Abstract
Aims
Our aim was to describe the electrocardiographic features of critical COVID-19 patients.
Methods and results
We carried out a multicentric, cross-sectional, retrospective analysis of 431 consecutive COVID-19 patients hospitalized between 10 March and 14 April 2020 who died or were treated with invasive mechanical ventilation. This project is registered on ClinicalTrials.gov (identifier: NCT04367129). Standard ECG was recorded at hospital admission. ECG was abnormal in 93% of the patients. Atrial fibrillation/flutter was detected in 22% of the patients. ECG signs suggesting acute right ventricular pressure overload (RVPO) were detected in 30% of the patients. In particular, 43 (10%) patients had the S1Q3T3 pattern, 38 (9%) had incomplete right bundle branch block (RBBB), and 49 (11%) had complete RBBB. ECG signs of acute RVPO were not statistically different between patients with (n = 104) or without (n=327) invasive mechanical ventilation during ECG recording (36% vs. 28%, P = 0.10). Non-specific repolarization abnormalities and low QRS voltage in peripheral leads were present in 176 (41%) and 23 (5%), respectively. In four patients showing ST-segment elevation, acute myocardial infarction was confirmed with coronary angiography. No ST-T abnormalities suggestive of acute myocarditis were detected. In the subgroup of 110 patients where high-sensitivity troponin I was available, ECG features were not statistically different when stratified for above or below the 5 times upper reference limit value.
Conclusions
The ECG is abnormal in almost all critically ill COVID-19 patients and shows a large spectrum of abnormalities, with signs of acute RVPO in 30% of the patients. Rapid and simple identification of these cases with ECG at hospital admission can facilitate classification of the patients and provide pathophysiological insights.
Keywords: ECG, Critically ill COVID-19, Right ventricular pressure overload
What’s new?
This is the first systematic analysis of electrocardiographic features in critically ill COVID-19 patients.
The vast majority of the patients had abnormal electrocardiogram at hospital admission.
Signs of right ventricular pressure overload are present in 30% of the patients. In patients mechanically ventilated, the values of positive end-expiratory pressure used seem not to be related to signs of right ventricular pressure overload.
The most frequent arrhythmias are atrial fibrillation/flutter (in 22% of the patients).
ST-T abnormalities related to acute myocarditis or acute coronary syndrome are rare.
Long QT interval and non-specific repolarization abnormalities are frequent even before starting specific drug therapy.
Introduction
Since the beginning of the COVID-19 pandemic, it was evident that patients with cardiovascular risk factors or previous cardiovascular disease had a poor outcome, as did patients with high values of troponin.1 In particular, increased troponin levels have been considered as an expression of ‘myocardial injury or damage’, including cases of acute coronary syndromes and myocarditis.2,3 Despite the availability of detailed descriptions of the clinical cardiovascular profile of COVID-19 patients, data on standard electrocardiogram (ECG) are scanty and today no detailed analysis of standard ECG in any cohort of patients is available. The assessment of ECG abnormalities of patients with COVID-19 could provide important prognostic information and pathophysiological insights into this still scarcely understood disease. ELCOVID is a large multicentre project aimed at assessing the standard ECG features of a large cohort of critically ill COVID-19 patients hospitalized in an endemic area of northern Italy.
Methods
This report is the first part of a three-step ongoing project on the acute and long-term cardiac involvement in COVID-19 patients. It has been approved by the local Ethics Committee and involves 13 hospitals in the Emilia Romagna region in northern Italy that has been heavily affected by the pandemic. This project is registered on ClinicalTrials.gov (identifier: NCT04367129).
We retrospectively analysed the ECG recorded at hospital admission of consecutive, critically ill COVID-19 patients, hospitalized from 10 March to 14 April 2020. Patients were considered ‘critically ill’ whether they died during hospitalization or underwent invasive mechanical ventilation. The inclusion criteria were: age >18 years; COVID-19 confirmed by severe acute respiratory syndrome coronavirus 2 RNA (SARS-CoV-2) detection at pharyngeal swab performed at hospital admission or during hospitalization; invasive mechanical ventilation or death during hospitalization; and 12-lead surface ECG recorded at hospital admission.
The exclusion criteria were: uncertain COVID-19 diagnosis or unavailability of 12-lead ECG.
All ECGs recorded at hospital admission were performed on patients during invasive ventilation, during non-invasive ventilation, or during high-flow oxygen support. Clinical data and high-sensitivity troponin I (Hs-TnI) values (when available) were also collected (Table 1). Cardiovascular disease, defined as a group of disorders of the heart and blood vessels (coronary heart disease, cerebrovascular disease, and peripheral arterial disease), was collected from medical record. Furthermore, we evaluated the medical therapy during hospitalization. ECG analysis was independently performed by two experienced cardiologists according to standard definitions and diagnostic criteria.4 All the parameters reported in Table 2 were assessed. For patients already in invasive mechanical ventilation at the time of ECG recording, positive end-expiratory pressure (PEEP) parameters were collected.
Table 1.
Demographic and clinical characteristics
| Overall (431) | Age ≤74 (228) | Age >74 (203) | P-value | |
|---|---|---|---|---|
| Male, n (%) | 296 (69) | 169 (74) | 128 (63) | 0.01 |
| Age, years (IQR) | 74 (64–82) | 65 (56–71) | 82 (79–87) | NA |
| Deaths, n (%) | 200 (46) | 58 (25) | 142 (70) | <0.001 |
| Hs-TnI, ng/L (IQR) | 22 (10–84) | 13 (8–75) | 67 (21–103) | 0.10 |
| Hypertension, n (%) | 194 (45) | 111 (49) | 83 (41) | 0.10 |
| Dyslipidaemia, n (%) | 57 (13) | 34 (15) | 23 (11) | 0.27 |
| Cigarette smoking, n (%) | 44 (10) | 27 (12) | 17 (8) | 0.23 |
| Diabetes, n (%) | 86 (20) | 44 (19) | 42 (21) | 0.71 |
| Previous cardiovascular disease, n (%) | 76 (18) | 40 (18) | 36 (18) | 0.95 |
| Chronic kidney disease (eGFR <60 mL/min/m2), n (%) | 69 (16) | 36 (16) | 33 (16) | 0.89 |
| Chronic obstructive pulmonary disease, n (%) | 36 (8) | 23 (10) | 13 (6) | 0.16 |
| History of atrial fibrillation/flutter, n (%) | 21 (5) | 12 (5) | 9 (4) | 0.69 |
| Medication during hospitalization | ||||
| Heparin, n (%) | 216 (50) | 121 (53) | 95 (47) | 0.19 |
| Hydroxychloroquine, n (%) | 286 (66) | 162 (71) | 124 (61) | 0.029 |
| Antiviral drug, n (%) | 276 (64) | 153 (67) | 123 (61) | 0.16 |
| Tocilizumab, n (%) | 27 (6) | 17 (8) | 10 (5) | 0.27 |
eGFR, estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration); Hs-TnI, high-sensitivity troponin I; IQR, interquartile range; NA, not applicable.
Table 2.
Electrocardiographic characteristics
| Overall (431) | Age ≤74 (228) | Age >74 (203) | P-value | |
|---|---|---|---|---|
| Abnormal electrocardiogram, n (%): | 401 (93) | 201 (88) | 200 (99) | <0.001 |
| Atrial fibrillation/flutter, n (%) | 96 (22) | 33 (15) | 63 (31) | <0.001 |
| Heart rate, b.p.m. (SD) | 87 ± 24 | 88 ± 26 | 87 ± 22 | 0.09 |
| Low QRS voltage peripheral leads, n (%) | 23 (5) | 8 (4) | 15 (7) | 0.07 |
| PR interval, ms (SD) | 161 ± 33 | 158 ± 33 | 164 ± 34 | 0.37 |
| QRS interval, ms (SD) | 99 ± 23 | 95 ± 19 | 103 ± 26 | <0.001 |
| QRS >120 ms, n (%) | 81 (19) | 29 (13) | 52 (26) | <0.001 |
|
Pathological Q waves, n (%): Anterior/anterolateral, n (%): Inferior/inferolateral, n (%): |
38 (9) 19 (4) 28 (6) |
13 (6) 7 (3) 10 (4) |
25 (12) 12 (6) 18 (9) |
0.02 0.15 0.06 |
| Left ventricular hypertrophy, n (%) | 7 (2) | 3 (1) | 4 (2) | 0.59 |
| Right ventricular hypertrophy, n (%) | 3 (1) | 3 (1) | 0 (0) | 0.10 |
| Isolated S1Q3T3 pattern, n (%) | 43 (10) | 28 (12) | 15 (7) | 0.09 |
| Left anterior hemiblock, n (%) | 32 (7) | 10 (4) | 22 (11) | 0.01 |
| Incomplete RBBB, n (%) | 38 (9) | 23 (10) | 15 (7) | 0.32 |
|
RBBB, n (%) +Left anterior hemiblock, n(%) +S1Q3T3, n (%) |
49 (11) 6 (2) 18 (5) |
17 (8) 2 (1) 3 (1) |
32 (16) 4 (2) 15 (7) |
0.007 0.33 0.002 |
| LBBB, n (%) | 18 (4) | 9 (4) | 9 (4) | 0.80 |
|
Pathological negative T waves, n (%) Anterior T waves, n (%) Inferior T waves, n (%) |
62 (14) 22 (5) 24 (5) |
25 (11) 7 (3) 12 (5) |
37 (18) 15 (7) 12 (6) |
0.03 0.04 0.77 |
| QTc, ms (SD) | 449 ± 47 | 443 ± 41 | 456 ± 52 | 0.03 |
| QTc >460 ms, n (%) | 166 (38) | 74 (33) | 92 (45) | 0.006 |
| Non-specific RA, n (%) | 176 (41) | 96 (42) | 75 (40) | 0.27 |
Hs-TnI, high sensitivity troponin I; IQR, interquartile range; LBBB, left bundle branch block; QTc, QT interval corrected with Bazett formula; RA, repolarization abnormalities; RBBB, right bundle branch block; SD, standard deviation.
Statistical analysis
Comparison was performed with χ2 test (Yates correction) between categorical variables, with Student’s test between continuous variables with normal distribution, and Kruskal–Wallis test between continuous variables with non-normal distribution. A P-value <0.05 was considered significant. Statistical analyses were performed with SPSS Statistics version 25.0 (IBM Corporation).
Results
A total of 431 consecutive patients were included. Demographic and clinical characteristics are summarized in Table 1. ECG features are summarized in Table 2. A total of 296 (69%) patients were male and 200 patients (46%) died during hospitalization. In 104 patients, ECG was recorded during invasive mechanical ventilation and PEEP range from 7.5 to 20 cmH2O {median value 10 [interquartile range (IQR) 8–12] cmH2O}. A total of 153 patients received non-invasive mechanical ventilation during ECG recording, and PEEP values were constantly less than 10 cmH2O. The remaining 174 patients received high-flow oxygen support.
Based on the median age (74 years), two subgroups were compared for the main clinical and ECG characteristics (Tables 1 and2). No differences in clinical characteristic were observed in the two subgroups of patients, except for hydrocloroquine that was more frequently used in younger patients (71% vs. 61%, P = 0.029).
ECG was abnormal in 93% of the patients and in 99% of those >74 years old. Atrial fibrillation or flutter was detected in 96 (22%) patients and was more frequent in those >74 years old (31% vs. 15%, P < 0.001). History of atrial fibrillation/flutter was present in 9 (9%) of the patients with atrial fibrillation/flutter. QRS interval >120 ms was found in 19% and pathological Q waves in 9% of the patients; both these characteristics were statistically more frequent in the more elderly subgroup. Left ventricular hypertrophy (LVH), right ventricular hypertrophy, and left anterior hemiblock (LAH) were diagnosed in 2, 1, and 7% of the patients, respectively. Only LAH was more frequent in those >74 years old (11% vs. 4%, P= 0.01). S1Q3T3 pattern, in isolation or associated with right bundle branch block (RBBB), or isolated RBBB (complete or incomplete) were considered signs of acute right ventricular pressure overload (RVPO), and 130 (30%) patients had ECG signs of acute RVPO. In particular, 43 patients (10%) had an isolated S1Q3T3 pattern (Figure 1), 38 (9%) incomplete RBBB, and 49 (11%) complete RBBB. Complete RBBB, but not S1Q3T3 pattern, was more prevalent in patients >74 years old (16% vs. 8%, P = 0.007). RBBB associated with S1Q3T3 pattern (Figure 2) was more frequent in patients >74 years old (7% vs. 1%, P = 0.002) whereas RBBB associated with LAH was not different between the subgroups. Finally, ECG signs of acute RVPO were not statistically different between patients with (n = 104) or without (n = 327) invasive mechanical ventilation during ECG recording (36% vs. 28%, P = 0.10). Of note, in the subgroup of patients with ECG recording during invasive mechanical ventilation, no differences in PEEP values were present between patients with and without ECG signs of RVPO (Figure 3).
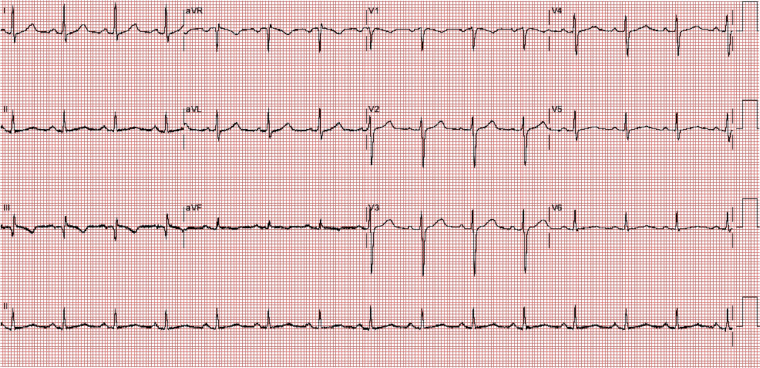
Figure 1.
Spectrum of electrocardiogram abnormalities associated with right ventricular pressure overload: presence of S1Q3T3 pattern and sinus rhythm.
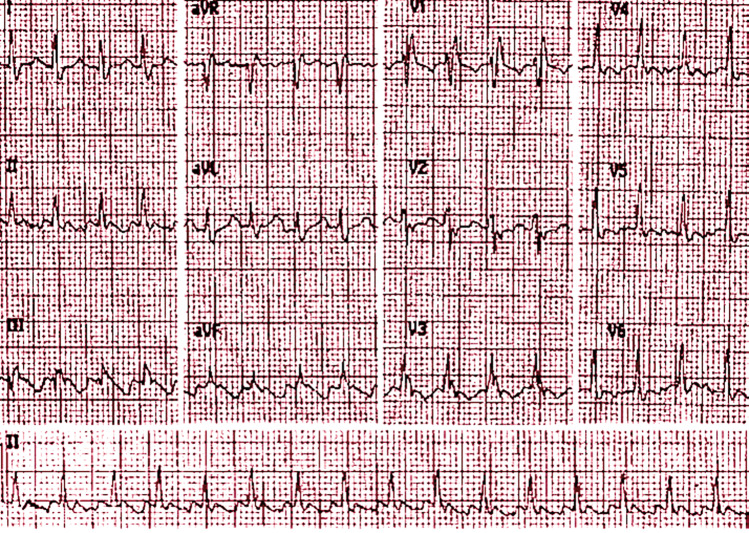
Figure 2.
Spectrum of electrocardiogram abnormalities associated with right ventricular pressure overload: presence of the S1Q3T3 pattern associated with right bundle branch block and atrial flutter.
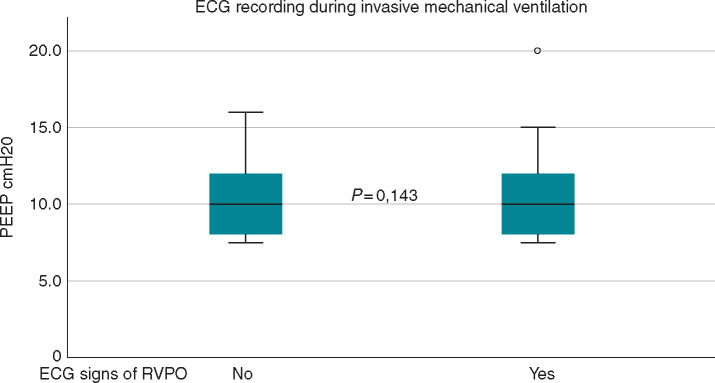
Figure 3.
No differences in positive end-expiratory pressure (PEEP) values between patients with and without ECG signs of right ventricular pressure overload (RVPO).
Pathological negative T waves and QT-corrected interval (QTc) >460 ms were present in 62 (14%) and 166 (38%) patients, respectively, both more frequent in patients >74 years old. Non-specific repolarization abnormalities and low QRS voltage in peripheral leads were present in 176 (41%) and 23 (5%), respectively, without differences between the subgroups (Table 2). Four patients showed ST-segment elevation suggesting ST elevation acute myocardial infarction (anterior in one patient, anterolateral in one patient, and inferior in the other two patients), that was confirmed with coronary angiography. No ST-T abnormality suggestive of acute myocarditis was detected.
Hs-TnI was available in 110 patients, with a median value of 22 ng/L. Two subgroups were considered according to the value of 5 times upper reference limit (Hs-TnI ≤100 ng/L and Hs-TnI >100 ng/L). ECG features were not different between the subgroups (Table 3).
Table 3.
Hs-TnI subgroups
| Overall (110) | Hs-TnI ≤5 URL (100 ng/L) (86) | Hs-TnI >5 URL 100 ng/L) (24) | P-value | |
|---|---|---|---|---|
| Male, n (%) | 80 (73) | 64 (74) | 16 (67) | 0.45 |
| Age, years (IQR) | 69 (60–78) | 68 (60–78) | 71 (59–76) | 0.88 |
| Deaths, n (%) | 38 (35) | 28 (33) | 10 (42) | 0.41 |
| Hs-TnI, ng/L (IQR) | 22 (10–84) | 15 (9 – 41) | 331 (152–671) | NA |
| Abnormal electrocardiogram, n (%): | 97 (88) | 75 (87) | 22 (92) | 0.55 |
| Atrial fibrillation/flutter, n (%) | 18 (16) | 11 (13) | 7 (29) | 0.055 |
| Heart rate, b.p.m. (SD) | 89 ± 27 | 88 ± 26 | 92 ± 32 | 0.58 |
| Low QRS voltage peripheral leads, n (%) | 3 (3) | 2 (2) | 1 (4) | 0.60 |
| PR interval, ms (SD) | 162 ± 34 | 162 ± 36 | 163 ± 25 | 0.92 |
| QRS interval, ms (SD) | 99 ± 26 | 99 ± 26 | 99 ± 26 | 0.98 |
| QRS >120 ms, n (%) | 21 (19) | 16 (19) | 5 (21) | 0.81 |
|
Pathological Q waves, n (%): Anterior/anterolateral, n (%): Inferior/inferolateral, n (%): |
7 (6) 4 (4) 7 (6) |
6 (7) 3 (4) 6 (7) |
1 (4) 1 (4) 1 (4) |
0.62 0.87 0.62 |
| Left ventricular hypertrophy, n (%) | 4 (4) | 3 (4) | 1 (4) | 0.87 |
| Right ventricular hypertrophy, n (%) | NA | NA | NA | NA |
| Isolated S1Q3T3 pattern, n (%) | 11 (10) | 8 (9) | 3 (13) | 0.64 |
| Left anterior hemiblock, n (%) | 7 (6) | 6 (7) | 1 (4) | 0.62 |
| Incomplete RBBB, n (%) | 10 (9) | 9 (10) | 1 (4) | 0.34 |
|
RBBB, n (%) +Left anterior hemiblock, n (%) +S1Q3T3, n (%) |
11 (10) 0 (0) 3 (3) |
10 (12) 0 (0) 2 (2) |
1 (4) 0 (0) 1 (4) |
0.28 NA 0.62 |
| LBBB, n (%) | 6 (6) | 2 (2) | 4 (17) | 0.006 |
|
Pathological negative T waves, n (%)| Anterior T waves, n (%) inferior T waves, n (%) |
18 (16) 6 (6) 4 (4) |
14 (16) 3 (3) 4 (5) |
4 (17) 3 (12) 0 (0) |
0.96 0.086 0.28 |
| QTc, ms (SD) | 386 ± 62 | 456 ± 45 | 447 ± 41 | 0.36 |
| QTc >460 ms, n (%) | 49 (46) | 38 (44) | 11 (46) | 0.89 |
| Non-specific RA, n (%) | 50 (46) | 43 (50) | 7 (29) | 0.070 |
Hs-TnI, high-sensitivity troponin I; IQR, interquartile range; LBBB, left bundle branch block; QTc, QT interval corrected with Bazett formula; RA, repolarization abnormalities; RBBB, right bundle branch block; SD, standard deviation, URL, upper reference limit.
Discussion
This is the first study that describes the ECG features of a large series of critically ill COVID-19 patients.
The main and unexpected finding is the high frequency of ECG signs suggesting acute RVPO (Figures 1 and2) and the low prevalence of repolarization abnormalities suggesting acute myocarditis. This finding is consistent with other echocardiographic reports of smaller series recently published.5,6 In particular, about one-third of the patients had the S1Q3T3 pattern, in isolation or associated with a RBBB. Notably the prevalence of RBBB in the general age-matched population is <4%.7 We interpret these abnormalities as related to an acute RVPO, similarly to those recorded in ‘classic’ pulmonary embolism.8 The high frequency of atrial fibrillation or atrial flutter (22%) as compared with the general population could have similar interpretation.9 The most plausible substrate for this ECG evidence of acute RVPO is thrombotic microvascular pulmonary occlusion, possibly due to diffuse endothelial damage or, in some cases, true, albeit unappreciated, venous thrombo-embolic events.10 These findings are in line with similar hypotheses generated by the evidence of increased coagulation markers in selected critically ill COVID-19 patients and by recent autoptic descriptions.10–16 Of course hypoxaemia-induced vasoconstriction of pulmonary vessels cannot be excluded in selected cases.
An alternative explanation may be that invasive mechanical ventilation in a context of ‘atypical acute respiratory distress syndrome (ARDS)’ such as COVID-19 pneumonia induces these ECG abnormalities.17 This, anyhow, seems less probable, at least in the majority of cases. It is known that invasive mechanical ventilation with elevated PEEP can eventually determine transient echocardiographic signs of acute RVPO in patients with ARDS.18,19 However, in COVID-19 pneumonia, lung compliance is higher and PEEPs used in the majority of cases are definitely lower.20 Unfortunately, ECG data during invasive mechanical ventilation in ARDS and in COVID-19 pneumonia are lacking in the literature. In our study, the prevalence of ECG signs of acute RVPO was not statistically different between patients with or without invasive mechanical ventilation during ECG recording. In particular, in the subgroup of patients with ECG recording during invasive mechanical ventilation, median PEEP was 10 cmH2O and only one patient had a high PEEP value of 20 cmH2O. Finally, the PEEP values between patients with and without ECG signs of RVPO were similar (Figure 3). Therefore, the hypothesis that elevated PEEP during invasive mechanical ventilation contributed to ECG signs of RVPO is unlikely.
Many considerations need to be taken into account regarding our patient population in order to correctly interpret the results. First, our patients were older than those previously reported in Chinese and Italian cohorts.21,22 Age could partially explain why ECG was abnormal in the vast majority (>90%) of the patients. Other possible reasons could be the co-existence of previous ECG abnormalities. In the absence of a pre-hospitalization ECG (unavoidable limitation in this context of an emergency and ‘protected’ access to the emergency room), it is difficult to distinguish between pre-existing and new-onset COVID-19-related abnormalities. Nevertheless, it is reasonable to consider LVH, LAH, and pathological Q waves as pre-existing. Non-specific repolarization abnormalities and mild QTc prolongation were the single most frequent findings (41% and 38%, respectively) and can be attributed to critical illness and hypoxaemia. Notably, all the ECGs were recorded at hospital admission, before starting any drug potentially affecting coagulation and ECG parameters, including repolarization and QT.
Our study also shows the heterogeneity and therefore the elusiveness of the concept of acute ‘myocardial injury’ often used in recent COVID-19 literature to interpret the high troponin values. No ST-T wave abnormalities suggestive of acute myocarditis were present, and the main ECG abnormalities were equally distributed among patients with high and low Hs-TnI values. Low QRS voltages in the peripheral leads were detected in 5% of the patients. This non-specific finding could be the expression of myocardial oedema or pre-existing chronic pulmonary disease, or could be related to invasive mechanical ventilation. Histological studies are needed to accurately characterize the meaning of these alterations. Pending these studies, it is worth recalling that in the majority of reported cases, the diagnosis is based on clinical criteria, while the use of more robust diagnostic tools such as cardiac magnetic resonance or endomyocardial biopsy tended to be avoided in order to maintain spread of infection as low as possible among workers and other patients. This awareness must also guide cardiological follow-up after discharge.
Limitations
In this retrospective cross-sectional study dealing with critically ill patients hospitalized in an emergency, a single ECG was collected at hospital admission without data on ECG follow-up. Furthermore, previous ECG was not available to discriminate new-onset and pre-existing abnormalities. Data on troponin were not systematically collected. The selection of critically ill COVID-19 patients precludes evaluation of the prognostic role of clinical and ECG variables. This should be explored in future studies on this topic.
Conclusions
This is the first systematic analysis of standard ECG in critically ill COVID-19 patients. The ECG of these patients is rarely normal and shows a large spectrum of abnormalities. Signs of acute RVPO are evident in 30% of the patients. Rapid and simple identification of these cases with ECG at hospital admission can facilitate classification of the patients and provide pathophysiological insights.
Acknowledgements
ELCOVID Group. Azienda Ospedaliero-Universitaria di Ferrara ‘Arcispedale S. Anna’: Matteo Bertini, Roberto Ferrari, Gabriele Guardigli, Michele Malagù, Francesco Vitali, Ottavio Zucchetti, Emanuele D’Aniello, Carlo Alberto Volta, Luca Di Ienno, Federico Gibiino, Giovanni Zuliani, Roberto Manfredini, Roberto Zoppellari. Università degli Studi di Ferrara: Claudio Rapezzi. Ospedale del Delta, Lagosanto, Ferrara: Biagio Sassone, Giovanni Pasanisi, Erminio Righini. Ospedale degli Infermi, Rimini: Giancarlo Piovaccari, Daniele Grosseto. Ospedale Bufalini, Cesena: Alessandro Corzani, Marco Marconi. Ospedale Morgagni-Pierantoni, Forlì: Marcello Galvani. Ospedale S. Maria della Scaletta, Imola: Paolo Ortolani, Rossella Ferrara, Giovanni Vitale, Claudia Camanini. Maria Cecilia Hospital, Cotignola, Ravenna: Paolo Cimaglia, Lorenzo Filippo Mantovani. Ospedale S. Maria delle Croci, Ravenna: Andrea Rubboli, Annamaria Di Cesare, Maurizio Fusari. Ospedale di Bentivoglio, Bentivoglio, Bologna: Gianfranco Tortorici, Agnese Milandri. Ospedale Maggiore C. A. Pizzardi, Bologna: Gianni Casella, Laura Cardelli, Rodolfo Francesco Massafra. Ospedale Civile di Guastalla, Reggio Emilia: Elisa Guerri. Arcispedale S. Maria Nuova, Reggio Emilia: Alessandro Navazio, Massimo Pantaleoni. Ospedale Guglielmo da Saliceto, Piacenza: Luca Rossi, Andrea Biagi, Giovanni Quinto Villani, Matteo Villani. Ospedale Civile di Castel San Giovanni, Piacenza: Daniela Aschieri.
Conflict of interest: none declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Raffo M. et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B. et al. ST-segment elevation in patients with Covid-19 – a case series. N Engl J Med 2020;382:2478–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Surawicz B, Knilans T. Chou’s Electrocardiography in Clinical Practice 6th ed. Saunders; 2008. [Google Scholar]
- 5. Mahmoud-Elsayed HM, Moody WE, Bradlow WM, Khan-Kheil AM, Hudsmith LE, Steeds RP. Echocardiographic findings in Covid-19 pneumonia. Can J Cardiol 2020;doi: 10.1016/j.cjca.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Argulian E, Sud K, Vogel B, Bohra C, Garg VP, Talebi S. et al. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging 2020;doi: 10.1016/j.jcmg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thrainsdottir IS, Hardarson T, Thorgeirsson G, Sigvaldason H, Sigfusson N. The epidemiology of right bundle branch block and its association with cardiovascular morbidity—the Reykjavik study. Eur Heart J 1993;14:1590–1596. [DOI] [PubMed] [Google Scholar]
- 8. Rodger M, Makropoulos D, Turek M, Quevillon J, Raymond F, Rasuli P. et al. Diagnostic value of the electrocardiogram in suspected pulmonary embolism. Am J Cardiol 2000;86:807–809. [DOI] [PubMed] [Google Scholar]
- 9. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ. et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F. et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E. et al. Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020;75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schaller T, Hirschbühl K, Burkhardt K, Braun G, Trepel M, Märkl B. Postmortem examination of patients with COVID-19. JAMA 2020;323:2518–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A. et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 2020;doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X. et al. CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020;46:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M. et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost 2020;18:1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care 2020;24:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Repessé X, Charron C, Vieillard-Baron A. Acute cor pulmonale in ARDS: rationale for protecting the right ventricle. Chest 2015;147:259–265. [DOI] [PubMed] [Google Scholar]
- 19. Mitaka C, Nagura T, Sakanishi N, Tsunoda Y, Amaha K. Two-dimensional echocardiographic evaluation of inferior vena cava, right ventricle, and left ventricle during positive-pressure ventilation with varying levels of positive end-expiratory pressure. Crit Care Med 1989;17:205–210. [DOI] [PubMed] [Google Scholar]
- 20. Xu Z, Shi L, Wang Y, Zhang J, Huang L. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A. et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.