Abstract
Aims
The global COVID-19 pandemic is caused by the SARS-CoV-2 virus entering human cells using angiotensin-converting enzyme 2 (ACE2) as a cell surface receptor. ACE2 is shed to the circulation, and a higher plasma level of soluble ACE2 (sACE2) might reflect a higher cellular expression of ACE2. The present study explored the associations between sACE2 and clinical factors, cardiovascular biomarkers, and genetic variability.
Methods and results
Plasma and DNA samples were obtained from two international cohorts of elderly patients with atrial fibrillation (n = 3999 and n = 1088). The sACE2 protein level was measured by the Olink Proteomics® Multiplex CVD II96 × 96 panel. Levels of the biomarkers high-sensitive cardiac troponin T (hs-cTnT), N-terminal probrain natriuretic peptide (NT-proBNP), growth differentiation factor 15 (GDF-15), C-reactive protein, interleukin-6, D-dimer, and cystatin-C were determined by immunoassays. Genome-wide association studies were performed by Illumina chips. Higher levels of sACE2 were statistically significantly associated with male sex, cardiovascular disease, diabetes, and older age. The sACE2 level was most strongly associated with the levels of GDF-15, NT-proBNP, and hs-cTnT. When adjusting for these biomarkers, only male sex remained associated with sACE2. We found no statistically significant genetic regulation of the sACE2 level.
Conclusions
Male sex and clinical or biomarker indicators of biological ageing, cardiovascular disease, and diabetes are associated with higher sACE2 levels. The levels of GDF-15 and NT-proBNP, which are associated both with the sACE2 level and a higher risk for mortality and cardiovascular disease, might contribute to better identification of risk for severe COVID-19 infection.
Keywords: COVID-19, ACE2, Biomarker, Cardiovascular disease, Atrial fibrillation, Age
Introduction
The rapidly spreading COVID-19 pandemic1–3 caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)3,4 invades mainly cells in the respiratory tract, which might lead to a life-threatening pneumonitis.5 The most recent pandemic with the H1N1 influenza virus mainly affected young adults without any sex difference.6,7 In contrast, COVID-19 is more severe at older age, with comorbidities, i.e. diabetes mellitus, hypertension, and vascular disease, and in men.1,8–10
The SARS-CoV-2 virus adheres to the pulmonary alveolar epithelial cells by its surface spike protein, which binds to the cell membrane-bound angiotensin-converting enzyme 2 (ACE2).5,11–13 In this context ACE2 functions as a receptor following activation of the SARS-CoV-2 spike protein by the cellular transmembrane protease serine 2 (TMPRSS2).14 After entering the cell, the SARS-CoV-2 virus replicates rapidly, leading to high early virus loads.15 The soluble ACE2 (sACE2) protein is shed into the circulation.14,16,17 In healthy subjects and patients with stable chronic diseases, the sACE2 level probably reflects the expression and availability of ACE2 at the cellular level. Currently there is limited knowledge on the regulation of ACE2 expression and circulating sACE2 levels in humans. Recently we measured the levels of sACE2 protein in plasma, as part of a project on multiplex proteomics in relations to outcomes in two large global cohorts of patients with atrial fibrillation (AF). Now we have taken the opportunity to explore the levels of sACE2 and their relationship to risk factors for COVID-19 and genetic variability in these two cohorts. The extended knowledge on the interplay between sACE2 levels and clinical manifestations of biomarkers reflecting cardiovascular dysfunction, might improve the understanding of the higher risk of COVID-19 in males and the elderly with cardiovascular disease.
Materials and methods
Cohorts
The ARISTOTLE trial included 18 201 patients with AF and an increased risk of stroke who were randomized to a median of 1.9 years treatment with warfarin or apixaban. Biomarker samples at baseline were available from 14 611 participants and, for economic reasons, a random sample of 3999 patients was used for the multiplex proteomics analysis including the sACE2 measurements in the current study. The RE-LY trial included 18 113 patients with AF and an increased risk of stroke to a median of 2.0 years treatment with dabigatran or warfarin. Biomarker samples drawn at baseline were available in 8548, out of which a random sample of 1088 was used for the multiplex proteomics analysis including the sACE2 measurements for this study. The details of the trials and the biomarker programmes have previously been published.18–20 The trials complied with the Declaration of Helsinki and were approved by appropriate ethics committees at all sites. All patients provided written informed consent.
Samples
In the ARISTOTLE19,20 and the RE-LY18 trials, patients provided blood samples at randomization into vacutainer tubes containing EDTA, which were centrifuged immediately. Plasma was pipetted into freezing tubes in aliquots and stored at –70°C until analyses.
Biochemical methods
The analysis of the ACE2 level in plasma was performed at the Clinical Biomarkers Facility, Science for Life Laboratory, Uppsala University, Uppsala, Sweden. The determination of the sACE2 protein level was performed using Proximity Extension Assay (PEA) with the Olink Proteomics® Multiplex CVDII96 × 96 panel.21 The plasma concentrations of high-sensitive cardiac troponin T (hs-cTnT), N-terminal probrain natriuretic peptide (NT-proBNP), and growth differentiation factor 15 (GDF-15) were determined by Roche immunoassays using a Cobas Analytics e601 (Roche Diagnostics, Penzberg, Germany).18–20 High sensitive C-reactive protein (CRP) was analysed using a particle-enhanced immunoturbidimetric assay, (Abbott, Abbott Park, IL, USA) and high-sensitivity interleukin-6 (IL-6) by sandwich ELISA immunoassays (R&D Systems Inc., Minneapolis, MN, USA). D-dimer was determined by an enzyme immunoassay (Asserachrome, Stago, France). Cystatin C was analysed with the ARCHITECT system ci8200 (Abbott Laboratories) using the particle-enhanced turbidimetric immunoassay (PETIA) from Gentian (Moss, Norway). Estimated glomerular filtration rate (eGFR) was calculated based on centrally determined creatinine levels using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. The conventional biomarker analyses were performed at the Uppsala Clinical Research Center Laboratory, Uppsala University, Uppsala, Sweden.
Statistical methods
The associations between sACE2 and a number of pre-specified variables (Table 1) were analysed using linear regression models with sACE2 as outcome. As sACE2 is measured on the OLINK CVDII chip, the measuring unit is NPX, representing a log2 scale of some original unit of the concentration. Thus, parameter estimates from the regression models should be interpreted as the difference in mean log2 concentration of sACE2. Hence, an estimate of 1 would indicate a doubling of concentration on the unknown original scale.
Table 1.
Baseline characteristics in the ARISTOTLE and RELY cohorts
| Cohort | ARISTOTLE | RE-LY |
|---|---|---|
| Patients | n = 3999 | n = 1088 |
| Age, (years) median (IQR) | 70.0 (63.0–76.0) | 72.0 (67.0–77.0) |
| Sex: male | 63.0% (2519) | 62.8% (683) |
| Body mass index, kg/m2 | 28.6 (25.4–32.7) [19] | 27.9 (24.9–31.2) [2] |
| Current smoker | 8.8% (353) | 7.5% (82) |
| Diabetes | 25.0% (998) | 21.1% (230) |
| Hypertension | 87.7% (3506) | 79.0% (859) |
| Congestive heart failure | 31.2% (1247) | 29.1% (317) |
| Prior myocardial infarction | 13.0% (518) | 16.8% (183) |
| Prior peripheral arterial disease | 4.8% (193) | 3.6% (39) |
| Prior stroke/TIA | 18.4% (736) | 20.3% (221) |
| Medications | ||
| Prior beta-blocker | 66.5% (2660) | 61.4% (668) |
| Prior aspirin | 41.7% (1667) | 40.5% (441) |
| Prior alopidogrel | 3.9% (154) | 5.0% (54) |
| Prior statin | 41.3% (1652) | 42.2% (459) |
| Prior ACE inhibitor | 50.9% (2036) | 49.9% (543) |
| Prior ARB | 23.8% (952) | 21.2% (231) |
| Prior ACE inhibitor or ARB | 71.6% (2864) | 68.5% (745) |
| Prior amiodarone | 13.5% (538) | 12.9% (140) |
| Biomarkers | ||
| NT-proBNP (ng/L) | 697.5 (368.0–1248.8) [3] | 845.5 (391.8–1470.0) [0] |
| hs-cTnT (ng/L) | 10.8 (7.4–16.6) | 12.1 (7.7–19.5) |
| GDF-15 (ng/L) | 1371.0 (966.0–2060.0) | 1474.5 (1097.5–2147.5) |
| CRP (mg/L) | 2.2 (1.0–4.6) [4] | 2.5 (1.2–5.5) [357] |
| IL-6 (ng/L) | 2.3 (1.5–4.0) [1] | 2.4 (1.5–3.9) [358] |
| eGFR (CKD-EPI) (mL/min) | 56.3 (45.5–68.6) [1] | 65.7 (54.9–76.9) [9] |
| Cystatin C (mg/L) | 1.0 (0.8–1.2) [4] | 1.0 (0.8–1.2) [357] |
| D-dimer (μg/L) | 521.0 (332.0–859.0) [26] | 511.5 (322.2–888.0) [358] |
| Soluble ACE2 (sACE2) (NPX) | 3.9 (3.5–4.4) | 4.4 (3.9–4.8) |
TIA, transient ischaemic attack; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; NT-proBNP, N-terminal probrain natriuretic peptide; hs-cTnT, high-sensitive cardiac troponin T; GDF-15, growth differentiation factor 15; CRP, C-reactive protein; IL-6, interleukin-6; eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Numbers in square brackets represent missing data.
We fitted unadjusted models including each of the variables of interest as well as multivariable models including (i) all ‘clinical’ variables (age, sex, body mass index, current smoker, hypertension, diabetes, prior myocardial infarction, prior coronary revascularization, prior stroke/transient ischaemic attack, prior peripheral arterial disease, congestive heart failure); (ii) all clinical variables and treatments at baseline (beta-blocker, aspirin, clopidogrel, statin, ACE inhibitor or angiotensin receptor blocker, amiodarone); and (iii) all clinical variables, treatments at baseline, and standard biomarkers (NT-proBNP, hs-cTnT, GDF-15, cystatin C, and IL-6).
All continuous variables were represented by restricted cubic splines with four knots placed at the marginal 5th, 35th, 65th, and 95th sample percentiles. For these variables, comparisons of the mean sACE2 were made for the difference between the third and the first sample quartile. Associations between sACE2 and each variable in the equations were presented graphically as predicted values with corresponding 95% confidence intervals, holding all other variables constant at the median value for continuous variables, ‘male’ for sex, and ‘no’ for prior diseases or treatments at baseline.
As there were 358 patients with missing values for CRP, IL-6, cystatin C, and D-dimer in RE-LY (Table 1), multiple imputation was used for the RE-LY data. The proportion of subjects with non-complete data was ∼35%. Therefore, 35 imputed datasets were created with predictive mean matching using the mice function in the R add-on package mice.22 Analyses were performed on the imputed datasets and combined using the function fit.mult.impute in the Hmisc23 package. In a sensitivity analysis, all analyses were performed on the complete cases only in RE-LY, with the results materially unaltered (data not shown).
All analyses were done following the regression modelling strategies outlined by Harrell24 using R (version 3.6.1).25 In particular, the packages Hmisc and rms,23,24 and mice22,26 were used.
Genetic methods
Participation in the ARISTOTLE and RE-LY genetic substudies with whole blood sampling for DNA extraction was voluntary and based on signing a separate consent form. The genotyping was performed in a subset of 5620 patients in ARISTOTLE using the Illumina Global Screening Array (24v2) and a subset of 3076 patients in RE-LY using the Illumina Human610-quad chip (Illumina, San Diego, CA, USA). Quality controls were performed using the whole-genome association analysis toolset PLINK v1.9 (https://www.cog-genomics.org/plink/)27 [sex check, call rate single nucleotide polymorphism (SNP) and individual >98%, minor allele frequency (MAF) >0.001, and Hardy–Weinberg equilibrium (HWE) P-value >1 × 10–8]. Imputation of genotypes was performed using the Haplotype reference consortium v1.1, utilizing Eagle v2.3.3 for phasing28 and PBWT for imputation.29Post-imputation, the data were filtered (MAF >0.005 and IMPUTE2s Info metric >0.7) and converted to hard calls using Plink.27 As the random sampling for sACE2 analyses did not consider availability to whole-genome association study (GWAS), there were only 1583 and 289 subjects included both in the subset with measurements of sACE2 and in the subset with performance of GWAS analyses in the ARISTOTLE and RE-LY cohorts, respectively. Analyses of the GWAS were performed using linear regression implemented in Plink v1.9,27 adjusting for age, sex, and the first four genetic principal components. The X chromosome was modelled assuming inactivation of the X chromosome and assuming no inactivation of the X chromosome as a sensitivity analysis. Meta-analysis of the GWAS results was performed using a fixed effects model. The genetic results are reported on build 37 hg19.
Results
The baseline characteristics were fairly similar in both cohorts, as summarized in Table 1. There were no relevant differences between the random cohorts used for this study and the total cohorts of patients included in the biomarker substudies of the two trials (Supplementary material online, Tables S3a and S4b and b). Both populations were elderly, with median ages of 70 and 72 years in ARISTOTLE and RE-LY, respectively. There were 73% male patients in both cohorts. Body mass index was high, with three-quarters of the patients overweight. Hypertension was the most common risk factor; 88% in ARISTOTLE and 79% in the RE-LY cohort. Congestive heart failure was observed in 31% and 29%, and diabetes in 25% and 21% of the respective populations. The use of medications was similar between the cohorts. The biomarker levels were slightly higher in the RE-LY than in the ARISTOTLE population. However, the results in this study are only based on within-cohort analyses and no between-cohort comparisons were performed.
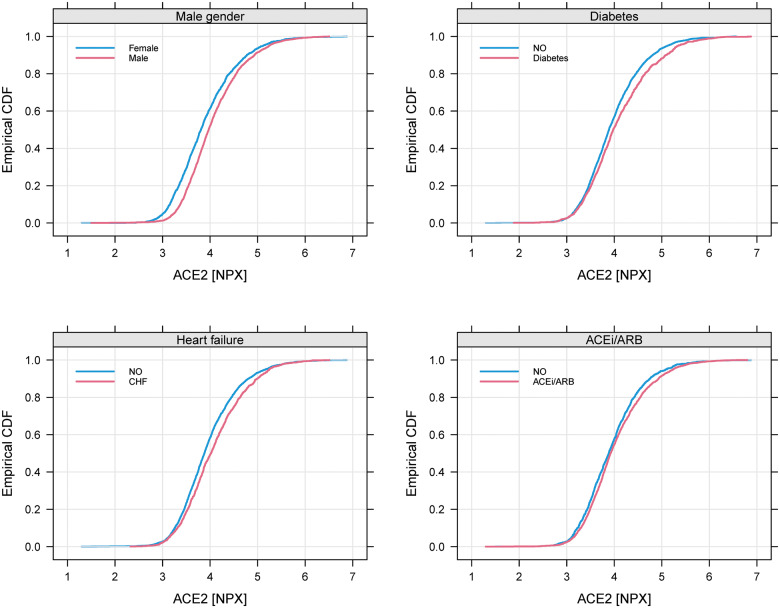
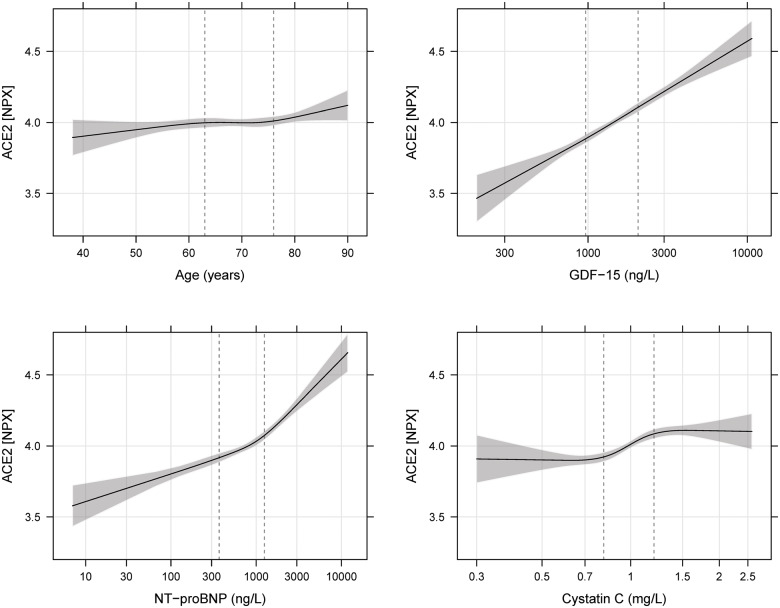
The distribution of all variables in the quartile groups of sACE2 in the ARISTOTLE cohort is shown in Table 2 and in the RE-LY cohort in Supplementary material online, Table S1. The most striking associations were that higher quartile groups of sACE2 contained higher proportions of males, and patients with diabetes, heart failure, prior myocardial infarction, and higher levels of cardiovascular biomarkers (i.e. NT-proBNP, hs-cTNT, and GDF-15). The distributions of sACE2 in relation to the most important categorical variables are outlined in Figure 1 for ARISTOTLE and in Supplementary material online, Figure S1 for RE-LY. For the most important continuous variables, an estimated smooth association with the mean sACE2 is shown in Figure 2 for ARISTOTLE and in Supplementary material online, Figure S2 for RE-LY. There was no statistically significant association between warfarin use within 7 days of randomization and the sACE2 levels.
Table 2.
Baseline characteristics in relation to quartile groups of sACE2 levels in the ARISTOTLE cohort
| Soluble ACE2 (NPX) | (1.30–3.55) | (3.55–3.91) | (3.91–4.37) | (4.37–6.87) |
|---|---|---|---|---|
| Patients | (n = 1000) | (n = 1000) | (n = 1000) | (n = 999) |
| Age (years) | 69.0 (61.8–75.0) | 70.0 (62.8–76.0) | 70.5 (63.0–77.0) | 70.0 (63.0–76.0) |
| Gender: male | 51.2% (512) | 64.7% (647 | 67.0% (670) | 69.1% (691) |
| Race: Caucasian | 84.7% (847) | 84.1% (841) | 83.9% (839) | 82.3% (822) |
| Black | 0.8% (8) | 1.2% (12) | 1.1% (11) | 1.3% (13) |
| American Indian | 0.2% (2) | 0.4% (4) | 0.3% (3) | 0.0% (0) |
| Asian | 13.5% (135) | 12.7% (127) | 13.7% (137) | 14.7% (147) |
| Other | 0.8% (8) | 1.6% (16) | 1.0% (10) | 1.7% (17) |
| Body mass index, kg/m2 | 28.7 (25.4–33.1) [3] | 28.7 (25.4–32.7) [4] | 28.6 (25.4–32.3) [2] | 28.4 (25.3–32.4) [10] |
| Current smoker | 8.1% (81) | 8.2% (82) | 9.4% (94) | 9.6% (96) |
| Diabetes | 22.4% (224) | 22.7% (227) | 23.4% (234) | 31.3% (313) |
| Hypertension | 87.5% (875) | 88.8% (888) | 86.5% (865) | 87.9% (878) |
| Congestive heart failure | 26.2% (262) | 29.7% (297) | 31.8% (318) | 37.0% (370) |
| Prior myocardial infarction | 9.5% (95) | 13.5% (135) | 13.8% (138) | 15.0% (150) |
| Prior peripheral arterial disease | 3.9% (39) | 4.8% (48) | 5.3% (53) | 5.3% (53) |
| Prior stroke/TIA | 17.9% (179) | 19.1% (191) | 19.7% (197) | 16.9% (169) |
| Beta-blocker | 64.2% (642) | 66.7% (667) | 64.5% (645) | 70.7% (706) |
| Aspirin | 42.7% (427) | 40.6% (406) | 40.3% (403) | 43.1% (431) |
| Clopidogrel | 3.5% (35) | 3.1% (31) | 5.0% (50) | 3.8% (38) |
| Statin | 41.1% (411) | 39.7% (397) | 42.0% (420) | 42.4% (424) |
| ACE inhibitor (ACEi) | 47.9% (479) | 52.2% (522) | 50.0% (500) | 53.6% (535) |
| Angiotensin receptor blocker (ARB) | 22.7% (227) | 23.9% (239) | 22.4% (224) | 26.2% (262) |
| ACEi or ARB | 68.2% (682) | 73.0% (730) | 69.4% (694) | 75.9% (758) |
| Amiodarone | 17.1% (171) | 13.2% (132) | 12.2% (122) | 11.3% (113) |
| NT-proBNP (ng/L) | 538.0 (241.5–926.0) [1] | 645.0 (336.5–1119.0) [1] | 754.0 (418.0–1346.0) [1] | 915.0 (479.0–1673.0) [0] |
| hs-cTnT (ng/L) | 8.8 (6.3–13.1) | 10.3 (7.4–15.2) | 11.9 (8.0–17.4) | 13.1 (8.6–20.3) |
| GDF-15 (ng/L) | 1195.5 (827.8–1715.8) | 1258.5 (907.5–1852.8) | 1431.0 (1013.0–2104.8) | 1722.0 (1172.5–2546.5) |
| CRP (mg/L) | 2.0 (0.9–4.0) [1] | 2.0 (1.0–4.6) [2] | 2.5 (1.1–4.8) [1] | 2.4 (1.1–5.2) [0] |
| IL-6 (ng/L) | 2.1 (1.3–3.3) [0] | 2.2 (1.4–3.7) [0] | 2.3 (1.5–4.0) [0] | 2.8 (1.7–4.9) [1] |
| eGFR (CKD-EPI) (mL/min) | 59.3 (47.5–72.4) [0] | 56.2 (45.2–67.3) [0] | 55.1 (44.8–67.4) [0] | 55.5 (43.9–67.5) [1] |
| Cystatin C (mg/L) | 0.9 (0.8–1.1) [1] | 1.0 (0.8–1.2) [2] | 1.0 (0.8–1.2) [1] | 1.0 (0.9–1.3) [0] |
| D-dimer (μg/L) | 493.0 (326.0–809.0) [11] | 523.0 (327.0–812.0) [3] | 525.0 (329.0–895.5) [5] | 548.0 (353.8–907.2) [7] |
TIA, transient ischaemic attack; NT-proBNP, N-terminal probrain natriuretic peptide; hs-cTnT, high-sensitive cardiac troponin T; GDF-15, growth differentiation factor 15; CRP, C-reactive protein; IL-6, interleukin-6; eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Numbers in square brackets represent missing data.
Figure 1.
Cumulative distribution of soluble angiotensin-converting enzyme 2 (ACE2) for different subgroups in ARISTOTLE. CHF, Congestive heart failure; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Figure 2.
Estimated mean soluble angiotensin-converting enzyme 2 (ACE2) with 95% pointwise confidence interval for different levels of the most important continuous variables in ARISTOTLE. GDF-15, growth differentiation factor 15; NT-proBNP, N-terminal probrain natriuretic peptide.
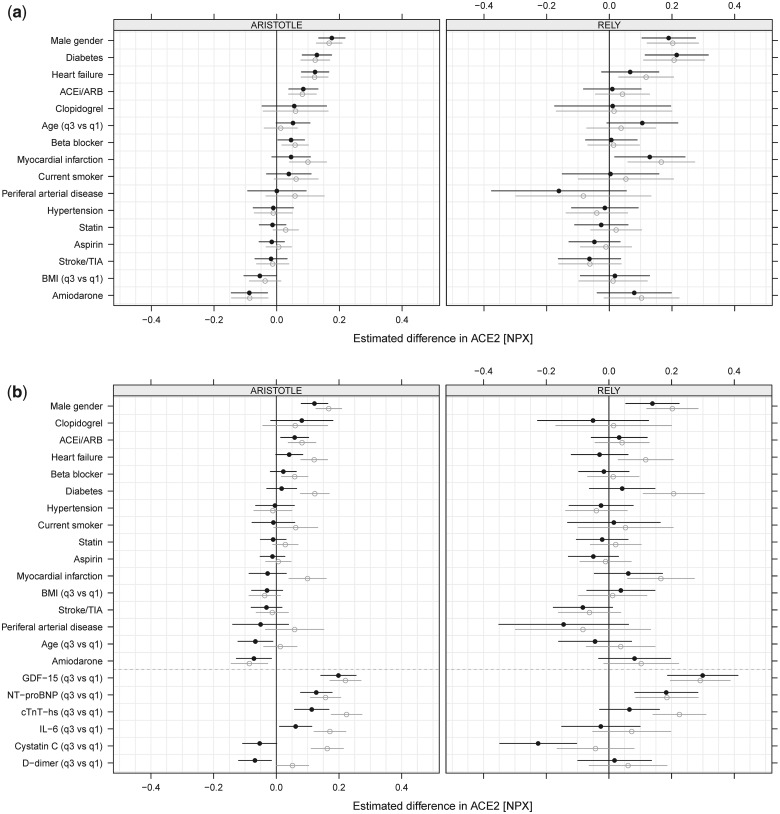
The crude and adjusted effect estimates on the effects of different variables on the sACE2 level are summarized in Figure 3A and B, and Supplementary material online, Table S2. In unadjusted analyses and after adjustment for clinical variables and medical treatment, male sex, diabetes, congestive heart failure, prior myocardial infarction, and age were consistently associated with higher sACE2 levels in both cohorts (Figure 3A; Supplementary material online, Table S2). When also adjusting for biomarker levels, only male sex and higher levels of the biomarkers GDF-15, NT-proBNP, and hs-cTnt were statistically significantly associated with higher sACE2 levels in both cohorts (Figure 3B; Supplementary material online, Table S2). However, the baseline use of an ACE inhibitor/angiotensin blocker and the level of hs-cTnT were statistically significantly associated with higher sACE2 in ARISTOTLE and directionally consistent, although not statistically significant, in the RE-LY study (Figure 3B; Supplementary material online, Table S2).
Figure 3.
(A) Estimated difference of soluble angiotensin-converting enzyme 2 (sACE2) based on the models with clinical variables and treatments for ARISTOTLE (left panel) and RE-LY (right panel). (B) Estimated difference of sACE2 based on the models with clinical variables, treatments, and biomarkers for ARISTOTLE (left panel) and RE-LY (right panel). Unadjusted results (grey, open circles) and adjusted results (black, filled circles). Variables are sorted after estimated effect size in ARISTOTLE. Note that effect sizes for continuous variables are, for a comparison between the third and the first quartile in both studies, estimated using only the ARISTOTLE data. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; TIA, transient ischaemic attack; BMI, body mass index. NT-proBNP, N-terminal probrain natriuretic peptide; cTnT-hs, high-sensitive cardiac troponin T; GDF-15, growth differentiation factor 15.
The genetic analyses showed no statistically significant associations between SNP variability and the sACE2 level when using screening across the whole genome or when focusing on the ACE2-related genetic area on the chromosome (Supplementary material online, Figure S4A and B, Table S3a and b). The results were similar whether modelled assuming inactivation of the X chromosome or not.
Discussion
The global COVID-19 pandemic is caused by the SARS-CoV-2 virus4 entering human cells using ACE2 as a receptor at the cell surface.11,12,16,17,30 The present study took the opportunity to explore factors associated with circulating levels of sACE2 in two large global cohorts of elderly patients with AF and increased risk of stroke, and thereby with an enrichment of risk factors for COVID-19 infection. The results showed that higher levels of sACE2 were associated with male sex, cardiovascular disease, diabetes, and older age, which are also the main risk factors for complications and mortality of COVID-19 infections. There were no statistically significant associations between the level of sACE2 and any of the medications for cardiovascular disease in both cohorts, although there was a trend for a higher level of sACE2 in patients using an ACE inhibitor or angiotensin receptor blocker. The sACE2 level was most strongly associated with the levels of biomarkers reflecting biological ageing, cardiovascular and renal dysfunction, and diabetes, i.e. GDF-15, NT-proBNP, and hs-cTnT. When information on these biomarkers was available, the only remaining statistically significant association between sACE2 level and clinical characteristics was the influence of male sex. An increased plasma level of sACE2 might be a reflection of a more widespread expression of ACE2 in many cells including the very richly perfused alveolar epithelium. As SARS-CoV-2 is using ACE2 as a receptor to enter cells, the increased risk of complications of COVID-19 in men and in patients with cardiovascular disease and diabetes and higher biological age might be related to higher cellular availability of ACE2. The indication that male sex and clinical or biomarker indicators of biological ageing, cardiovascular disease, and diabetes might be associated with a specific mechanism leading to higher risk of more severe SARS-CoV-2 infection might be useful for risk stratification concerning COVID-19. Accordingly, beyond male sex and prior cardiovascular disease, higher levels of GDF-15 and NT-proBNP, which are associated both with a higher sACE2 level and a higher risk for mortality and cardiovascular complications, might contribute to better identification of risk for severe COVID-19 infection.
Cellular ACE2
ACE2, a homologue of ACE, degrades angiotensin (Ang-I) and Ang-II to produce Ang-(1-9) and Ang-(1-7) which have vasodilatory properties by decreasing the levels of Ang-II and increasing the levels of Ang-(1-7).30 ACE2 is expressed by a multitude of cells including endothelial, renal, myocardial, liver, and pulmonary cells. ACE2 is the internalization receptor for SARS-CoV-2 and thus functions as the main port of entry for the virus causing COVID-19.11,13,14,31 Currently there are several research projects investigating whether the ACE2 pathway might be utilized as a treatment target for SARS-CoV-2 infection. Hypothetically, infusion of an abundance of ACE2 might compete with the binding of SARS-CoV-2 to cell membranes, while TMPRSS2 inhibitors might prevent the activation of the spike protein and thereby impair the binding to the ACE2 receptor.12,32
Soluble ACE2
sACE2 protein, containing the catalytic site of the enzyme, is shed into the circulation after the cleavage of the membrane-bound molecule by a disintegrin and metalloprotease 17 (ADAM17) or tumour necrosis factor-α-converting enzyme (TACE).11,16,17,30 There is limited knowledge of the regulation of ACE2 expression in different cells and also of the association between circulating sACE2 levels and the cellular levels of ACE2 in humans. So far, the few published studies on circulating sACE2 concern measurements of sACE2 activity, which in a few small studies has been shown to be higher in older individuals, males, and those with cardiovascular disease.30,33,34 In another study in patients with chronic kidney disease, the ACE2 activity was also higher in males and was associated with atherosclerotic lesions.35 In a study of patients with chronic coronary artery disease, there was higher sACE2 activity in males and an association with cardiovascular events during 10 years follow-up.36 The sACE2 activity has also been found to be elevated in diabetes with vascular complications.37 Recently it was reported from two cohorts of 2017 and 1698 patients with heart failure that male sex was the strongest clinical predictor of the sACE2 level measured by the same method as in the current study.38 Therefore, our results concerning the sACE2 protein level are in accordance with previous findings of an association between ACE2 and male sex and the occurrence of cardiovascular disease and diabetes.
Clinical risk factors
During the last months, >2.1 million people have been diagnosed with COVID-19, of which >146 088 have died (WHO Covid-19 Situation Report-89, 18 April 2020 at 10.00 h CET). Based on a few scientific reports and a wealth of information on the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) websites, it is well established that both occurrence and severity of COVID-19 increase with age, male sex, and comorbidities such as cardiovascular diseases and diabetes.1,8,9 Based on the current demonstration of associations between sACE2 and these risk factors, it might be hypothesized that males and elderly persons with cardiovascular disease or diabetes have an increased expression of ACE2 leading to binding of more SARS-CoV-2 virus particles and, accordingly, a more severe infection. The increased rate of complications and poor outcomes of COVID-19 in these groups may also be driven by unopposed renin–angiotensin–aldosterone system (RAAS) activation due to SARS-CoV2-mediated down-regulation of the previously up-regulated ACE2. Future studies measuring sACE2 pre- and post-infection are therefore warranted to investigate if sACE2 might have a role as a predictor of outcomes in patients with COVID-19 infections.
ACE inhibitors
Concerns have been raised about the influence of ACE inhibitors and angiotensin receptor blockers on the expression of ACE2. In our study, there was an association between renin–angiotensin system (RAS) inhibitor treatment and the sACE2 level in the larger cohort and a trend in the same direction in the smaller cohort. Therefore, these results support the need for further investigation of the effects of RAS inhibitors on ACE2 expression and COVID-19 infection.13,39
Cardiovascular biomarkers
The present data showed that the cardiovascular biomarkers GDF-15, NT-proBNP, and hs-cTnT had the strongest associations with the sACE2 level and stronger associations than any of the clinical characteristics. Also two previous small studies of ACE2 activity reported statistically significant associations between sACE2 activity, heart failure, AF, myocardial function, and a higher level of BNP.33,34 Several previous reports have documented that these biomarkers are stronger indicators of cardiovascular dysfunction and disease than clinical risk factors. Both in elderly healthy indiviuals40,41 and in patients with previous cardiovascular disease,18–20,42–44 these biomarkers are strong risk indicators for cardiovascular outcomes such as myocardial infarction, congestive heart failure, stroke, and cardiovascular death. NT-proBNP and hs-cTnT are specific indicators of myocardial dysfunction and necrosis, and also increase with age. GDF-15 is an unspecific indicator of cellular stress, inflammation, and biological ageing, which, besides being elevated with cardiovascular dysfunction, is also increased with diabetes mellitus, renal dysfunction, and increased chronological age. These biomarkers therefore seem to reflect biological ageing, as well as cardiovascular dysfunction and diabetes, which might be the reason why chronological age is not statistically significantly associated with sACE2 after multivariable adjustment. The close association between biomarkers and the sACE2 level suggests that biological ageing and cardiovascular disease and dysfunction might lead to increased ACE2 expression and a potentially higher risk for SARS-CoV-2 binding and more severe COVID-19 infection.
GWAS
In this study we were unable to show any genetic regulation of the sACE2 level either in women or in men. In a previous small study of 213 subjects,45 there was, in the 111 women, but not in the men, an association between reduced level of sACE2 and rare alleles of ACE2 rs2074192 and rs2106809, which, however, was not verified in the present analyses. Neither were there any differences between the sACE2 level in different ethnic or geographic groups in these analyses (data not shown). Therefore, these data do not support that different expression of ACE2 might explain any differences in COVID-19 infection between different geographic areas or different ethnic groups.
Limitations
The present study has several limitations. The main limitation is that currently there is no information about the association between ACE2 bound to cell membranes and circulating sACE2 levels. There is also no information on the expression of ACE2 in different cells and organs and the sACE2 level. In addition, it is unknown whether the variability of cellular ACE2 and/or sACE2 has any influence on the risk for COVID-19 infection or its severity. The investigated populations of patients with AF at increased risk of stroke contain mainly high-risk elderly patients with a median age of 70–72 years and 79–88% with hypertension, making it not feasible to evaluate the associations between sACE2 and hypertension, AF, and the full range of age. These data were generated in cohorts of mainly white European ancestry and therefore these results might be generalizable only to similar populations.
Conclusions
In two large international cohorts of elderly patients with AF, the level of circulating sACE2 was associated with male sex, cardiovascular disease, diabetes, and age. The sACE2 level was most strongly associated with the levels of the biomarkers GDF-15 and NT-proBNP, reflecting biological ageing and cardiovascular and renal dysfunction, and diabetes. As SARS-CoV-2 is using ACE2 as a receptor to enter cells, the increased risk of complications of COVID-19 in men, those of higher biological age, and those with cardiovascular disease and diabetes might be related to a higher sACE2 level reflecting a higher cellular availability of ACE2.
Implications
Measurements of the routinely available biomarkers GDF-15 and NT-proBNP, which are associated both with higher sACE2 level and a higher risk for mortality and cardiovascular disease, might be useful in better identifying patients who are at high risk for severe COVID-19 infection.
Supplementary material
Supplementary material is available online at European Heart Journal.
Funding
This work was supported by the Swedish Foundation for Strategic Research. The ARISTOTLE trial (ClinicalTrials.gov Identifier: NCT00412984) was funded by Bristol-Myers Squibb Co., Princeton, NJ, USA, and Pfizer Inc., New York, NY, USA. The RE-LY (ClinicalTrials.gov Identifier: NCT00262600) trial was funded by Boehringer Ingelheim, Ingelheim, Germany.
Supplementary Material
Acknowledgements
The authors would like to thank all study participants and the investigators of the ARISTOTLE and RE-LY trials.
Conflict of interest: L.W. reports grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, Roche Diagnostics, and Merck & Co; and two patents involving GDF-15 licensed to Roche Diagnostics (EP2047275B1 and US8951742B2). J.L. and N.E. report grants from Bristol-Myers Squibb/Pfizer. Z.H. has received lecture/consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, and Roche Diagnostics; and consulting fees from Merck Sharp & Dohme. J.W.E. has received grants and honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, Eli Lilly, GlaxoSmithKline, Janssen, and Sanofi. M.D.E. has received grants and consultant fees from Boehringer Ingelheim, Bristol Myers-Squibb, and Pfizer; and consultant fees from Boston Scientific, Anthos Therapeutic, and Alta Therapeutics. C.B.G. has received grants and consulting/speaker fees from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen Pharmaceuticals, Pfizer, AstraZeneca, and Novartis; grants from Daichii-Sankyo, AKROS, and GlaxoSmithKline; and consulting/speaker fees from Bayer Corp, Boston Scientific Corp, Abbvie, Espero BioPharma, Merck, NovoNordisk, Roche Diagnostics, Rho Diagnostics, Sirtex, and Verseon. R.D.L. has received grants and consulting fees from Bristol-Myers Squibb, Pfizer, GlaxoSmithKline, Medtronic PLC, and Sanofi; and consulting fees from Amgen, Bayer, and Boehringer Ingelheim. S.Y. reports grants, speaker fees, and travel expenses from Boehringer Ingelheim. J.O. reports grants and consulting fees from Roche Diagnostics, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer, Sanofi, and Daichii-Sankyo. A.S. has received grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, and Roche Diagnostics; and consultancy fees from Olink Proteomics.
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 2. Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol 2020;92:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, Duan G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 2020;12;372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fowlkes AL, Arguin P, Biggerstaff MS, Gindler J, Blau D, Jain S, Dhara R, McLaughlin J, Turnipseed E, Meyer JJ, Louie JK, Siniscalchi A, Hamilton JJ, Reeves A, Park SY, Richter D, Ritchey MD, Cocoros NM, Blythe D, Peters S, Lynfield R, Peterson L, Anderson J, Moore Z, Williams R, McHugh L, Cruz C, Waters CL, Page SL, McDonald CK, Vandermeer M, Waller K, Bandy U, Jones TF, Bullion L, Vernon V, Lofy KH, Haupt T, Finelli L. Epidemiology of 2009 pandemic influenza A (H1N1) deaths in the United States, April–July 2009. Clin Infect Dis 2011;52 Suppl 1:S60–S68. [DOI] [PubMed] [Google Scholar]
- 7. Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, Bandaranayake D, Breiman RF, Brooks WA, Buchy P, Feikin DR, Fowler KB, Gordon A, Hien NT, Horby P, Huang QS, Katz MA, Krishnan A, Lal R, Montgomery JM, Mølbak K, Pebody R, Presanis AM, Razuri H, Steens A, Tinoco YO, Wallinga J, Yu H, Vong S, Bresee J, Widdowson MA. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 2012;12:687–695. [DOI] [PubMed] [Google Scholar]
- 8. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2badmitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, Saemundsdottir J, Sigurdsson A, Sulem P, Agustsdottir AB, Eiriksdottir B, Fridriksdottir R, Gardarsdottir EE, Georgsson G, Gretarsdottir OS, Gudmundsson KR, Gunnarsdottir TR, Gylfason A, Holm H, Jensson BO, Jonasdottir A, Jonsson F, Josefsdottir KS, Kristjansson T, Magnusdottir DN, le Roux L, Sigmundsdottir G, Sveinbjornsson G, Sveinsdottir KE, Sveinsdottir M, Thorarensen EA, Thorbjornsson B, Löve A, Masson G, Jonsdottir I, Möller AD, Gudnason T, Kristinsson KG, Thorsteinsdottir U, Stefansson K. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med 2020;382:2302–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sargiacomo C, Sotgia F, Lisanti MP. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging (Albany NY) 2020;12:6511–6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jia HP, Look DC, Tan P, Shi L, Hickey M, Gakhar L, Chappell MC, Wohlford-Lenane C, McCray PB., Jr. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol 2009;297:L84–L96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020;46:586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 2020;318:H1084–H1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol 2014;88:1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chu H, Chan JF, Wang Y, Yuen TT, Chai Y, Hou Y, Shuai H, Yang D, Hu B, Huang X, Zhang X, Cai JP, Zhou J, Yuan S, Kok KH, To KK, Chan IH, Zhang AJ, Sit KY, Au WK, Yuen KY. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis 2020;doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chhabra KH, Chodavarapu H, Lazartigues E. Angiotensin converting enzyme 2: a new important player in the regulation of glycemia. IUBMB Life 2013;65:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol 2020;92:726–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, Reilly PA, Vinereanu D, Siegbahn A, Yusuf S, Wallentin L. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation 2012;125:1605–1616. [DOI] [PubMed] [Google Scholar]
- 19. Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, Horowitz JD, Hylek EM, Lopes RD, Åsberg S, Granger CB, Siegbahn A, ARISTOTLE Investigators. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation 2014;130:1847–1858. [DOI] [PubMed] [Google Scholar]
- 20. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Christersson C, Ezekowitz J, Gersh BJ, Hanna M, Hohnloser S, Horowitz J, Huber K, Hylek EM, Lopes RD, McMurray JJ, Granger CB. N-terminal pro-B-type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation). J Am Coll Cardiol 2013;61:2274–2284. [DOI] [PubMed] [Google Scholar]
- 21. Assarsson E, Lundberg M, Holmquist G, Björkesten J, Thorsen SB, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, Andersson AC, Lindstedt P, Stenvang J, Gullberg M, Fredriksson S. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Software 2011;45:1–67. [Google Scholar]
- 23. Harrell FE., Jr. R package version 4.2-0. 2019. https://CRAN.R-project.org/package=Hmisc
- 24. Harrell FE., Jr Regression Modeling Strategies, with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed.Springer-Verlag; 2015. [Google Scholar]
- 25. R Core Team. R: A Language and Environment for Statistical Computing; 2019. https://www.R-project.org
- 26. van Buuren S. Flexible Imputation of Missing Data. 2nd ed.Chapman & Hall, CRC Press; 2018. [Google Scholar]
- 27. Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 2015;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loh PR, Danecek P, Palamara PF, Fuchsberger C, Reshef YA, Finucane HK, Schoenherr S, Forer L, McCarthy S, Abecasis GR, Durbin R, Price AL. Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet 2016;48:1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Durbin R. Efficient haplotype matching and storage using the positional Burrows–Wheeler transform (PBWT). Bioinformatics 2014;30:1266–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel SK, Velkoska E, Freeman M, Wai B, Lancefield TF, Burrell LM. From gene to protein—experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front Physiol 2014;5:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao Y, Feng Y, Liu M, Chen L, Meng Q, Tang X, Wang S, Liu L, Li L, Shen W, Zhang H. Single-cell RNA sequencing analysis reveals alginate oligosaccharides preventing chemotherapy-induced mucositis. Mucosal Immunol 2020;13:437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Epelman S, Tang WH, Chen SY, Van Lente F, Francis GS, Sen S. Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin–angiotensin–aldosterone system. J Am Coll Cardiol 2008;52:750–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Úri K, Fagyas M, Mányiné Siket I, Kertész A, Csanádi Z, Sándorfi G, Clemens M, Fedor R, Papp Z, Édes I, Tóth A, Lizanecz E. New perspectives in the renin–angiotensin–aldosterone system (RAAS) IV: circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure. PLoS One 2014;9:e87845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anguiano L, Riera M, Pascual J, Valdivielso JM, Barrios C, Betriu A, Clotet S, Mojal S, Fernández E, Soler MJ; investigators from the NEFRONA study. Circulating angiotensin converting enzyme 2 activity as a biomarker of silent atherosclerosis in patients with chronic kidney disease. Atherosclerosis 2016;253:135–143. [DOI] [PubMed] [Google Scholar]
- 36. Ramchand J, Patel SK, Srivastava PM, Farouque O, Burrell LM. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS One 2018;13:e0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soro-Paavonen A, Gordin D, Forsblom C, Rosengard-Barlund M, Waden J, Thorn L, Sandholm N, Thomas MC, Groop PH; FinnDiane Study Group. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens 2012;30:375–383. [DOI] [PubMed] [Google Scholar]
- 38. Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, Cleland JGF, Rienstra M, Friedrich AW, Samani NJ, Ng LL, Dickstein K, Lang CC, Filippatos G, Anker SD, Ponikowski P, Metra M, van Veldhuisen DJ, Voors AA. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors. Eur Heart J 2020;41:1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA., Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wallentin L, Zethelius B, Berglund L, Eggers KM, Lind L, Lindahl B, Wollert KC, Siegbahn A. GDF-15 for prognostication of cardiovascular and cancer morbidity and mortality in men. PLoS One 2013;8:e78797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zethelius B, Berglund L, Sundström J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlöv J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med 2008;358:2107–2116.18480203 [Google Scholar]
- 42. Hagström E, Held C, Stewart RA, Aylward PE, Budaj A, Cannon CP, Koenig W, Krug-Gourley S, Mohler ER, 3rd, Steg PG, Tarka E, Östlund O, White HD, Siegbahn A, Wallentin L; STABILITY Investigators. Growth differentiation factor 15 predicts all-cause morbidity and mortality in stable coronary heart disease. Clin Chem 2017;63:325–333. [DOI] [PubMed] [Google Scholar]
- 43. Lindholm D, Lindbäck J, Armstrong PW, Budaj A, Cannon CP, Granger CB, Hagström E, Held C, Koenig W, Östlund O, Stewart RAH, Soffer J, White HD, de Winter RJ, Steg PG, Siegbahn A, Kleber ME, Dressel A, Grammer TB, März W, Wallentin L. Biomarker-based risk model to predict cardiovascular mortality in patients with stable coronary disease. J Am Coll Cardiol 2017;70:813–826. [DOI] [PubMed] [Google Scholar]
- 44. Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Held C, Hylek EM, Lopes RD, Yusuf S, Granger CB, Siegbahn A, Wallentin L; ARISTOTLE and RE-LY Investigators. A biomarker-based risk score to predict death in patients with atrial fibrillation: the ABC (age, biomarkers, clinical history) death risk score. Eur Heart J 2018;39:477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen YY, Zhang P, Zhou XM, Liu D, Zhong JC, Zhang CJ, Jin LJ, Yu HM. Relationship between genetic variants of ACE2 gene and circulating levels of ACE2 and its metabolites. J Clin Pharm Ther 2018;43:189–195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.