Abstract
Infusion centres are a central part in the management of patients with inflammatory bowel disease [IBD] and could be a source of transmission of SARS-COV-2. Here we aimed to develop global guidance for best practices of infusion centres for IBD patients and to determine the impact of the COVID-19 pandemic on these centres. Under the auspices of the International Organization for the Study of Inflammatory Bowel Disease [IOIBD], a task force [TF] was formed, an online survey was developed to query infusion centre protocols during COVID-19, and recommendations were made, based on TF experience and opinion. Recommendations focus mainly on patients screening, infusion centres re-organization, personnel protection, and protocol modifications such as shortening infusion duration or replacing it with subcutaneous alternatives. Implementing these recommendations will hopefully reduce exposure of both IBD patients and care givers to SARS-COV-2 and improve the function and safety of infusion centres during the COVID-19 pandemic as well as potential future threats.
Keywords: COVID, biologics, infusion centers
1. Introduction
The global emergence of SARS-COV-2 virus and the COVID-19 pandemic has led to rethinking how we deliver medical service. This has affected many patient populations including those with inflammatory bowel diseases [IBD].1,2 Early in the pandemic there were concerns regarding how vulnerable IBD patients may have been given their disease state and the medications they are exposed to. Over time, there has been a greater understanding of conditions that promoted the spread of the virus and concurrently measures that could decrease transmission and protect both patients and healthcare providers [HCPs].
Infusion centres have become central to the way care is delivered to patients with IBD. Globally, these centres may be part of hospitals, private offices or free standing. The global standards and operating practices may vary. However, it was quickly identified that these centres could be a source of transmission of SARS-COV-2 and that guidance to decrease the risk to patients and HCPs was paramount. Therefore, under the auspices of the International Organization for the Study of Inflammatory Bowel Disease [IOIBD] a task force [TF] was formed to develop global guidance for best practices of infusion centres caring for IBD patients and to determine the impact of the COVID-19 pandemic on these centres.
The specific assignments were to point out the challenges and suggest how they may be addressed, to study the actions taken in various areas in the world and various health systems, and to follow up the effects of modified infusion centre protocols, if modifications took place. Beyond relevance to the COVID-19 pandemic and expected future waves until a vaccine is found, the insights and recommendations may be relevant to future pandemics. This is specifically meaningful as widespread infectious diseases may be one of the 21st century’s global challenges.
2. Methods
Six TF members were appointed [I.D., J.O.L., C.O., G.K., M.T.A., R.P.], representing Europe, North America and Israel. A survey was developed to query infusion centre protocols during COVID-19. A total of 36 IOIBD members replied. The data are presented graphically.
After identifying the key challenges, recommendations for infusion centre operation were made, based on TF experience and opinion. These were discussed in two calls with IOIBD membership [n = 89], as well as additional IBD experts. Recommendations were then modified according to comments made, and uploaded to the IOIBD site in order to provide immediate, practical, comprehensive recommendations, until evidence-based ones can be generated [Table 1—abbreviated recommendations].
Table 1.
Main recommendations for infusion center guidance regarding COVID-19 and IBD
| Category | Main recommendations infusion center guidance re COVID-19 and IBD date: May 6, 2020 |
|---|---|
| Patients | 1. Pre-screen for symptoms/exposure 24–48 h before scheduled infusion 2. Self-isolate if respiratory symptoms, anosmia, abrupt change in gastrointestinal symptoms 3. If positive SARS-COV-2 infection, schedule infusion only after a negative swab, or 2 weeks after symptoms resolve 4. Patients must come unaccompanied when possible |
| Clinic | 1. Staff pooling 2. Separate from other departments/patients 3. Rescreen patients and temperature check at check-in 4. Social distancing and hygiene: 2 m between staff/patients, between patients; clean chairs between patients 5. Personnel protection: gloves and surgical masks. Where applicable full personal protection equipment including N95 masks and waterproof gown should be readily available 6. Patients should use nose–mouth masks 7. To limit contact time in clinic consider: [a] Rapid infusion protocols [b] Switching any pre-meds to injectable or oral [c] Take pre-meds at home as required 8. Update contact details at each visit to facilitate contact tracing in event a patient is/becomes SARS-COV2-positive |
| Personnel | 1. Daily personal symptom assessment, health statement and temperature check 2. Glove removal and hand washing between patient encounters 3. Log off where personnel is working/has worked if individual works in multiple locations |
| General | 1. Infusion clinic—an essential service 2. Prioritize SARS-COV2 tests for personnel and patients 3. Postpone/avoid unnecessary laboratory/therapeutic drug monitoring tests 4. Provide COVID-IBD-related information [no hard copies] 5. Provide access to guidance/help/support lines 6. Extend operating hours to accommodate infusions 7. Home infusions are discouraged due to safety issues, medical and logistic efficiency https://www.ioibd.org/ioibd-update-on-covid19-for-patients-with-crohns-disease-and-ulcerative-colitis/ |
These recommendations were divided into four categories:
Patients
Personnel
Infusion centre operations
General considerations
3. Results and Guidance
3.1. Survey of IBD experts highlights changes in infusion centre dynamics since the start of the COVID-19 pandemic
A survey was disseminated to the IOIBD membership, with specific questions related to modifications of infusion centres in their own sites. The results of the survey were discussed in an international call with 89 IOIBD members. Based on comments made, another round of the survey was distributed. The summary of both rounds, with 36 responses [representing a 69% response rate out of IOIBD active gastroenterologists], are presented.
The responders represent a global practice, with 11 from North America [eight USA, three Canada], 17 Europe, two Israel, and six from Hong Kong, New Zealand, Brazil, China and India.
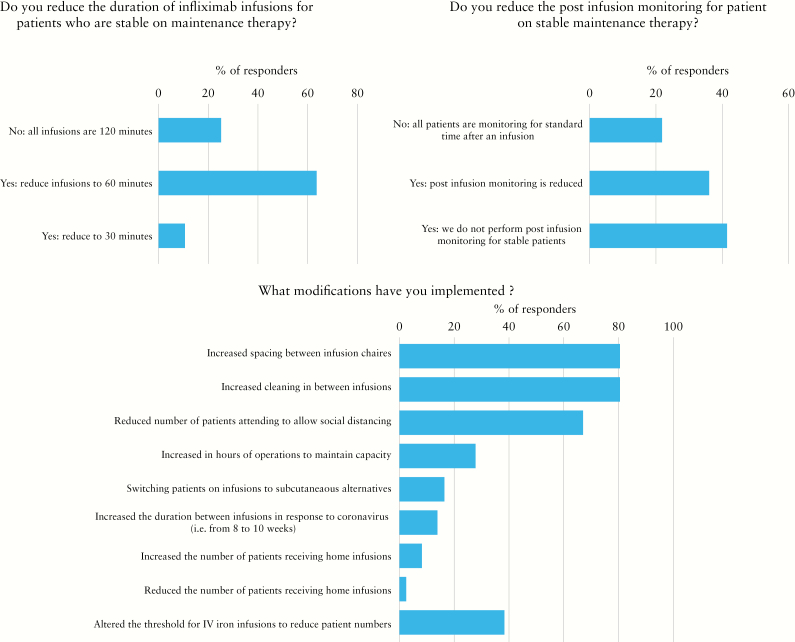
In the pre-COVID-19 era, approximately half [19/36, 53%] the infusion centres accepted patients from different specialties rather than patients with IBD alone, and were staffed by general nurses [20/36, 55.6%], providing over 50 infusions/week in 19/36 [55.3%]. In only 6/36 [16.6%] centres, all from the USA, more than 10% of infusions were home infusions. In the vast majority of centres, evaluation of clinical disease activity, laboratory tests and therapeutic drug monitoring were performed routinely. In 23/36 [64%], the duration of infliximab infusion was shortened to 60 minutes or less, and in 15/36 [41/7%] centres, those patients would be discharged immediately after infusion, without further monitoring in patients who are clinically stable.
During the COVID19 pandemic, 5/36 centres had to change location to a site remote from the acute care facility; this did not require a change in staffing. However, in 12/36 [33.3%] the capacity of the centre was reduced, without significant change of the case mix. Interestingly, while all centres screened patients on site for signs/symptoms of SARS-COV-2 infection and for a history of exposure, previsit screening calls were performed in 70%. Multiple modifications have taken place during the COVID-19 pandemic. Those changes related to several aspects of infusion centre operations, logistics and protocols. Specifically, 29/36 [80.6%] increased spacing between patients and increased cleaning between infusions. In 24/36 [66.7%] there was reduced number of patients in the centre to allow for social distancing, and for this reason 10/36 [27.8%] increased the hours of operation [Figure 1].
Figure 1.
Changes in infusion centre dynamics since the start of the COVID-19 pandemic.
Importantly, treatment protocol modifications with potential clinical implications were made. Those included changing intravenous to subcutaneous preparations where applicable [i.e. subcutaneous infliximab instead of intravenous] in 6/36 [16.7%] of centres, and increasing intervals between a patient’s infusions in 5/36 [14%]. Changes were also made to infusion protocols; in 5/33 [15.2%] centres, stable patients had shorter infusion durations, post-infusion monitoring was reduced, and premedication was switched to the oral route, where applicable. In almost all centres there has always been a telephone help line and email address for patients. About half the infusion centres produced patient information sheets in response to the COVID-19 pandemic. The estimated cancellation of infusions was less than 10% in the majority of centres [26/36, 72%], but at the time the survey was sent, no report of disease exacerbation due to these changes was noted.
4. Guidance for Patients Attending Infusion Centers for the Management of their IBD
Based on the survey results and our TF deliberations we have generated guidance for infusions of IBD-related medications. The guidelines are based on survey results and expert opinion. We have intentionally made these broad to encompass differences in practice settings internationally.
Patients should be contacted and screened 24–48 h prior to an infusion appointment for any symptoms that may be consistent with COVID-19. This includes sore throat, cough, fever, shortness of breath or difficulty breathing. Atypical symptoms of loss of smell/taste as well as any relevant contact history should also be reviewed. Any abrupt change in intestinal symptoms should be captured. A change in GI symptoms should be discussed with the patient’s gastroenterologist.
Patients exhibiting any symptoms suggestive of COVID-19 should be encouraged to self-isolate, referred for SARS-COV-2 testing if available and their infusion cancelled. A follow-up call in 72 h is recommended to review clinical status. If symptoms persist, the patient’s appointment should be delayed at least an additional 14 days and should only proceed on the advice of a physician.
If a patient has had documented infection with SARS-COV-2, the patient should return to the centre only after the patient has had two consecutive negative swabs if resources for testing permit. Antibody testing may also be informative, with IgG antibodies against the virus suggesting immunity. Otherwise the patient may return to the centre 2 weeks after symptoms resolve.
Patients should come unaccompanied when possible to reduce the number of individuals entering the infusion unit.
Patients should wear a nose–mouth mask.
5. Infusion Centre Operations
Infusion centres and their administration may be different across jurisdictions. They may be hospital-based, community-based or in private practice. Often, the services offered may be shared across multiple disciplines.
To ease and simplify operations, a central schedule and pooling of resources [including staff] should be considered. This should include cross-specialty training of infusion nurses so that they are comfortable with different protocols [where possible protocols for each drug should be reviewed so that they are the same across specialities].
If the infusion centre is within/next to a medical admission department or active gastrointestinal department, an alternative location should be sought, where possible, to enable minimal exposure for IBD patients visiting the infusion centre to patients potentially infected with SARS-COV-2.
Patients attending the centre should be re-screened for symptoms and have a temperature check before entering the centre. Patients exhibiting symptoms and/or fever should be isolated away from the main infusion area, wear a surgical mask, and recommended to self-isolate and referred for SARS-COV-2 testing if available. The infusion should be rescheduled consistent with recommendations above.
-
Infusion centres should re-organize their layout to comply with recommendations of personal hygiene and social distancing. This includes the following:
a. a minimum of 2 m distance during check-in or check-out
b. a minimum of 2 m distance between infusion chairs or beds
c. a minimum of 2 m distance between chairs/stations for laboratory draws
d. chairs should be cleaned between patients, and/or disposable covers used.
Personnel should use surgical masks and gloves for protection. Where applicable, full personal protective equipment [PPE] should be readily available [including gloves, gowns, ±eye protection and surgical mask].
Personnel should be appropriately trained with the donning and doffing procedures of PPE.
Personnel are required to adhere to proper hand hygiene before and between any patient contact. This includes the removal of gloves/gowns and handwashing between patient encounters.
When possible, patients receiving infliximab infusions should be transitioned to a shorter rapid [30–60 min] infusion protocol to limit time within the clinic and increase clinic capacity.
Any pre-medication that is necessary should be converted to oral administration or subcutaneous medication once again to limit time in the clinic.
Consider changing infusion into subcutaneous injections of the same drug, where applicable [e.g. subcutaneous infliximab, subcutaneous vedolizumab].
In patients with adequate drug levels, consider increasing intervals between infusions, where applicable.
A mechanism should exist for reporting and allowing for contact tracing of any patient or personnel who test positive for COVID-19. Any contacts should be isolated for a period of 10–14 days according to regional guidelines.
6. Personnel in Infusion Centres
Personnel working in infusion centres should closely monitor themselves for any symptoms that may reflect infection with SARS-COV-2. Personnel should not be attending work if they have any symptoms suggestive of COVID-19 regardless of how minor. A self-isolation period of 14 days or approval by the local authorities [infectious diseases/occupational health] should be mandatory before returning to work.
Daily temperature checks should be done for all employees entering into the infusion centre area before starting a shift.
-
Self-health forms signed by personnel should be considered [where not obligatory], including the date and a declaration that:
a. the person does not have any symptoms
b. the person has a normal temperature.
7. General
All infusion services whether hospital-based, outpatient or private centres are deemed ‘essential services’. Thus, they should keep operating during any lockdown.
Prioritize SARS-COV-2 tests for personnel and symptomatic patients to avoid [a] isolation of all centre personnel and patients, and [b] a 14-day delay in a specific patient’s scheduled infusions due to the need to self-isolate.
Consider merging infusion facilities with other [rheumatology, dermatology, oncology] infusion centres to increase resilience in the service.
Physician/nurse in charge should go over patients’ routine testing. Unnecessary laboratory tests and/or therapeutic drug monitoring should be postponed to avoid burden on laboratories.
Information/education should be provided by the IBD or the infusion centre. It should NOT include brochures or handouts. Rather, links to suggested websites or posts should be advertised.
A hotline to respond to patients’ questions should be available by e-mail and phone [operating hours: work hours].
Extending hours of the infusion centre should be considered to allow for adequate social distancing and at the same time allow for continued capacity.
-
A change to home infusions should not be routinely recommended due to:
a. inability to properly screen personnel and patients
b. the increased possibility of spreading infection to patients and their households
c. the difficulty with tracing and tracking
d. difficulty to ensure personnel training
e. inefficient use of personnel in times of extreme shortage.
This provides guidance for what is thought to be the best practices during the COVID-19 pandemic. The TF recognizes that local, regional, and national differences and disparities exist. Therefore, this guidance should be integrated with local, regional and national guidelines.
8. Discussion
During this COVID-19 pandemic, HCP and health systems have had to address unprecedented challenges. In IBD, the operation of infusion centres required specific attention due to several distinct features. First, the rapid spread of the SARS-COV-2 pandemic across the world and within specific countries has not left time for systematic evidence-based changes to established protocols and recommendations. Patients with IBD receiving intravenous biologics are dependent on timely scheduled infusions, as a delay of even a few days may affect treatment efficacy and immunogenicity.3–5 In addition, patients on immune-modifying medication including biological therapy are more susceptible to infection. The safety profile of specific biologics is relevant because vedolizumab6/ustekinumab7 may be safer than infliximab. However, during the initial stages of the COVID-19 pandemic there was no information regarding viral spread; thus all biologics might have been considered risk factors for susceptibility to infection and/or worse COVID-19 outcomes. Preliminary outcomes from the SECURE-IBD registry suggest that tumour necrosis factor antagonists do not appear to be associated with severe COVID-19.8 Other issues that made the development of infusion centre guidance critical is that medical teams are considered a population at risk for infection with SARS-COV-2.9 Thus, the teams operating an infusion centre are also at risk. Therefore, there is concern that staff will serve as conveyors of infection to their susceptible patients, or vice versa, that patients will ‘import’ infection from their environment to the infusion centre. In some overloaded health systems, staff members were often diverted to emergency care facilities, thus decreasing resources in infusion centres.
The IOIBD is composed of experts from around the globe who treat patients with IBD. Importantly, the centres represented in the survey are all tertiary academic referal centres, infusing thousands of patients per week combined. A rapid response to the challenges related to COVID-19 was deemed to be of the highest importance by the organization in order to ensure patient and HCP safety. In a series of meetings [virtual] and surveys conducted between March 20 and April 25, 2020, practical guidelines were drafted and made available at the IOIBD.org website. Those related to infusion centre operations are presented here [Table 1]. It was soon recognized that the COVID-19 pandemic may have a vast effect not only on current operations but on future patient care and clinical outcomes. Thus, in addition to providing practical guidelines, a survey to assess the immediate impact of the pandemic was conducted. One immediate surprise was that only 70% of centres performed previsit screening, highlighting the importance of practical guidelines.
A third of the centres saw a reduction in capacity despite no significant changes in the case mix. This may have been due to several reasons. Most notably, the majority of the infusion centres [80.6%] increased spacing between patients and implemented more stringent cleaning protocols between infusions. In addition, 14% reported increasing the time between infusions. This necessitated a reduction in patient volume and an increase in operation hours to allow physical distancing. Operationally, this may lead to specific allocation of resources such as ensuring staff availability after hours, payment of over-time and re-scheduling efforts.
In an effort to limit patient attendance at infusion centres, several changes were implemented, specifically, a change of intravenous drugs to subcutaneous ones where applicable. Such modification was seen in 16.7% of centres. Although IBD clinicians supported the concept of using subcutaneous versions of intravenous biologics [i.e. infliximab and vedolizumab subcutaneously], these are not available in all countries. To this end the IOIBD appealed to drug approval agencies around the world, requesting expedited review of subcutaneous substitution of intravenous drugs.
The IOIBD will follow up with further surveys to assess the consequences that result from changes in infusion centre protocols. Such implications were indeed noticed. Addressing patient anxiety is of utmost importance in times of uncertainty. The use of telephone helplines, web-based fora and non-printed information is advisable, and was performed by most centres. Whether post-traumatic effects will also affect IBD patients’ health remains to be determined in further studies.
At the time of writing, COVID-19 seems to be contained in most areas of the world. At this point, it is important to assess specific outcomes in patients with IBD treated with biologics, and to note which infusion centre modifications had a negative or positive consequence. An example may be the use of home infusions which were implemented in several American centres [10%], but not utilized in other countries. The general recommendation of the IOIBD was to refrain from diverting patients to receive home infusions. This was mainly due to safety concerns for patients and HCPs, as well as the need to avoid sending trained staff to patients’ homes [or worse—having untrained staff perform infusions of biologics]. Follow up of clinical implications is one of the important research gaps for future studies.
There are several areas to consider for future pandemics, including development of subcutaneous substitutes for existing intravenous drugs; providing operation standards for infusion centres, including size, staffing positions, equipment and sanitation requirements; redefining treatment protocols and follow up; and addressing patient and staff psycosocial sequaelae.
In summary, widespread infections may be one of the risks of the 21st century. While the challenges of the COVID-19 pandemic have been unprecedented, a second wave is expected by many experts before a vaccine is found. Furthermore, other similar challenges may unfortunately occur in the future. Thus, being more prepared, and evaluating outcomes of the current event, may be of particular importance. The current paper represents one attempt at outlining a universal protocol.
Supplementary Material
Funding
None.
Conflict of Interest
I.D. has served as a consultant/speaker for: Janssen, Abbvie, Takeda, Pfizer, Genentech/Roche, Arena, Neopharm, Gilead, Celltrion, Celgene/BMS, Rafa Laboratories, Ferring, Falk Pharma, MSD, Protalix, Nestle, DSM, Abbott, Medtronic/Given Imaging, Sublimity, Sandoz. I.D. has received research grant support from Pfizer, Altman research. R.P. has served as a consultant/speaker for: AI4GI, AbbVie, Arena Pharmaceuticals Amgen, Atlantic Healthcare, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Cosmo Technologies, Eagle, Eisai Medical Research, Elan, EnGene, Eli Lilly, Ferring,Genentech, Sanofi Genzyme, Gilead, Given Imaging, GlaxoSmithKline, Janssen, Merck & Co., Merck Research Laboratories, MerckSerono, Novo Nordisk, PDL Biopharma, Pfizer, Robarts Clinical Trials, Prometheus Laboratories, Protagonist, Receptos, Salix, Sandoz, Shire Pharmaceuticals, Sigmoid Pharma, Sublimity, Takeda, Theradvance. G.G.K. has received honoraria for speaking or consultancy from Abbvie, Janssen, Pfizer, and Takeda. He has received research support from Ferring, Janssen, Abbvie, GlaxoSmith Kline, Merck and Shire. G.G.K. has been a consultant for Gilead. G.G.K. shares ownership of a patent: TREATMENT OF INFLAMMATORY DISORDERS, AUTOIMMUNE DISEASE, AND PBC. UTI Limited Partnership, assignee. Patent WO2019046959A1. PCT/CA2018/051098. 7 Sept. 2018. C.O. has consulted for Shire and Pfizer. J.O.L. has served as consultant and an advisory board participant for: AbbVie, Alergan [Warner Chilcott], Atlantic Healthcare, Celgene, Celtrion, Ferring, Gilead, GSK, Janssen, Lilly, MSD, Napp, Pfizer, Shire, Takeda and Vifor Pharma. Received speaker fees and sponsorship for academic meetings: from AbbVie, Alergan [Warner Chilcott], Ferring, Janssen, MSD, Napp, Norgine, Pfizer, Shire, Tillott’s, Takeda. Received investigator-led research grants from Abbvie, Gilead, Pfizer, Shire and Takeda. M.T.A. has consulted for Janssen, Prometheus Bioscience, Takeda, Focus Medical Communications, Pfizer, Boehringer Ingelheim Pharmaceuticals, Gilead, Imedex, Cornerstone Health, Inc, Landos Biophama, UCB Biopharma SRL, Eli Lilly and Cosmo Biopharma. M.T.A. has received grant support from Prometheus Bioscience, Takeda and Pfizer.
Author Contributions
I.D., M.T.A. and R.P. envisioned and designed the study, J.O.L. drafted the survey, I.D. and R.P. analysed data and drafted the manuscript. All authors reviewed and approved the study.
References
- 1. Mao R, Liang J, Shen J, et al.. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol 2020. doi: 10.1016/S2468-1253(20)30076-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danese S, Cecconi M, Spinelli A. Management of IBD during the COVID-19 outbreak: resetting clinical priorities. Nat Rev Gastroenterol Hepatol 2020. doi: 10.1038/s41575-020-0294-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colombel JF, Rutgeerts PJ, Sandborn WJ, et al.. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014;12:414–22.e5. [DOI] [PubMed] [Google Scholar]
- 4. Colombel JF, Reinisch W, Mantzaris GJ, et al.. Randomised clinical trial: deep remission in biologic and immunomodulator naïve patients with Crohn’s disease–a SONIC post hoc analysis. Aliment Pharmacol Ther 2015. doi: 10.1111/apt.13139 [DOI] [PubMed] [Google Scholar]
- 5. Rubin DT, Uluscu O, Sederman R. Response to biologic therapy in Crohn’s disease is improved with early treatment: an analysis of health claims data. Inflamm Bowel Dis 2012;18:2225–31. [DOI] [PubMed] [Google Scholar]
- 6. Colombel JF, Sands BE, Rutgeerts P, et al.. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017;66:839–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanauer SB, Sandborn WJ, Feagan BG, et al.. IM-UNITI: three-year efficacy, safety, and immunogenicity of ustekinumab treatment of Crohn’s disease. J Crohn’s Colitis 2019;14:23–32. [DOI] [PubMed] [Google Scholar]
- 8. Brenner EJ, Ungaro RC, Gearry RB, et al.. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology 2020. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang D, Xu H, Rebaza A, Sharma L, Dela Cruz CS. Protecting health-care workers from subclinical coronavirus infection. Lancet Respir Med 2020. doi: 10.1016/S2213-2600(20)30066-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.