Abstract
Natural products have been used by humans since antiquity for both egregious and beneficial purposes. Regarding the latter, these products have long been valued as a rich source of phytochemicals and developed into numerous life-saving pharmaceutical agents. Today, the sales and use of natural products with purported medicinal qualities continue to increase worldwide. However, natural products are not subject to the same premarket testing requirements as pharmaceutical agents, creating critical gaps in scientific knowledge about their optimal use. In addition, due to the common misperception that “natural” means “safe,” patients may supplement or replace their prescription medications with natural products, placing themselves at undue risk for subefficacious pharmacotherapy or potentially toxic exposure. Collectively, with few exceptions, researchers, health care providers, and educators lack definitive information about how to inform consumers, patients, and students in the health professions on the safe and optimal use of these products. Recognition of this deficiency by key stakeholders, including the three pillars of biomedical research—industry, academia, and government—has facilitated multiple collaborations that are actively addressing this fundamental knowledge gap. This special issue contains a collection of articles highlighting the challenges faced by researchers in the field and the use of various experimental systems and methods to improve the mechanistic understanding of the disposition and drug interaction potential of natural products. Continued refinement of existing, and development of new, approaches will progress toward the common overarching goal of improving public health.
SIGNIFICANCE STATEMENT
Natural products with purported medicinal value constitute an increasing share of the contemporary health care market. Natural products are not subject to the same premarket testing requirements as drug products, creating fundamental scientific knowledge gaps about the safe and effective use of these products. Collaborations among industrial, academic, and governmental researchers in multiple disciplines are anticipated to provide the definitive information needed to fill these gaps and improve public health.
Introduction
According to the US National Institutes of Health National Center for Complementary and Integrative Health (NCCIH), natural products encompass a large and diverse group of substances that are produced from a variety of sources, including plants, bacteria, fungi, insects, arachnids, marine organisms, and higher-order animals (https://www.nccih.nih.gov/grants/natural-products-research-information-for-researchers). The term also includes complex mixtures produced by these sources, isolated compounds derived therefrom, as well as vitamins, minerals, probiotics, and special diets for medical conditions or health outcomes. As detailed in the proceeding sections, a host of other related terms, which vary with country or jurisdiction, are defined to convey to readers that these terms have distinct meanings and should not be used interchangeably. As such, the meaning of any term used in a publication or presentation should be clear at the outset and used consistently throughout (Paine and Roe, 2018). Before delving into this complexity of natural products, a brief history of the use of these products, specifically those that are plant-derived, is presented to underscore the necessity of continued research in the drug metabolism/pharmacokinetics space.
Brief History of Use.
As classily stated by the renowned natural products chemists Drs. James Robbers, Marilyn Speedie, and Vero Tyler, “Plants have been faithful servants to humans from the beginning of time” (Robbers et al., 1996). One of the earliest, most famous examples of an egregious use, circa 400 BC, involved the philosopher Socrates, who was sentenced to death by drinking a tea made from poison hemlock (Conium maculatum) when found guilty of heresy (Ober, 1977; Paine and Roe, 2018). A modern example of a deliberate fatal poisoning involved ricin, a constituent from the seeds of the castor oil plant (Ricinus communis), which was used to assassinate the Bulgarian dissident George Markov in 1978. Fans of the popular television series Breaking Bad, which aired from 2008 to 2013, will recall Walter White’s sinister use of ricin. Although intentional poisonings with natural products have waned exponentially since the era of Socrates, attempts, some fatal, have been reported sporadically into the 21st century (http://www.bbc.com/earth/story/20150817-earths-most-poisonous-plants). Fortunately, the beneficial uses of natural products far outweigh the nefarious uses, notably the development of a multitude of isolated constituents and analogs derived therefrom as life-saving or life-prolonging drug products, including aspirin, atropine, digoxin, morphine, quinidine, statins, and a plethora of antimicrobial and chemotherapeutic agents (Robbers et al., 1996).
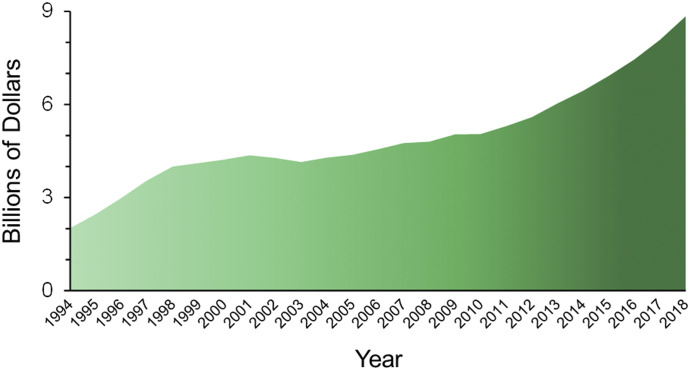
Nowadays, based on the (mis)perception that “natural” implies “safe,” consumers are turning increasingly to natural products to supplement, sometimes replace, their prescribed pharmacotherapies. The often-unqualified claims promoting the health benefits of these products, including those marketed on social media, have only enhanced this practice. Consequently, global sales of natural products have increased steadily during the past two decades, particularly in industrialized countries in which patients are accustomed to evidence-based Western medicine. Sales of herbal products alone in the United States have more than quadrupled since passage of the Dietary Supplement Health and Education Act (DSHEA) in 1994 (Table 1), from approximately $2 billion to nearly $9 billion in 2018 (Fig. 1). Because these products have become an appreciable component of the health care market, research on their optimal use must keep pace with rising popularity to mitigate public harm.
TABLE 1.
Glossary of country- or jurisdiction-specific terms related to natural products
| Country or jurisdiction | Term | Definition | Source (accessed July 15, 2020) |
|---|---|---|---|
| United States | Dietary supplement | A product (other than tobacco) in the United States that is intended to supplement the diet; contains one or more dietary ingredients (including vitamins, minerals, herbs or other botanicals, amino acids, and other substances) or their constituents; is intended to be taken by mouth as a pill, capsule, tablet, or liquid; and is labeled on the front panel as being a dietary supplement. | https://ods.od.nih.gov/factsheets/list-all/ |
| United States | Botanical | A plant or plant part valued for its medicinal or therapeutic properties, flavor, and/or scent. Products made from botanicals used for health purposes may be called herbal products, botanical products, or phytomedicines. Herbs are a subset of botanicals. | https://ods.od.nih.gov/factsheets/list-all/ |
| NA | Phytochemicals or phytochemical constituents | Chemical compounds produced by plants. | https://www.health.harvard.edu/staying-healthy/fill-up-on-phytochemicals |
| United States | DSHEA | A 1994 statute of the US federal legislation that defines and regulates dietary supplements. Under the DSHEA, manufacturers and distributors of dietary supplements and dietary ingredients are prohibited from marketing products that are adulterated or misbranded. Firms are responsible for evaluating the safety and labeling of their products before marketing to ensure that they meet all the requirements of the DSHEA and FDA regulations. | https://www.fda.gov/food/dietary-supplements |
| United States | Functional foods and nutraceuticals | Terms widely used in the marketplace. Foods associated with these terms are regulated in the United States by the FDA under the authority of the federal Food, Drug, and Cosmetic Act, albeit these products are not specifically defined by law. | https://www.fda.gov/food/food-labeling-nutrition |
| United States | New dietary ingredient | A dietary ingredient that was not sold in the United States as a dietary supplement before October 15, 1994. The FDA requires specific safety information from a manufacturer intending to market a dietary supplement containing a new dietary ingredient. This information is not required for older dietary supplement ingredients. | https://www.fda.gov/food/new-dietary-ingredients-ndi-notification-process/new-dietary-ingredients-dietary-supplements-background-industry#:∼:text=What%20is%20a%20%22new%20dietary,the%20FD%26C%20Act)%2C%2021%20U.S.C. |
| China | Traditional Chinese medicine | A 3000-year-old holistic system of medicine combining the use of medicinal herbs, acupuncture, food therapy, massage, and therapeutic exercise. Chinese physicians look for the underlying causes of imbalance in the “yin” and “yang” that lead to disharmony in the “qi” energy in the body. Traditional Chinese medicine addresses how illness manifests in a patient and treats the patient, not the ailment or disease. | https://www.hopkinsmedicine.org/health/wellness-and-prevention/chinese-medicine |
| China | Health foods | Defined by the China Health Food Registration and Filing as food products that have specific health function or supply vitamins and (or) minerals with the goal of regulating body’s function. However, it is not used for the purpose of curing disease and causes no acute, subacute, or chronic health effect to human body. | http://www.cirs-reach.com/news-and-articles/health-food-registration-and-filing.html# |
| China | Nutrition supplement | Defined by the China Health Food Registration and Filing as food that replenishes the vitamins and (or) minerals but without providing energy or other active ingredients. | http://www.cirs-reach.com/news-and-articles/health-food-registration-and-filing.html#:∼:text=Nutrition%20supplement%3A,energy%20or%20other%20active%20ingredients. |
| European Union | Herbal medicinal products | Any medicinal product, exclusively containing as active ingredients one or more herbal substances, one or more herbal preparations, or a combination of the two. | https://ec.europa.eu/health/human-use/herbal-medicines_en |
| European Union | Food supplement | Defined by the European Food Safety Authority as concentrated sources of nutrients (i.e., mineral and vitamins) or other substances with a nutritional or physiologic effect that are marketed in “dose” form (e.g., pills, tablets, capsules, liquids in measured doses). | https://www.efsa.europa.eu/en/topics/topic/food-supplements |
| Japan | Foods with health claims | Foods that comply with the specifications and standards established by the Ministry of Health, Labor and Welfare and are labeled with certain nutritional or health functions. These foods are categorized into two groups, according to differences in purpose and function: Foods with Nutrient Function Claims, defined as foods that are labeled with the functions of nutritional ingredients (vitamins and minerals), and Foods for Specified Health Uses, defined as foods officially approved to claim their physiologic effects on the human body. | https://www.mhlw.go.jp/english/topics/foodsafety/fhc/ |
| Canada | Natural health products | Defined by Health Canada as vitamins and minerals, herbal remedies, homeopathic medicines, traditional medicines such as traditional Chinese medicines, probiotics, and other products such as amino acids and essential fatty acids. | https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription.htm |
| Australia | Complementary medicines | Defined by the Australian Government Department of Health Therapeutic Goods Administration as “traditional” or “alternative” medicines including vitamin, mineral, herbal, aromatherapy, and homoeopathic products. | https://www.tga.gov.au/complementary-medicines |
NA, not applicable.
Fig. 1.
Sales of herbal products in the United Sates since passage of the Dietary Supplement Health and Education Act in 1994. Adapted from data reported by Blumenthal et al. (2006) and Smith et al. (2019).
This special issue of Drug Metabolism and Disposition includes an array of contributed articles describing integrative approaches to assess the mechanisms of disposition of natural products and select constituents and to predict pharmacokinetic natural product-drug interactions. Continued and new collaborative efforts among researchers in the industrial, governmental, and academic communities are expected to fill fundamental knowledge gaps, generating definitive information about how best to manage pharmacotherapeutic regimens that are supplemented with natural products.
Definitions.
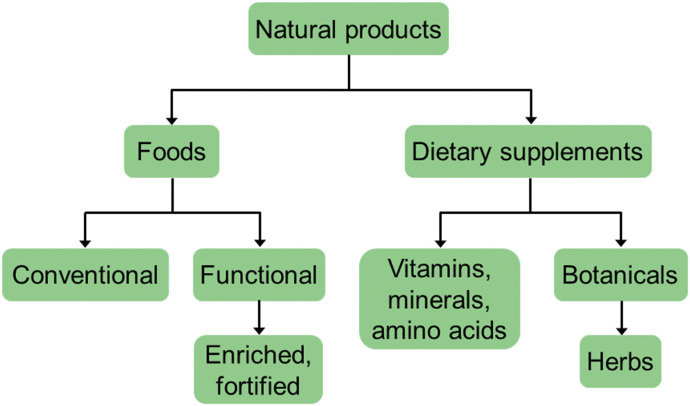
As evidenced throughout this special issue, a variety of terms related to natural products are used, potentially creating confusion. As such, a glossary of these terms is provided (Table 1). Those specific to the United States are further categorized to emphasize the distinct meanings (Fig. 2). As mentioned earlier, the meaning of a given term should be clear and used consistently in publications and presentations (Paine and Roe, 2018).
Fig. 2.
The natural products “umbrella” according to terms used by authorities in the United States. Definitions of select terms, along with those specific to other countries/jurisdictions, are provided in Table 1.
Regulatory Aspects.
Another common misperception of natural products, particularly in the United States, is that they are not regulated. The degree of premarketing requirements varies by country or jurisdiction, with perhaps those in the United States being the most lenient, at least for dietary supplements marketed before passage of the DSHEA. Regulations in other countries/jurisdictions are nicely detailed in a recent review (Roytman et al., 2018). Further details about the DSHEA and associated lessons learned during the last 25+ years are provided in a recent review by Gurley et al. (2018). Those pertinent to this special issue include the following:
Significant disconnects exist between in vitro assessments of biologic effects and in vivo realities.
Human clinical pharmacokinetic parameters (e.g., clearance, bioavailability, elimination half-life, etc.) for the most unique phytoconstituents are unknown.
Human clinical study design/conduct flaws and lack of standardization have hampered determination of potential botanical dietary supplement efficacy.
Education regarding both the positive and negative aspects of botanical dietary supplements remains lamentably inadequate among health care professionals.
Collaborative research efforts between the dietary supplement industry, regulatory agencies, and academia can benefit all parties and ultimately better serve public interests.
In spite of significant restrictions on labeling, which broadly limit product claims and alert consumers to the lack of testing, evaluation, and US Food and Drug Administration (FDA) oversight, the use of botanical dietary supplements continues to expand, and concomitant use with prescription medications remains a common practice.
The nuances specific to natural products research with respect to drug metabolism/pharmacokinetics and drug interaction potential are apparent, providing the impetus for this special issue. The primary goal is to increase awareness of these challenges and encourage researchers to develop and apply current and innovative experimental systems and methods to advance the field. The following sections summarize the contributed articles using these approaches.
Experimental Systems and Methods.
Like new chemical entities (NCEs), the disposition of natural product constituents is commonly influenced by a multitude of drug metabolizing enzymes and transporters expressed in a variety of organs, including the intestine, liver, kidney, and brain. Likewise, multiple experimental systems and methods are used to understand the disposition mechanisms and predict the drug interaction liability of natural products in humans. This special issue highlights a variety of approaches involving a variety of natural products, biochemical targets (i.e., drug metabolizing enzymes and transporters), and mechanisms of natural product-drug interactions (Table 2).
TABLE 2.
Summary of contributed original research articles to this special issue of Drug Metabolism and Disposition that illustrate the use of various experimental approaches to assess the disposition, drug interaction potential, or hepatoprotective effects of select natural products and constituents
| Natural product studied | Common use(s) | Biochemical target(s) | Experimental system(s) or approaches | Authors | ||
|---|---|---|---|---|---|---|
| Common name(s) | Latin name | Constituent(s) studied | ||||
| Cannabis, marijuana | Cannabis sativa | CBD, THC | Multiple | CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A | HLMs | Bansal et al. |
| Chrysin | NA | Chrysin, chrysin-7-glucuronide, chrysin-7-sulfate | Anxiety, depression, hypertension, maintaining testosterone levels | CYP2C9, CYP2C19, CYP2D6, CYP3A4, OATP1A2, OATP1B1, OATP1B3, OATP2B1, BCRP, MRP2, P-gp | CypExpress kits; OATP-overexpressing A431 cells; BCRP-, MRP2-, and P-gp–expressing membrane vesicles | Mohos et al. |
| Cinnamon | Cinnamomum spp. | Cinnamaldehyde, 2-methoxy-cinnamaldehyde | Cooking spice, glucose control | CYP2A6 | HLMs, purified CYP2A6 | Espiritu et al. |
| Goldenseal | Hydrastis canadensis | Berberine, (-)-β-hydrastine, hydrastinine | Common cold, upper respiratory tract infections, gastrointestinal disorders | CYP2C9, CYP2D6, CYP3A | HLMs, rCYP3A4, rCYP3A5 | McDonald et al. |
| Gotu kola, Indian pennywort | Centella asiatica | NA | Cognitive decline | CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19 CYP2D6, CYP3A | HLMs, human hepatocytes | Wright et al. |
| Turmeric | Curcuma longa | Curcumin glucuronide | Cooking spice, chemoprevention, multiple others | MRP3 | Membrane vesicles, Mrp3 knockout and wild type mice | Jia et al. |
| Yan hu suo, corydalis, Asian corydalis | Corydalis yanhusuo | Dehydrocorydaline | Coronary heart, disease, analgesia | OCT1, OCT3, OCTN1, OCTN2, P-gp | Transfected MDCK cells, primary neonatal rat cardiomyocytes, mice | Chen et al. |
| Zuojin pill | Rhizoma coptidis/Fructus evodiae (6:1 blend) | Berberine, coptisine, evodiamine, rutaecarpine | Gastrointestinal disorders | CYP2D6/Cyp2d1 | HLMs, rCYP2D6, RLMs, rats | Li et al. |
| NA | Schisandra chinensis | Lignans | Liver diseases | Multiple | Metabolomics, proteomics, lipidomics | Yan et al. |
| Multiple | Multiple | NA | Multiple | CYP3A | Cryopreserved human intestinal mucosa | Loretz et al. |
CBD, cannabidiol; MDCK, Manine-Darby canine kidney; NA, not applicable; OCTN, organic cation/carnitine transporter; rCYP, recombinant P450; RLM, rat liver microsome; THC, (−)-trans-Δ9-tetrahydrocannabinol.
Human Liver Microsomes.
Human liver microsomes (HLMs) represent a mainstay in vitro tool to characterize the metabolic pathways of natural product constituents, most commonly pathways involving the cytochromes P450 (P450s) and UDP-glucuronosyltransferases. In addition, HLMs are frequently used to determine the kinetics of such constituents as inhibitors of these enzymes. Bansal et al. (2020) evaluated the reversible and time-dependent inhibition (TDI) kinetics of the two major cannabinoids contained in cannabis, cannabidiol and (−)-trans-Δ9-tetrahydrocannabinol, toward CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A activity using HLMs and established probe substrates. The IC50 and KI values were corrected for extensive nonspecific binding to labware and incubation proteins to generate true (unbound) values that were used to predict pharmacokinetic cannabinoid-drug interactions in vivo using established mechanistic static models. Results suggested that the drug interaction potential of both cannabinoids might have been previously underestimated. McDonald et al. (2020) took a similar approach to evaluate three indole alkaloids contained in goldenseal—berberine, (-)-β-hydrastine, and hydrastinine—as potential time-dependent inhibitors of CYP2C9, CYP2D6, and CYP3A activity. All of these alkaloids contain methylenedioxyphenyl rings, known structural alerts for TDI of P450s. The effects of each alkaloid on the tested P450s were varied, with berberine showing strong TDI of CYP2D6 activity. Berberine additionally showed allosteric effects on CYP3A activity, which could attenuate the inhibitory effects of goldenseal on this enzyme in vivo.
Espiritu et al. (2020) used a multipronged approach to elucidate the mechanisms of CYP2A6-mediated TDI of nicotine metabolism in HLMs by two constituents in cinnamon, cinnamaldehyde and 2-methoxycinnamaldehyde. Molecular dynamic simulations indicated multiple ligand binding within the enzyme active site. Deconvoluted mass spectra indicated apoprotein modification by both constituents that was unaffected by glutathione. Heme degradation by 2-methoxycinnamaldehyde was detected via liquid chromatography–tandem mass spectrometry. These observations, combined with the TDI kinetics of each constituent recovered via numerical methods, suggested potential clinically relevant pharmacokinetic interactions between cinnamon and nicotine, as well as the CYP2A6 substrate and anticancer agent letrozole.
Zuojin pill is a traditional Chinese medicine (TCM) consisting of two commonly used herbs, Rhizoma coptidis and Fructus evodiae (Li et al., 2020). The prescription antidepressant venlafaxine undergoes extensive metabolism by CYP2D6 to form the active metabolite, O-desmethylvenlafaxine. Because Zuojin pill had been reported previously to inhibit CYP2D6 activity in vitro and to increase the plasma area under the concentration-time curve of the CYP2D6 probe drug dextromethorphan in healthy CYP2D6 extensive metabolizers, Li et al. (2020) hypothesized that Zuojin pill could interact with venlafaxine by inhibiting CYP2D6. Using HLMs, Zuojin pill showed strong inhibition of venlafaxine depletion and O-desmethlyvenlafaxine formation. Coptisine and berberine, constituents of R. coptidis, represented potential candidate inhibitors. Similar trends were observed using rat liver microsomes, albeit the magnitudes of effect were comparatively weaker, which were attributed to the well known species differences in P450 activity. The collective observations, including those from a pharmacokinetic study in rats and the previously reported Zuojin pill–dextromethorphan interaction in human subjects, suggested Zuojin pill could precipitate a pharmacokinetic interaction with venlafaxine when coadministered to patients.
A well characterized water extract of Centella asiatica, specifically CAW-R61J, is currently undergoing clinical development as a botanical drug to treat cognitive decline (Wright et al., 2020), a common affliction among the elderly population. As part of their Investigational New Drug (IND) application for CAW-R61J, Wright et al. (2020) characterized the P450 inhibition properties of this promising natural product using HLMs and standard procedures. CAW-R61J was a weak reversible inhibitor and was not a time-dependent inhibitor of the P450s tested (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A), suggesting low risk for CAW-R61J–precipitated pharmacokinetic drug interactions via P450 inhibition. These data, which should not be viewed as “negative,” provided critical safety information for CAW-R61J to support the IND application.
Isolated Enzyme Systems.
Isolated drug metabolizing enzymes, both recombinant and purified forms, represent another mainstay in vitro tool to aid in the identification of metabolic pathways and assess the inhibition kinetics of xenobiotics, as well as a variety of other mechanistic applications. McDonald et al. (2020) used recombinant CYP3A4 and CYP3A5 to gain additional mechanistic insight into the allosteric effects observed with berberine on CYP3A activity (midazolam hydroxylation) in HLMs, whereas Espiritu et al. (2020) used purified CYP2A6 to assess the mechanism of TDI of the cinnamon constituents toward CYP2A6.
Chrysin, a flavonoid contained in honey and many dietary supplements, undergoes extensive phase II conjugation in humans, producing chrysin-7-sulfate and chrysin-7-glucuronide. Mohos et al. (2020) compared the inhibitory effects of the two conjugated metabolites with chrysin on CYP2C9, CYP2C19, CYP2D6, and CYP3A4 using CypExpress kits, which are permeabilized and stabilized dried yeast powder preparations containing full length, unmodified, human P450s and recombinant human NADPH oxidoreductase (https://www.oxfordbiomed.com/tech-resources/resources/cypexpress). The sulfate conjugate was a strong inhibitor of CYP2C9, whereas the glucuronide conjugate showed minimal to no inhibition of the P450s tested (Mohos et al., 2020). Because systemic peak concentrations of chrysin-7-sulfate have been reported to greatly exceed chrysin concentrations in humans after oral administration of chrysin (Walle et al., 2001; Mohos et al., 2020), the authors concluded that chronic consumption of dietary supplements containing high amounts of chrysin could precipitate pharmacokinetic interactions with CYP2C9 substrates.
Human Hepatocytes.
Unlike HLMs, human hepatocytes contain native receptors and drug metabolizing enzymes, as well as transporters, and remain the gold standard for assessing the induction of drug metabolizing enzymes, particularly the P450s, by NCEs and other xenobiotics (Fahmi and Ripp, 2010; Dvorak, 2016; https://www.fda.gov/regulatory-information/search-fda-guidance-documents/vitro-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions). In addition to characterizing the P450 inhibitory effects of CAW-R61J described earlier, Wright et al. (2020) assessed the P450 inductive effects of this natural product. Using cryopreserved sandwich-cultured human hepatocytes, CAW-R61J showed no induction of the P450s tested (CYP1A2, CYP2C9, CYP3A), as assessed by the lack of effect on both mRNA and catalytic activity. As with the P450 inhibition data, this useful—not negative—information provided critical knowledge about the safety of CAW-R61J in support of an IND application.
In Vitro Transporter-Expressing Systems.
Because transporters function to move xenobiotics into or out of cells across a basolateral or apical membrane, HLMs and other subcellular tissue fractions cannot be used to assess transporter-mediated disposition of xenobiotics. Thus, transporter-expressing systems are often used, including membrane vesicles and transfected cell lines.
Multidrug resistance-associated protein (MRP) 3 is an efflux transporter expressed on the basolateral membrane of enterocytes, hepatocytes, and other cell types. Curcumin, a major polyphenol contained in turmeric, undergoes extensive first-pass metabolism to form curcumin-O-glucuronide, the primary metabolite detected in plasma and feces (Jia et al., 2020). This glucuronide is eliminated from the body via MRP2- and breast cancer resistance protein (BCRP)–mediated biliary excretion. Because glucuronide conjugates are typical substrates for both MRP2 and MRP3, Jia et al. (2020) hypothesized that curcumin-O-glucuronide could also be a substrate for MRP3 for eventual excretion by the kidneys. Using MRP3-expressing inside-out membrane vesicles, they showed that the uptake of the glucuronide was higher in the presence of ATP compared with the presence of AMP. These in vitro data supported results from in vivo mouse studies, which showed that systemic plasma concentrations of the glucuronide were much lower, and corresponding liver-to-plasma ratios much higher, in Mrp3 knockout compared with wild type mice. Based on the combined data, the authors concluded that curcumin-O-glucuronide is a substrate for MRP3/Mrp3.
In addition to examining the inhibitory effects of chrysin-7-sulfate and chrysin-7-glucuronide on the P450s described earlier, Mohos et al. (2020) tested the effects of these conjugates on human transporters using A431 cells transfected with uptake transporters and inside-out membrane vesicles expressing efflux transporters. Both conjugates were shown to inhibit the uptake transporters organic anion transporting polypeptide (OATP) 1A2, OATP1B1, OATP1B3, and OATP2B1 and the efflux transporter BCRP. The sulfate conjugate was generally more potent than the glucuronide conjugate. As postulated for CYP2C9, chronic consumption of dietary supplements containing high amounts of chrysin could precipitate interactions with OATP and BCRP substrates.
The TCM Corydalis yanhusuo (Table 2) contains the major active constituent, dehydrocorydaline, which has been shown to accumulate in the heart after oral administration of C. yanhusuo or dehydrocorydaline to mice. Using Manine-Darby canine kidney cells transfected with various human transporters, Chen et al. (2020) showed dehydrocorydaline to be a substrate for organic cation transporter (OCT) 1 and OCT3, which are uptake transporters expressed on the basolateral membrane of cells, including cardiomyocytes. Subsequent experiments with neonatal rat cardiomyocytes, a pharmacokinetic study in mice, and transporter inhibitors further supported the notion that the accumulation of dehydrocorydaline in mouse heart is mediated at least in part by Oct1/3.
The in vitro transporter systems described above, along with others, are detailed further in the minireview by Cao et al. (2020), which is focused on TCMs, and constituents thereof, as a rich source of potential multidrug resistance reversal agents. Specifically, the efflux transporter P-glycoprotein (P-gp) is known to reduce intracellular concentrations of anticancer drugs in tumor cells overexpressing P-gp, which can lead to reduced efficacy of the anticancer drug. Development of effective small molecule P-gp inhibitors has largely failed in the clinic due to nonspecific or nonselective distribution to nontarget organs (Callaghan et al., 2014; Kumar and Jaitak, 2019). The identification of P-gp inhibitors from TCMs could lead to safer multidrug resistance reversal agents.
In Vitro Intestinal Models.
The intestine is arguably the major extrahepatic site for xenobiotic-drug interactions, particularly for natural products, as the associated constituents typically do not achieve sufficient concentrations in the portal or systemic circulation after oral administration to precipitate interactions. The limited concentrations are typically due to poor absorption and/or extensive first-pass metabolism. In addition, the complement of drug metabolizing enzymes and transporters differs from that of the liver, often precluding liver-derived models to elucidate biochemical determinants of natural product constituent disposition in the intestine. The minireview by Li (2020) details currently available enteric cell systems to assess the metabolism and drug interaction potential of xenobiotics. These systems include the immortalized human colon adenocarcinoma cell line Caco-2; human stem cell–derived enterocyte models; and primary cell models, specifically human intestinal slices, cryopreserved human enterocytes, and cryopreserved human intestinal mucosa. Advantages and disadvantages of each system are provided. The author concluded that the primary cell models represent appropriate models for the assessment of intestinal metabolism and drug interaction potential of orally administered xenobiotics, including natural products. Loretz et al. (2020) applied a cryopreserved human intestinal mucosa model to evaluate the CYP3A inhibitory, thus drug interaction potential, of 29 commercially available herbal products and compared them to grapefruit juice, a well established clinically relevant inhibitor of enteric CYP3A. The inhibitory effects of four of these products (green tea extract, horehound, St. John’s wort, and valerian root) were similar to those for grapefruit juice, suggesting these products could precipitate interactions with orally administered CYP3A substrates.
Omics.
Acetaminophen overdose is the most common cause of acute liver failure in Western countries (Yan et al., 2020). Despite decades of research into the mechanisms of this life-threatening event, treatment options remain limited. Although N-acetylcysteine is an effective antidote, treatment should begin within 24 hours after overdose. As such, alternative treatments of acetaminophen overdose are urgently needed. Lignans contained in the TCM Schisandra chinensis have been shown to exhibit hepatoprotective effects. Yan et al. (2020) used a multi-omics approach (metabolomics, proteomics, lipidomics) to determine the mechanism of acetaminophen-induced acute liver failure and hepatoprotective effects of a Schisandra lignan extract in mice. Results indicated that the liver failure was associated with lipid regulation and that Schisandra lignan extract exerts hepatoprotective effects by decreasing intrahepatic diglyceride and triglyceride concentrations. The authors concluded that this multi-omics approach could be used to determine the biologic mechanism(s) of other TCMs and to develop an approach for the prevention and treatment of acute liver failure.
Human Subjects.
As with NCEs, human subjects represent the definitive experimental system to investigate the pharmacokinetics, pharmacodynamics, and drug interaction liability of natural products. However, obvious disadvantages, notably cost and time, preclude routine testing. In addition, clinical testing of natural products adds complexities that typically are not encountered with NCEs, particularly with respect to product integrity (Kellogg et al., 2019; Weber and Hopp, 2020). Nevertheless, when warranted based on the collective in vitro, preclinical, and safety data, clinical studies involving natural products are feasible. The NCCIH is a major federal funder of natural products research in the United States (https://www.nccih.nih.gov/), supporting a broad portfolio that includes clinical trials. The commentary by Weber and Hopp (2020) describes an overview of key aspects covering product integrity and clinical research design considerations, as well as NCCIH’s current investments and funding opportunities.
Natural Product-Drug Interactions.
A number of experimental approaches to assess potential pharmacokinetic natural product-drug interactions are described in the preceding sections. The majority of natural products examined were evaluated as precipitants (“perpetrators”) of interactions with prescription drugs acting as the object (“victim”) drugs, i.e., whose pharmacokinetics could be altered by the natural product. All involved inhibition of P450s and/or transporters, illustrating the preponderance of these proteins as biochemical targets of natural product-drug interactions, akin to drug-drug interactions. Other potential targets include non-P450 phase I enzymes, phase II enzymes, and understudied or unidentified enzymes and transporters.
Emerging Biochemical Targets.
Carboxylesterases (CESs) are non-P450 phase I enzymes that represent understudied biochemical targets of natural product-drug interactions. The minireview by Qian and Markowitz (2020) focuses on CES1, the major esterase expressed in human liver, which catalyzes the hydrolysis of a variety of drugs, toxins, and endogenous compounds. Based on their systematic literature search, the authors identified three cannabinoids, several constituents in botanical dietary supplements, and two TCMs that could precipitate CES1-mediated interactions by inhibiting the enzyme. As with natural products in general, translation of these in vitro assessments is hampered by the scarce human pharmacokinetic data available, as noted earlier in this commentary. These and other limitations, challenges, and future directions are provided.
In Vitro to In Vivo Extrapolation.
In vitro to in vivo extrapolation approaches are used routinely during early drug development to predict the magnitude of potential drug-drug interactions involving an NCE as either a precipitant or object drug. These approaches include basic and mechanistic static models detailed in a US Food and Drug Administration guidance document (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/vitro-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions). Three of the contributed original research articles, those involving cannabinoids (Bansal et al., 2020), cinnamon (Espiritu et al., 2020), and goldenseal (McDonald et al., 2020), describe the use of mechanistic static models to assess the likelihood of a given natural product-drug interaction. At least one constituent of each natural product was predicted to precipitate at least one pharmacokinetic interaction. Because these models are static, such predictions tend to be overestimated. Nevertheless, the models provide a relatively efficient means to guide next steps, e.g., development of physiologically based pharmacokinetic models or clinical evaluation to determine clinical relevance or identify patient populations most susceptible to the interaction.
Data Dissemination.
Dissemination of scientific data to the research community and other stakeholders beyond journal publications and presentations at professional and scientific meetings has come to the forefront due in part to the information being readily accessible via internet sources, including social media. As noted by Weber and Hopp (2020), the NCCIH is supporting a repository that houses data generated by the Center of Excellence for Natural Product Drug Interaction Research (NaPDI Center), whose mission is to provide leadership and guidance on the optimal design and conduct of pharmacokinetic natural product–drug interaction studies (Paine et al., 2018). Birer-Williams et al. (2020) describe this new user-friendly online repository, which is accessible through a public access portal. Two high-priority natural products currently under study by the NaPDI Center, cannabis and kratom, are used as case studies to illustrate the types of data that are being entered into the repository, which range from chemical characterization of the natural product study materials to pharmacokinetic data obtained from clinical studies. The repository, which will be housing data produced by research groups in addition to the NaPDI Center, is expected to generate new hypotheses and facilitate multidisciplinary collaborations to help inform which natural products merit evaluation for drug interaction liability in a rigorous, expeditious manner.
Final Remarks
As another lesson learned since passage of the DSHEA, Gurley et al. (2018) elegantly stated, “Complex phytochemical mixtures beget complex research problems and solutions.” Although the research community has gained a wealth of knowledge during the last two-plus decades about the challenges unique to natural products research, health care providers and consumers are still without definitive knowledge about the optimal use of the majority of these products. Likewise, health care educators lack definitive information to deliver to their students. Continued partnerships among the natural products and pharmaceutical industries, governmental agencies, and academia, coupled with robust experimental approaches and data dissemination strategies will assuredly help fill these fundamental knowledge gaps to achieve the ultimate goal of improving public health.
Acknowledgments
The author thanks Deena Hadi for her assistance in preparing and finalizing the manuscript. The author dedicates this article to Dr. David P. Paine.
Abbreviations
- BCRP
breast cancer resistance protein
- CES
carboxylesterase
- DSHEA
Dietary Supplement Health and Education Act
- FDA
US Food and Drug Administration
- HLM
human liver microsome
- IND
Investigational New Drug
- MRP
multidrug resistance-associated protein
- NaPDI Center
Center of Excellence for Natural Product Drug Interaction Research
- NCCIH
National Center for Complementary and Integrative Health
- NCE
new chemical entity
- OATP
organic anion transporting polypeptide
- OCT
organic anion transporter
- P-gp
P-glycoprotein
- P450
cytochrome P450
- TCM
traditional Chinese medicine
- TDI
time-dependent inhibition
Authorship Contribution
Wrote the manuscript: Paine.
Footnotes
This work was supported in part by National Institutes of Health National Center for Complimentary and Integrative Health [Grant U54 AT008909]. The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
References
- Bansal S, Maharao N, Paine MF, Unadkat JD. (2020) Predicting the potential for cannabinoids to precipitate pharmacokinetic drug interactions via reversible inhibition or inactivation of major cytochromes P450. Drug Metab Dispos DOI: 10.1124/dmd.120.000073 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birer-Williams C, Gufford BT, Chou E, Alilio M, VanAlstine S, Morley RE, McCune JS, Paine MF, Boyce RD. (2020) A new data repository for pharmacokinetic natural product-drug interactions: from chemical characterization to clinical studies. Drug Metab Dispos DOI: 10.1124/dmd.120.000054 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal M, Cavaliere C, Ferrier GKL. (2006) Total sales of herbal supplements in United States show steady growth. HerbalGram 71:64–66. [Google Scholar]
- Callaghan R, Luk F, Bebawy M. (2014) Inhibition of the multidrug resistance P-glycoprotein: time for a change of strategy? Drug Metab Dispos 42:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Shi Y, Cai Y, Hong Z, Chai Y. (2020) The effects of traditional Chinese medicine on P-glycoprotein mediated multidrug resistance and methods to study the herb–P-glycoprotein interactions. Drug Metab Dispos. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li C, Yi Y, Du W, Jiang H, Zeng S, Zhou H. (2020) Organic cation transporter 1 and 3 contribute to the high accumulation of dehydrocorydaline in the heart. Drug Metab Dispos DOI: 10.1124/dmd.120.000025 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Dvorak Z. (2016) Opportunities and challenges in using human hepatocytes in cytochromes P450 induction assays. Expert Opin Drug Metab Toxicol 12:169–174. [DOI] [PubMed] [Google Scholar]
- Espiritu MJ, Chen J, Yadav J, Larkin M, Pelletier RD, Chan JM, GC JB, Natesan S, Harrelson JP. (2020) Mechanisms of herb-drug interactions involving cinnamon and cytochrome P450 2A6: focus on time-dependent inhibition by cinnamaldehyde and 2-methoxycinnamaldehyde. Drug Metab Dispos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmi OA, Ripp SL. (2010) Evaluation of models for predicting drug-drug interactions due to induction. Expert Opin Drug Metab Toxicol 6:1399–1416. [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Yates CR, Markowitz JS. (2018) “…Not intended to diagnose, treat, cure or prevent any disease.” 25 years of botanical dietary supplement research and the lessons learned. Clin Pharmacol Ther 104:468–483. [DOI] [PubMed] [Google Scholar]
- Jia Y-M, Zhu T, Zhou H, Ji J-Z, Tai T, Xie H-G. (2020) MRP3 is responsible for the efflux transport of curcumin glucuronide from hepatocytes to the blood. Drug Metab Dispos DOI: 10.1124/dmd.119.089193 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Kellogg JJ, Paine MF, McCune JS, Oberlies NH, Cech NB. (2019) Selection and characterization of botanical natural products for research studies: a NaPDI center recommended approach. Nat Prod Rep 36:1196–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Jaitak V. (2019) Natural products as multidrug resistance modulators in cancer. Eur J Med Chem 176:268–291. [DOI] [PubMed] [Google Scholar]
- Li AP. (2020) In vitro human cell-based experimental models for the evaluation of enteric metabolism and drug interaction potential of drugs and natural products. Drug Metab Dispos DOI: 10.1124/dmd.120.000053 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Li Y, Li J, Yan DM, Wang Q, Jin JY, Tan B, Qiu F. (2020) Influence of Zuojin Pill on the metabolism of venlafaxine in vitro and in rats and associated herb-drug interaction. Drug Metab Dispos DOI: 10.1124/dmd.120.000048 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Loretz C, Ho D, Alam N, Mitchell W, Li AP. (2020) Application of cryopreserved human intestinal mucosa and cryopreserved human enterocytes in the evaluation of herb-drug interactions: evaluation of CYP3A inhibitory potential of grapefruit juice and commercial formulations of 29 herbal supplements. Drug Metab Dispos DOI: 10.1124/dmd.120.000033 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- McDonald MG, Tian D-D, Thummel KE, Paine MF, Rettie AE. (2020) Modulation of major human liver microsomal cytochromes P450 by component alkaloids of goldenseal: time-dependent inhibition and allosteric effects. Drug Metab Dispos DOI: 10.1124/dmd.120.091041 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohos V, Fliszár-Nyúl E, Ungvári O, Bakos É, Kuffa K, Bencsik T, Zsidó BZ, Hetényi C, Telbisz Á, Özvegy-Laczka C, et al. (2020) Effects of chrysin and its major conjugated metabolites chrysin-7-sulfate and chrysin-7-glucuronide on cytochrome P450 enzymes, and on OATP, P-gp, BCRP and MRP2 transporters. Drug Metab Dispos DOI: 10.1124/dmd.120.000085 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Ober WB. (1977) Did Socrates die of hemlock poisoning? N Y State J Med 77:254–258. [PubMed] [Google Scholar]
- Paine MF, Roe AL. (2018) “Green medicine”: the past, present, and future of botanicals. Clin Pharmacol Ther 104:410–415. [DOI] [PubMed] [Google Scholar]
- Paine MF, Shen DD, McCune JS. (2018) Recommended approaches for pharmacokinetic natural product-drug interaction research: a NaPDI center commentary. Drug Metab Dispos 46:1041–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Markowitz JS. (2020) Natural products as modulators of CES1 activity. Drug Metab Dispos DOI: 10.1124/dmd.120.000065 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Robbers JE, Speedie MK, Tyler VE. (1996) Pharmacognosy and Pharmacobiotechnology, 9th ed, Williams & Wilkins, Baltimore, MD. [Google Scholar]
- Roytman MM, Poerzgen P, Navarro V. (2018) Botanicals and hepatotoxicity. Clin Pharmacol Ther 104:458–469. [DOI] [PubMed] [Google Scholar]
- Smith T, Gillespie M, Eckl V, Knepper J, Morton-Reynolds C. (2019) Herbal supplement sales in US increase by 9.4% in 2018. HerbalGram 123:62–73. [Google Scholar]
- Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV. (2001) Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol 51:143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber WJ, Hopp DC. (2020) NCCIH perspectives on clinical research involving natural products. Drug Metab Dispos DOI: 10.1124/dmd.120.000071 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KM, Alcazar Magaña A, Laethem RM, Moseley CL, Banks TT, Maier CS, Stevens JF, Quinn JF, Soumyanath A. (2020) Centella asiatica water extract shows low potential for CYP-mediated drug interactions. Drug Metab Dispos DOI: 10.1124/dmd.120.090860 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Guo H, Ding Q, Shao Y, Kang D, Yu T, Li C, Huang H, Du Y, Wang H, et al. (2020) Multi-omics profiling reveals protective function of Schisandra lignans against acetaminophen-induced hepatotoxicity. Drug Metab Dispos DOI: 10.1124/dmd.120.000083 [published ahead of print]. [DOI] [PubMed] [Google Scholar]