogen receptor status and its intratumoral heterogeneity in patients with breast cancer by using 18F‐fluoroestradiol PET/CT. Clin Nucl Med 2017;42:421–427. [DOI] [PubMed] [Google Scholar]
- 66. Aide N, Lasnon C, Veit‐Haibach P et al. EANM/EARL harmonization strategies in PET quantification: From daily practice to multicentre oncological studies. Eur J Nucl Med Mol Imaging 2017;44:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information.


