Abstract
Background
Targeted therapies and checkpoint blockade therapy (CBT) have shown efficacy for patients with Hodgkin lymphoma (HL) in the relapsed and refractory (R/R) setting, but once discontinued owing to progression or side effects, it is unclear how successful further therapies will be. Moreover, there are no data on optimal sequencing of these treatments with standard therapies and other novel agents. In a multicenter, retrospective analysis, we investigated whether exposure to CBT could sensitize HL to subsequent therapy.
Materials and Methods
Seventeen centers across the U.S. and Canada retrospectively queried medical records for eligible patients. The primary aim was to evaluate the overall response rate (ORR) to post‐CBT treatment using the Lugano criteria. Secondary aims included progression‐free survival (PFS), duration of response, and overall survival (OS).
Results
Eighty‐one patients were included. Seventy‐two percent had stage III–IV disease, and the population was heavily pretreated with a median of four therapies before CBT. Most patients (65%) discontinued CBT owing to progression. The ORR to post‐CBT therapy was 62%, with a median PFS of 6.3 months and median OS of 21 months. Post‐CBT treatment regimens consisted of chemotherapy (44%), targeted agents (19%), immunotherapy (15%), transplant conditioning (14%), chemotherapy/targeted combination (7%), and clinical trials (1%). No significant difference in OS was found when stratified by post‐CBT regimen.
Conclusion
In a heavily pretreated R/R HL population, CBT may sensitize patients to subsequent treatment, even after progression on CBT. Post‐CBT regimen category did not impact OS. This may be a novel treatment strategy, which warrants further investigation in prospective clinical trials.
Implications for Practice
Novel, life‐prolonging treatment strategies in relapsed and refractory (R/R) Hodgkin lymphoma (HL) are greatly desired. The results of this multicenter analysis concur with a smaller, earlier report that checkpoint blockade therapy (CBT) use in R/R HL may sensitize patients to their subsequent treatment. This approach may potentially enhance therapeutic options or to bridge patients to transplant. Prospective data are warranted prior to practice implementation. As more work is done in this area, we may also be able to optimize sequencing of CBT and novel agents in the treatment paradigm to minimize treatment‐related toxicity and thus improve patient quality of life.
Keywords: Checkpoint blockade, Hodgkin lymphoma, Relapsed, Sensitization, Immunotherapy
Short abstract
This study investigated the outcome of checkpoint blockade therapy on subsequent treatment for patients with relapsed and refractory Hodgkin lymphoma in a large, multicenter, retrospective analysis.
Introduction
The majority of patients with Hodgkin lymphoma (HL) will be cured with standard therapy. However, approximately one third of patients will experience a relapse or be refractory to initial therapy (defined as progression of disease within 6 months). For these patients, only half will be salvaged with conventional therapies, including autologous stem cell transplant (SCT) [1]. Brentuximab vedotin (BV), an antibody‐drug conjugate targeting CD30, has shown response rates of 75% in relapsed or refractory HL but with complete remission in only 34% of patients [2, 3]. More recently, checkpoint inhibitors have shown striking activity in the relapsed and refractory setting, but the complete response rate is modest [4, 5].
A small, retrospective study of patients with HL showed that after anti–programmed death 1 (PD‐1) therapy, the overall response rate (ORR) to chemotherapy alone was 59% [6]. There are no other published studies evaluating this question. However, studies in other tumor types suggest that checkpoint blockade therapy (CBT) may have an impact beyond immediate efficacy, by enhancing patients’ response to subsequent chemotherapy. In non‐small cell lung cancer (NSCLC), one study demonstrated that salvage chemotherapy given after CBT achieved an ORR of 53.4%, as compared with an ORR of 34.9% to chemotherapy prior to CBT [7]. Another study showed that patients with NSCLC were more than three times more likely to achieve a partial response (PR) with salvage chemotherapy if they had prior exposure to CBT [8]. We have shown this same effect in a retrospective study of patients with non‐Hodgkin lymphoma (NHL), a disease where CBT does not appear to have much single‐agent activity [9, 10]. Given the significance of these findings, we investigated the outcome of CBT on subsequent treatment for patients with relapsed and refractory (R/R) HL in a large, multicenter, retrospective analysis.
Materials and Methods
After obtaining a waiver of patient consent and institutional review board approval (S18‐00122) at all institutions, data were retrospectively collected from 17 centers across the U.S. and Canada. Medical records of each institution were queried to identify patients with R/R HL, ages 16–90, who received CBT as second‐ or later‐line therapy between 2012 and 2017 and then received a subsequent systemic therapy because of progression of disease (PD) or toxicity. The primary aim of this study was to determine the ORR to post‐CBT therapy and to compare this with historical data. Secondary endpoints included progression free survival (PFS), duration of response (DOR), and overall survival (OS) to post‐CBT treatment. Overall survival was calculated from time of administration of post‐CBT through time of analysis or death, regardless of subsequent lines of therapy, including transplant. Patients with inadequate clinical data, those who discontinued CBT because of a complete response (CR) and never subsequently progressed, or patients whose best response to post‐CBT therapy could not be determined owing to death from another cause were excluded from this analysis. Responses were assessed by local investigators based on the Lugano criteria [11]. Progression‐free survival and OS to post‐CBT treatment were estimated using the Kaplan‐Meier method, and OS was stratified by post‐CBT treatment categories (standard chemotherapy, targeted therapy, other immunotherapy, SCT conditioning, chemotherapy/targeted therapy combination, or clinical trial). Log‐rank tests were performed to test for statistical significance. DOR to an individual's post‐CBT therapy was compared with their DOR to the treatment just prior to CBT using a paired Wilcoxon signed rank test [12]. Two‐sided p < .05 was considered to be statistically significant. Additionally, Kruskal‐Wallis test [13] and pairwise comparison with adjusted p value among all groups was performed to determine if there was any correlation between the number of prior regimens and outcome to post‐CBT therapy.
Results
Patient Characteristics
We identified 112 patients with R/R HL who were treated with CBT and received subsequent therapy. Twenty‐eight patients with CR from CBT were treated with transplant conditioning directly after CBT and never had a period of progression and thus were excluded from the analysis. Three additional patients were excluded because their response to post‐CBT therapy could not be assessed: one was lost to follow‐up, one died of progressive multifocal leukoencephalopathy, and one died of hyperacute graft‐versus‐host disease (GVHD) after allogeneic SCT.
The median age of the 81 included patients was 39 years (range 23–78); 41 (51%) patients were men and 40 (49%) were women. Twenty‐three patients (28%) had stage I–II disease, and 58 (72%) were stage III–IV. Forty patients (49%) underwent SCT prior to CBT: 31 autologous (ASCT) and 6 allogeneic (AlloSCT); 3 patients had both AlloSCT and ASCT prior, with AlloSCT most recently. Fifty‐eight (72%) had been previously treated with BV. Patients received a median of 4 (range 1–11) therapies prior to CBT, and the median DOR to the treatment immediately prior to CBT was 84 days (range 0–2,953; Table 1). Fifty‐nine patients (73%) were refractory to their immediate pre‐CBT therapy, whereas 22 patients (27%) had an initial response and subsequently relapsed. All patients had received prior chemotherapy.
Table 1.
Patient characteristics
| Characteristics | n (%) |
|---|---|
| No. of patients | 81 |
| Age, median (range), yr | 39 (21–78) |
| Male vs. female | 41 vs. 40 |
| Stage I–II | 23 (28) |
| Stage III–IV | 58 (72) |
| No. of therapies before CBT, median (range) | 4 (1–11) |
| No. of SCTs prior to CBT | 40 (49) |
| Auto | 31 |
| Allo | 6 |
| Both (with allo most recent) | 3 |
| Disease status prior to CBT | |
| Relapsed (responded for more than 6 mo) | 22 |
| Refractory (progressed within 6 mo) | 59 |
| DOR to most recent treatment prior to CBT, median (range), days | 84 (0–2,953) |
| No. of CBT infusions, median (range) | 8 (1–41) |
| Best response to CBT | |
| PD | 17 (21) |
| SD | 18 (22) |
| PR | 38 (47) |
| CR | 7 (9) |
| Indeterminate | 1 (1) |
| Reason for discontinuing CBT | |
| PD | 53 (65) |
| Toxicity | 13 (16) |
| Transplant prep | 10 (12) |
| Infection | 1 |
| Per protocol | 1 |
| CR after adding chemo | 1 |
| CR to CBT but PD after discontinuation | 2 |
| DOR to post‐CBT therapy, median (range), days | 169 (30–1,490) |
| Time between CBT and subsequent treatment, median (range), days | 47 (0–737) |
| No. of SCTs after CBT | 38 (47) |
| Auto | 16 |
| Allo | 22 |
Abbreviations: Allo, allogeneic; Auto, autologous; CBT, checkpoint blockade; CR, complete response; DOR, duration of response; PD, progression of disease; PR, partial response; SCT, stem cell transplant; SD, stable disease.
Response to CBT
Checkpoint inhibitors used were nivolumab (n = 52), pembrolizumab (n = 23), ipilimumab (n = 3) ipilimumab/nivolumab combination (n = 2), and CA170 (n = 1), which is an oral agent targeting programmed death ligand 1 (PD‐L1)/PD‐L2 and V‐domain Ig suppressor of T‐cell activation. Patients received a median of 8 infusions (range 1–41), and there was a median of 49 days (range 0–737) between treatment with last dose of CBT and initiation of post‐CBT therapy. Sixty‐five percent of patients discontinued CBT because of PD (n = 53); other causes included toxicity (n = 13), transplant preparation (n = 10), per clinical trial protocol (n = 1), infection (n = 1), CR after adding chemo (n = 1), or CR to CBT itself (with PD after discontinuation; n = 2). The ORR to CBT was 56%, including 7 CRs and 38 PRs. Eighteen patients (22%) had a best response of stable disease (SD), 17 (21%) PD, and 1 (1%) indeterminate (Table 1).
Efficacy of Post‐CBT Treatment
The most commonly used post‐CBT treatment regimen was cytotoxic chemotherapy (44%: 67% combination and 33% single agent), followed by targeted therapies (19%) including brentuximab vedotin, ibrutinib, and everolimus. Fifteen percent of patients received an alternate immunotherapy, including nivolumab, pembrolizumab, CAR‐T, lenalidomide, Lymphocyte‐activation gene 3 LAG‐3 inhibitors, and the investigational therapies MDR1 and TTI‐621, whereas 14% received conditioning regimens for SCT. One patient was treated on a clinical trial with a drug that did not fall into any other category (calcium channel blocker; Tables 2, 3).
Table 2.
Regimens
| Immediate pre‐CBT regimen | Immediate post‐CBT regimen | Post‐CBT treatment regimen category |
|---|---|---|
| GVD | BV/bendamustine | Chemotherapy + targeted therapy |
| Brentuximab vedotin | BV/bendamustine | Chemotherapy + targeted therapy |
| Gem‐OX | BV/bendamustine | Chemotherapy + targeted therapy |
| ABVD | BV/bendamustine | Chemotherapy + targeted therapy |
| Brentuximab vedotin | R‐AVD | Chemotherapy + targeted therapy |
| ABVD | R‐ICE | Chemotherapy + targeted therapy |
| ABVD | ICE | Standard chemotherapy |
| Brentuximab | Bendamustine | Standard chemotherapy |
| Everolimus | Bendamustine | Standard chemotherapy |
| Brentuximab vedotin | Gem‐Ox | Standard chemotherapy |
| Brentuximab vedotin | GND | Standard chemotherapy |
| Brentuximab vedotin | GND | Standard chemotherapy |
| Brentuximab vedotin | GND | Standard chemotherapy |
| Brentuximab vedotin | Bendamustine/gemcitabine | Standard chemotherapy |
| Brentuximab+umbralisib | Bendamustine | Standard chemotherapy |
| Transplant | GND | Standard chemotherapy |
| ABVD | GND | Standard chemotherapy |
| ABVD | ICE | Standard chemotherapy |
| Dacarbazine | ICE | Standard chemotherapy |
| BV/bendamustine | GND | Standard chemotherapy |
| Brentuximab vedotin | Gemcitabine | Standard chemotherapy |
| Bendamustine | Vinorelbine | Standard chemotherapy |
| Gemcitabine | ICE | Standard chemotherapy |
| GVD | Bendamustine | Standard chemotherapy |
| ABVD | ICE | Standard chemotherapy |
| Brentuximab | ESHAP | Standard chemotherapy |
| Chlorambucil/CCNU/dex | Bendamustine | Standard chemotherapy |
| ABVD | ICE | Standard chemotherapy |
| BV/bendamustine | GVD | Standard chemotherapy |
| Transplant | GND | Standard chemotherapy |
| Revlimid | Bendamustine | Standard chemotherapy |
| Brentuximab vedotin | GND | Standard chemotherapy |
| Brentuximab | Bendamustine | Standard chemotherapy |
| Brentuximab | GND | Standard chemotherapy |
| Transplant | Bendamustine | Standard chemotherapy |
| Brentuximab vedotin | GND | Standard chemotherapy |
| Bendamustine | GND | Standard chemotherapy |
| Brentuximab vedotin | GND | Standard chemotherapy |
| Brentuximab vedotin | Bendamustine | Standard chemotherapy |
| ABVD | ICE | Standard chemotherapy |
| ABVD | ICE | Standard chemotherapy |
| Brentuximab vedotin | Gemcitabine | Standard chemotherapy |
| Transplant | XRT followed by allo | Conditioning regimen for SCT |
| Bendamustine | Gem/Nav/BCNU/VP/CY | Conditioning regimen for SCT |
| ABVD | Gem/Nav/BCNU/VP/CY | Conditioning regimen for SCT |
| BV/bendamustine | Fludarabine/cyclophosphamide | Conditioning regimen for SCT |
| R‐ICE | Fludarabine/cyclophosphamide | Conditioning regimen for SCT |
| ABVD | Gem/Nav/BCNU/VP/CY | Conditioning regimen for SCT |
| ABVD | Gem/Nav/BCNU/VP/CY | Conditioning regimen for SCT |
| ABVD | Gem/Nav/BCNU/VP/CY | Conditioning regimen for SCT |
| ABVD | BEAM | Conditioning regimen for SCT |
| Brentuximab vedotin | TLI‐ATG conditioning | Conditioning regimen for SCT |
| Brentuximab vedotin | TLI‐ATG conditioning | Conditioning regimen for SCT |
| ABVD | Brentuximab vedotin | Targeted therapy |
| Transplant | Brentuximab vedotin | Targeted therapy |
| Transplant | Brentuximab vedotin | Targeted therapy |
| Everolimus | Brentuximab vedotin | Targeted therapy |
| R‐Benda‐Brentuximab Vedotin | Brentuximab vedotin | Targeted therapy |
| Brentuximab vedotin | Ibrutinib | Targeted therapy |
| INCB‐50465‐101 | Everolimus | Targeted therapy |
| Transplant | Brentuximab vedotin | Targeted therapy |
| Brentuximab vedotin | Ibrutinib/everolimus | Targeted therapy |
| Brentuximab vedotin | Brentuximab vedotin | Targeted therapy |
| Brentuximab vedotin | Brentuximab vedotin | Targeted therapy |
| ICE | Brentuximab vedotin | Targeted therapy |
| ICE | Brentuximab vedotin | Targeted therapy |
| GVD | BV + MDR1 | Targeted therapy |
| Transplant | BV + ibrutinib | Targeted therapy |
| GND | Nivolumab + LAG3 inhibitor | Other immunotherapy |
| Gemcitabine | Pembrolizumab | Other immunotherapy |
| Brentuximab vedotin | Nivolumab + LAG3 inhibitor | Other immunotherapy |
| Brentuximab vedotin | Nivolumab + LAG3 inhibitor | Other immunotherapy |
| Vinblastine | CAR‐T | Other immunotherapy |
| GVD | Lenalidomide | Other immunotherapy |
| Transplant | Nivolumab + LAG3 inhibitor | Other immunotherapy |
| IGEV | TTI‐621 | Other immunotherapy |
| IGEV | TTI‐621 | Other immunotherapy |
| Brentuximab vedotin | Nivolumab + LAG3 inhibitor | Other immunotherapy |
| Brentuximab vedotin | Nivolumab | Other immunotherapy |
| Brentuximab vedotin | Pembrolizumab + vorinostat | Other immunotherapy |
| Brentuximab vedotin | Calcium channel blocker | Clinical trial—other |
The exact regimens that patients received immediately prior to CBT and immediately after CBT are listed in this table. It also explains how each post‐CBT regimen was categorized.
Abbreviations: ABVD, doxorubicin + bleomycin + vinblastine + dacarbazine; AVD, doxorubicin + vinblastine + dacarbazine; BCNU, carmustine; BEAM, carmustine + etoposide + cytarabine + melphalan; BV, brentuximab vedotin; CAR‐T, chimeric antigen receptor ‐T cells; CBT, checkpoint blockade; CCNU, lomustine; CY, cyclophosphamide; ESHAP, etoposide + methylprednisolone + cisplatin + cytarabine; Gem, gemcitabine; GND, gemcitabine + navelbine + doxorubicin; GVD, gemcitabine + vinorelbine + pegylated liposomal doxorubicin; ICE, ifosfamide + carboplatin + etoposide + mesna; IGEV, ifosfamide + gemcitabine + vinorelbine; LAG3, lymphocyte‐activation gene 3; MDR1, multidrug resistance gene 1; Nav, navelbine; Ox, oxaliplatin; R, rituximab; SCT, stem cell transplant; TLI‐ATG, total lymphoid irradiation and antithymocyte globulin; VP, etoposide; XRT, radiation therapy.
Table 3.
Post‐CBT regimen category
| Regimen category | Patients, n (%) |
|---|---|
| Standard chemotherapy | 36 (44) |
| Targeted therapy | 15 (19) |
| Other immunotherapy | 12 (15) |
| Conditioning regimen for SCT | 11 (14) |
| Chemotherapy + targeted therapy | 6 (7) |
| Clinical trial—other | 1 (1) |
Most patients received standard chemotherapy, targeted therapy, other immunotherapy, or conditioning for SCT. Fewer patients proceeded with chemotherapy + targeted therapy or a clinical trial drug outside of these categories.
Abbreviations: CBT, checkpoint blockade; SCT, stem cell transplant.
The ORR to post‐CBT treatment was 62%, with 34 CRs (42%) and 16 PRs (20%). Ten patients (12%) had SD as their best response, and 19 (23%) PD. Two patients’ responses were indeterminate as it was too early in their treatment course (Fig. 1). The median DOR to post‐CBT therapy was 169 days for the entire cohort. Even though only 11 patients directly underwent transplant conditioning after CBT, 38 total patients (47%) ultimately proceeded to transplantation at any time after CBT: 22 AlloSCT and 16 ASCT (Table 1).
Figure 1.
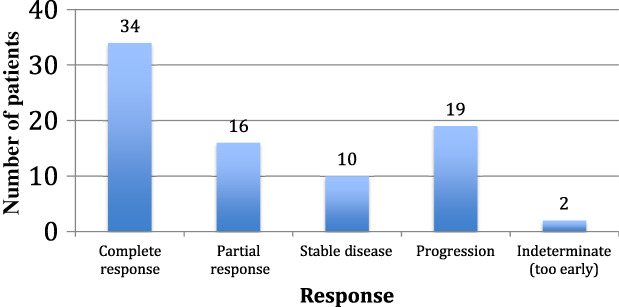
Response to the treatment regimen directly after checkpoint blockade therapy: complete response 34 (42%), partial response 16 (20%), stable disease 10 (12%), progression of disease 19 (23%), or indeterminate—too early to tell 2 (3%). The overall response rate was 62%.
Fifty‐six percent of patients (n = 45) in the analysis had had a response to CBT itself (CR or PR), and 76% (n = 34 of 45) of these patients responded to post‐CBT therapy. Of the 35 patients whose response to CBT was SD or PD, 15 (43%) responded to post‐CBT treatment (Fig. 2). The one patient with indeterminate response to CBT had a PR to post‐CBT therapy. Three patients who progressed through CBT had a CR to their next line of therapy; all of these patients’ post‐CBT regimens were chemotherapy. Seven patients (9%) progressed through both lines of therapy.
Figure 2.
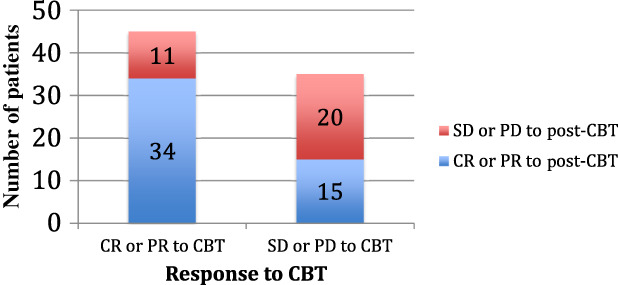
Comparison of responses to CBT and post‐CBT therapy. Of patients who had a response to CBT, 76% had a response to post‐CBT therapy as well. Only 43% of the patients who did not respond to CBT had a response to post‐CBT therapy.
Abbreviations: CBT, checkpoint blockade therapy; CR, complete response; PD, progression of disease; PR, partial response; SD, stable disease.
As of December 2018, 30 patients (45%) had not yet progressed on post‐CBT therapy, yielding a median PFS of 6.3 months (Fig. 3A). The median OS to post‐CBT therapy was 21 months (Fig. 3B); 14 (17%) patients expired: 10 from disease progression, 2 from transplant complications (hyperacute GVHD, veno‐occlusive disease), 1 from paraneoplastic syndrome, and 1 from carmustine‐related pneumonitis/pneumonia. No statistically significant difference in OS was found when stratified by post‐CBT regimen (Fig. 3C).
Figure 3.
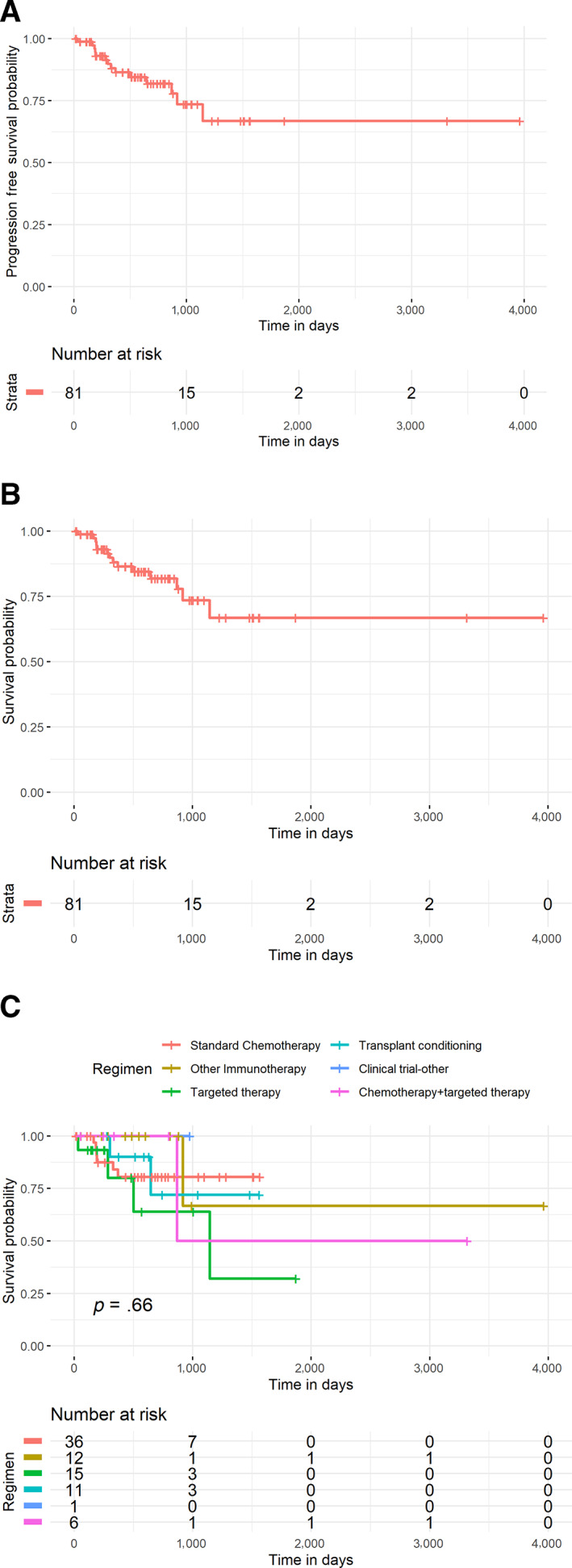
Kaplan‐Meier Curves (A): PFS to post‐CBT therapy. At a median follow‐up of 18 months, median PFS is 6.3 months. Thirty (45%) patients have not yet progressed. (B): OS to post‐CBT therapy. Median OS has not been reached. Sixty‐six (81%) patients remain alive. (C): OS by post‐CBT regimen. For standard chemotherapy, targeted therapy, transplant conditioning, other immunotherapy, chemotherapy + targeted therapy, and clinical trial drugs that do not fall into another category, no regimen was superior with regard to OS (p = .66).
Abbreviations: CBT, checkpoint blockade therapy; OS, overall survival; PFS, progression‐free survival.
Further statistical analysis showed that there was a significant correlation between the number of prior regimens and outcome to post‐CBT. However, a pairwise comparison failed to replicate this and showed no difference.
Discussion
More than 70% of patients with HL are cured with initial therapy [14, 15]. Autologous and AlloSCT may be curative for about half of patients with relapsed disease, but minimal disease burden is generally required to optimize outcomes [16, 17, 18]. Transplant is less successful for patients whose disease is chemotherapy resistant or multiply relapsed [19]. Brentuximab vedotin and PD‐1 inhibitors have improved outcomes in this setting, but most of these patients will ultimately relapse [18, 20, 21]. Novel approaches to minimize disease burden prior to transplant are needed. Our data show that checkpoint inhibitors may sensitize patients to subsequent therapy, even allowing for multiply relapsed and refractory patients to have a response to chemotherapy.
In addition to objective tumor response (62%), a significant number of additional patients (12%) were able to achieve SD with post‐CBT therapy. The median DOR for patients who achieved SD was 5.2 months. This was clinically meaningful as these patients had minimal disease burden with minor impairment of functional status during this time. Moreover, stable disease may represent a milestone for these patients, as the median OS for patients with HL who have failed ASCT is 20 months [22]. The median DOR to post‐CBT therapy for our entire population was 5.6 months as compared with a median DOR of 2.6 months to the line of therapy prior to CBT (p = .0003). In our study population, 40 patients (49%) received SCT prior to CBT (31 ASCT, 6 AlloSCT, and 1 both). Nearly the same number of patients (n = 38, 47%) were ultimately able to proceed to SCT (16 ASCT, 22 AlloSCT) after a meaningful response to post‐CBT therapy. The degree and depth of these responses suggest that CBT may be sensitizing patients to their subsequent treatments, perhaps through priming of the tumor microenvironment (TME) to subsequent direct tumor killing.
In the analysis of OS by post‐CBT treatment regimen category, no treatment type appeared to be superior, suggesting a generalized sensitization from CBT. However, our study was not powered to make this comparison. Most patients were treated with chemotherapy alone. Far fewer patients were treated with a combination of targeted therapy and chemotherapy, other immunotherapy, targeted therapies, or SCT conditioning. Moreover, most of the patients who received subsequent immunotherapy were enrolled in a clinical trial.
Statistical analysis ultimately failed to show a definitive correlation between the number of prior regimens and outcome after CBT. However, this may be due to a limited sample size or a weak correlation and should be evaluated prospectively in larger studies.
Checkpoint blockers remain active systemically for several months beyond the time of administration owing to a half‐life of 15–25 days [23, 24, 25]; side effects of CBT have been seen up to a year after discontinuation [26]. Murine studies suggest that chemotherapy increases the antitumor activity mediated by CBT by inducing immunological changes, which vary depending on the chemotherapeutic agent used [27]. In lung cancer and renal cell carcinoma, the addition of immunotherapy to chemotherapy and targeted agents has shown superior results to chemotherapy alone [28, 29]. Moreover, although CBT by itself has shown little activity in NHL, a retrospective study showed that the DOR to therapies given after CBT were longer than the DOR to the line of treatment immediately before CBT, suggesting a potentiation effect from the immunotherapy [9]. It is possible that in our study the CBT is working synergistically with the next line of therapy, despite its administration as an earlier line of therapy.
Another potential hypothesis is that the CBT alters the TME, making the tumor cell more susceptible to DNA damage, and more receptive to tumor killing once damage occurs within the tumor cells and antigen is released. In solid tumors it is understood that tumor mutational burden predicts immunotherapy response more accurately than PD‐L1 expression [30]. Chemotherapy can promote cytotoxic T‐lymphocyte function by increasing tumor antigen presentation and improving the penetration of cytotoxic T lymphocytes into the tumor parenchyma [31]. Studies in mice have shown that chemotherapy can be used to increase mutational load; when this leads to changes in immune cell infiltration, the mice were more responsive to CBT [32]. Whether the reverse sequence of therapies induces a greater response has not been studied. Further prospective correlative studies should help to elucidate this fascinating question.
Limitations of our study include the heterogeneity of prior treatments in our patient population, the retrospective nature of our analysis, and our small sample size. Additionally, all of our patients had adequate performance status to be considered for further treatment.
Our data suggest that patients with HL who relapse after CBT may have a good response to subsequent salvage therapy. Our study is not powered to make any recommendations as to which treatments may work synergistically with CBT or produce an optimal response. Prospective, randomized trials with thoughtful correlative analyses are needed to study this topic further. Now that CBT is used earlier in the repertoire of standard treatments, it may be easier to investigate this question. If our hypothesis is true, then we should expect to see longer PFS and DOR to later lines of chemotherapy when used after progression to CBT.
Conclusion
R/R HL presents a clinical challenge, and better treatment strategies are greatly desired to prevent these patients from ultimately succumbing to their disease. Our work suggests that CBT treatment may sensitize patients with HL to subsequent therapy, but prospective data are needed to validate this finding. As more work is done in this area, we may be able to optimize sequencing of CBT and novel agents in the treatment paradigm to minimize toxicity related to treatment and to optimize patient outcomes.
Author Contributions
Conceptions/design: Nicole A. Carreau, Catherine Diefenbach
Provision of study material or patients: Nicole A. Carreau, Philippe Armand, Reid Merryman, Ranjana H. Advani, Michael A. Spinner, Alex Herrera, Robert Chen, Sarah Tomassetti, Radhakrishnan Ramchandren, Muhammad S. Hamid, Sarit Assouline, Raoul Santiago, Nina Wagner‐Johnston, Suman Paul, Jakub Svoboda, Steven Bair, Stefan Barta, Yang Liu, Sunita Nathan, Reem Karmali, Madelyn Burkart, Pallawi Torka, Kevin David, Catherine Wei, Frederick Lansigan, Lukas Emery, Daniel Persky, Sonali Smith, James Godfrey, Julio Chavez, Catherine Diefenbach
Collection and/or assembly of data: Nicole A. Carreau, Orrin Pail, Philippe Armand, Reid Merryman, Ranjana H. Advani, Michael A. Spinner, Alex Herrera, Robert Chen, Sarah Tomassetti, Radhakrishnan Ramchandren, Muhammad S. Hamid, Sarit Assouline, Raoul Santiago, Nina Wagner‐Johnston, Suman Paul, Jakub Svoboda, Steven Bair, Stefan Barta, Yang Liu, Sunita Nathan, Reem Karmali, Madelyn Burkart, Pallawi Torka, Kevin David, Catherine Wei, Frederick Lansigan, Lukas Emery, Daniel Persky, Sonali Smith, James Godfrey, Julio Chavez, Catherine Diefenbach
Data analysis and interpretation: Nicole A. Carreau, Orrin Pail, Philippe Armand, Reid Merryman, Ranjana H. Advani, Michael A. Spinner, Alex Herrera, Robert Chen, Sarah Tomassetti, Radhakrishnan Ramchandren, Muhammad S. Hamid, Sarit Assouline, Raoul Santiago, Nina Wagner‐Johnston, Suman Paul, Jakub Svoboda, Steven Bair, Stefan Barta, Yang Liu, Sunita Nathan, Reem Karmali, Madelyn Burkart, Pallawi Torka, Kevin David, Catherine Wei, Frederick Lansigan, Lukas Emery, Daniel Persky, Sonali Smith, James Godfrey, Julio Chavez, Yuhe Xia, Andrea B. Troxel, Catherine Diefenbach
Manuscript writing: Nicole A. Carreau, Orrin Pail, Philippe Armand, Reid Merryman, Ranjana H. Advani, Michael A. Spinner, Alex Herrera, Robert Chen, Sarah Tomassetti, Radhakrishnan Ramchandren, Muhammad S. Hamid, Sarit Assouline, Raoul Santiago, Nina Wagner‐Johnston, Suman Paul, Jakub Svoboda, Steven Bair, Stefan Barta, Yang Liu, Sunita Nathan, Reem Karmali, Madelyn Burkart, Pallawi Torka, Kevin David, Catherine Wei, Frederick Lansigan, Lukas Emery, Daniel Persky, Sonali Smith, James Godfrey, Julio Chavez, Yuhe Xia, Andrea B. Troxel, Catherine Diefenbach
Final approval of manuscript: Nicole A. Carreau, Orrin Pail, Philippe Armand, Reid Merryman, Ranjana H. Advani, Michael A. Spinner, Alex Herrera, Robert Chen, Sarah Tomassetti, Radhakrishnan Ramchandren, Muhammad S. Hamid, Sarit Assouline, Raoul Santiago, Nina Wagner‐Johnston, Suman Paul, Jakub Svoboda, Steven Bair, Stefan Barta, Yang Liu, Sunita Nathan, Reem Karmali, Madelyn Burkart, Pallawi Torka, Kevin David, Catherine Wei, Frederick Lansigan, Lukas Emery, Daniel Persky, Sonali Smith, James Godfrey, Julio Chavez, Yuhe Xia, Andrea B. Troxel, Catherine Diefenbach
Disclosures
Philippe Armand: Merck, Bristol‐Myers Squibb, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics (C/A), Merck, Bristol‐Myers Squibb (H), Merck, Bristol‐Myers Squibb, Affimed, Adaptive, Roche, Tensha, Otsuka, Sigma Tau, Genentech (RF); Ranjana H. Advani: Seattle Genetics, Takeda (C/A), Seattle Genetics, Merck, Millenium (RF); Alex Herrera: Bristol‐Myers Squibb, Genentech, Merck, KiTE Pharma/Gilead, Adaptive Biotechnologies, Seattle Genetics (C/A), Bristol‐Myers Squibb, Genentech, AstraZeneca, Merck, Seattle Genetics, Gilead Sciences (RF); Robert Chen: Affimed, Bristol‐Myers Squibb, Pharmacyclics, Seattle Genetics, Millennium Pharmaceuticals, Merck & Co. (RF), Bristol‐Myers Squibb, Pharmacyclics, Seattle Genetics, Millennium Pharmaceuticals, Genentech Inc., Merck & Co. (C/A), Seattle Genetics (H), Seattle Genetics, Merck & Co. (other); Radhakrishnan Ramchandren: Bristol‐Myers Squibb, Seattle Genetics, Pharmacyclics LLC an AbbVie Company, Janssen (C/A), Seattle Genetics, Pharmacyclics LLC an AbbVie Company, Janssen, Merck (RF); Sarit Assouline: Janssen, Bristol‐Myers Squibb, Pfizer, Roche (H), Janssen (SAB), Bristol‐Myers Squibb, Roche, Novartis (RF), Bristol‐Myers Squibb, Pfizer, Roche (other); Nina Wagner‐Johnston: Celgene, Merck, Novartis, Astex (RF), Juno, Janssen, ADC Therapeutics (H, SAB), Janssen (C/A); Jakub Svoboda: Bristol‐Myers Squibb, Kite, Pharmacyclics, Kyowa, Seattle Genetics (C/A), Bristol‐Myers Squibb, Regeneron, Merck, TG Therapeutics, Pharmacyclics, Seattle Genetics (RF); Steven Bair: Bayer (C/A), Bayer, Celgene, Merck, Seattle Genetics, Takeda (RF), Janssen (SAB), Mundipharma, Seattle Genetics (H); Reem Karmali: Takeda, Bristol‐Myers Squibb (RF), Gilead/Kite, Celgene/Juno (SAB), AstraZeneca (other); Sonali Smith: Bristol‐Myers Squibb (C/A), Portola (H); Julio Chavez: Novartis, Genentech, Kite, Humanigen (C/A), Novartis, Genentech, Kite (SAB), Janssen, Genentech, Kite (other), Merck (RF); Catherine Diefenbach: Merck, Seattle Genetics, Bristol‐Myers Squibb, Genentech (C/A), Merck, Seattle Genetics, Incyte, Acerta, Millenium/Takeda, Denovo, Trillium, Bristol‐Myers Squibb (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This study was supported (in part) by funding from American Cancer Society grant MRSG‐14‐052‐01‐LIB to C.S.D. This study was previously presented in part at the American Society of Hematology Annual Meeting, December 2018, San Diego, California.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
For Further Reading: Reid W. Merryman, Nicole A. Carreau, Ranjana H. Advani et al. Impact of Treatment Beyond Progression with Immune Checkpoint Blockade in Hodgkin Lymphoma. The Oncologist 2020;25:e993–e997.
Abstract: Atypical response patterns following immune checkpoint blockade (ICB) in Hodgkin lymphoma (HL) led to the concept of continuation of treatment beyond progression (TBP); however, the longitudinal benefit of this approach is unclear. We therefore performed a retrospective analysis of 64 patients treated with ICB; 20 who received TBP (TBP cohort) and 44 who stopped ICB at initial progression (non‐TBP cohort). The TBP cohort received ICB for a median of 4.7 months after initial progression and delayed subsequent treatment by a median of 6.6 months. Despite receiving more prior lines of therapy, the TBP cohort achieved longer progression‐free survival with post‐ICB treatment (median, 17.5 months vs. 6.1 months, p = .035) and longer time‐to‐subsequent treatment failure, defined as time from initial ICB progression to failure of subsequent treatment (median, 34.6 months vs. 9.9 months, p = .003). With the limitations of a retrospective study, these results support the clinical benefit of TBP with ICB for selected patients.
References
- 1. Montanari F, Diefenbach C. Relapsed Hodgkin lymphoma: Management strategies. Curr Hematol Malig Rep 2014;9:284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Younes A, Gopal AK, Smith SE et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol 2012;30:2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yi JH, Kim SJ, Kim WS. Brentuximab vedotin: Clinical updates and practical guidance. Blood Res 2017;52:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hude I, Sasse S, Engert A et al. The emerging role of immune checkpoint inhibition in malignant lymphoma. Haematologica 2017;102:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin RJ, Diefenbach CS. Checkpoint inhibition in Hodgkin lymphoma: Saving the best for last? Oncology (Williston Park) 2016;30:914–920. [PMC free article] [PubMed] [Google Scholar]
- 6. Rossi C, Gilhodes J, Maerevoet M et al. Efficacy of chemotherapy or chemo‐anti‐PD‐1 combination after failed anti‐PD‐1 therapy for relapsed and refractory Hodgkin lymphoma: A series from Lysa centers. Am J Hematol 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7. Park SE, Lee SH, Ahn JS et al. Increased response rates to salvage chemotherapy administered after PD‐1/PD‐L1 inhibitors in patients with non‐small cell lung cancer. J Thorac Oncol 2018;13:106–111. [DOI] [PubMed] [Google Scholar]
- 8. Leger PD, Rothschild S, Castellanos E et al. Response to salvage chemotherapy following exposure to immune checkpoint inhibitors in patients with non‐small cell lung cancer. J Clin Oncol 2017;35(suppl 15):9084a. [Google Scholar]
- 9. Carreau NA, Armand P, Merryman RW et al. Checkpoint blockade treatment sensitises relapsed/refractory non‐Hodgkin lymphoma to subsequent therapy. Br J Haematol 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10. Phillips EH, Illidge TM. Is it time to rethink checkpoint blockade therapy in non‐Hodgkin lymphoma? Br J Haematol 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11. Cheson BD, Fisher RI, Barrington SF et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: The Lugano classification. J Clin Oncol 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jung SH. Rank tests for matched survival data. Lifetime Data Anal 1999;5:67–79. [DOI] [PubMed] [Google Scholar]
- 13. Hollander M, Wolfe DA. The One‐Way Layout 202. In: Nonparametric Statistical Methods, New York: John Wiley & Sons, 1973:115–120. [Google Scholar]
- 14. Merli F, Luminari S, Gobbi PG et al. Long‐term results of the HD2000 trial comparing ABVD versus BEACOPP versus COPP‐EBV‐CAD in untreated patients with advanced Hodgkin lymphoma: A study by Fondazione Italiana Linfomi. J Clin Oncol 2016;34:1175–1181. [DOI] [PubMed] [Google Scholar]
- 15. Johnson P, Federico M, Kirkwood A et al. Adapted treatment guided by interim PET‐CT scan in advanced Hodgkin's lymphoma. N Engl J Med 2016;374:2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rivas MM, Berro M, Prates MV et al. Allogeneic stem cell transplantation improves survival in relapsed Hodgkin lymphoma patients achieving complete remission after salvage treatment. Bone Marrow Transplant 2020;55:117–125. [DOI] [PubMed] [Google Scholar]
- 17. Shah GL, Moskowitz CH. Transplant strategies in relapsed/refractory Hodgkin lymphoma. Blood 2018;131:1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. LaCasce AS. Treating Hodgkin lymphoma in the new millennium: Relapsed and refractory disease. Hematol Oncol 2019;37(suppl 1):87–91. [DOI] [PubMed] [Google Scholar]
- 19. Hahn T, McCarthy PL, Carreras J et al. Simplified validated prognostic model for progression‐free survival after autologous transplantation for Hodgkin lymphoma. Biol Blood Marrow Transplant 2013;19:1740–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Badar T, Epperla N, Szabo A et al. Trends in postrelapse survival in classic Hodgkin lymphoma patients after experiencing therapy failure following auto‐HCT. Blood Adv 2020;4:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Merryman RW, LaCasce A. Novel agents and immune invasion in Hodgkin lymphoma. Hematol Am Soc Hematol Educ Program 2019;2019:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elshenawy MA, Shahzad Rauf M, Elhassan TAM et al. Survival analysis of patients with Hodgkin lymphoma who failed high dose chemotherapy and autologous stem cell transplant. Ann Hematol 2018;97:1229–1240. [DOI] [PubMed] [Google Scholar]
- 23. Keytruda (pembrolizumab) injection. Prescribing information. Available at https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Accessed February 2020.
- 24. Opdivo (nivolumab) injection. Prescribing information. Bristol‐Myers Squibb; 2018. Available at https://packageinserts.bms.com/pi/pi_opdivo.pdf. Accessed February 2020.
- 25. Yervoy (ipilimumab) injection. Prescribing information. Bristol‐Myers Squibb; 2018. Available at https://packageinserts.bms.com/pi/pi_yervoy.pdf. Accessed February 2020.
- 26. Haanen JBAG, Carbonnel F, Robert C et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017;28(suppl 4):iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 27. Cubas R, Moskalenko M, Cheung J et al. Chemotherapy combines effectively with anti–PD‐L1 treatment and can augment antitumor responses. J Immunol 2018;201:2273–2286. [DOI] [PubMed] [Google Scholar]
- 28. Lazzari C, Karachaliou N, Bulotta A et al. Combination of immunotherapy with chemotherapy and radiotherapy in lung cancer: Is this the beginning of the end for cancer? Ther Adv Med Oncol 2018;10:1758835918762094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rini BI, Plimack ER, Stus V et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal‐cell carcinoma. N Engl J Med 2019;380:1116–1127. [DOI] [PubMed] [Google Scholar]
- 30. Samstein RM, Lee C‐H, Shoushtari AN et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019;51:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramakrishnan R, Gabrilovich DI. Novel mechanism of synergistic effects of conventional chemotherapy and immune therapy of cancer. Cancer Immunol Immunother 2013;62:405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuczynski EA, Krueger J, Chow A et al. Impact of chemical‐induced mutational load increase on immune checkpoint therapy in poorly responsive murine tumors. Mol Cancer Ther 2018;17:869–882. [DOI] [PubMed] [Google Scholar]


