Abstract
Aims
Coronavirus disease 2019 (COVID-19) due to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has been associated with cardiovascular features of myocardial involvement including elevated serum troponin levels and acute heart failure with reduced ejection fraction. The cardiac pathological changes in these patients with COVID-19 have yet to be well described.
Methods and results
In an international multicentre study, cardiac tissue from the autopsies of 21 consecutive COVID-19 patients was assessed by cardiovascular pathologists. The presence of myocarditis, as defined by the presence of multiple foci of inflammation with associated myocyte injury, was determined, and the inflammatory cell composition analysed by immunohistochemistry. Other forms of acute myocyte injury and inflammation were also described, as well as coronary artery, endocardium, and pericardium involvement. Lymphocytic myocarditis was present in 3 (14%) of the cases. In two of these cases, the T lymphocytes were CD4 predominant and in one case the T lymphocytes were CD8 predominant. Increased interstitial macrophage infiltration was present in 18 (86%) of the cases. A mild pericarditis was present in four cases. Acute myocyte injury in the right ventricle, most probably due to strain/overload, was present in four cases. There was a non-significant trend toward higher serum troponin levels in the patients with myocarditis compared with those without myocarditis. Disrupted coronary artery plaques, coronary artery aneurysms, and large pulmonary emboli were not identified.
Conclusions
In SARS-CoV-2 there are increased interstitial macrophages in a majority of the cases and multifocal lymphocytic myocarditis in a small fraction of the cases. Other forms of myocardial injury are also present in these patients. The macrophage infiltration may reflect underlying diseases rather than COVID-19.
Keywords: Myocarditis, Macrophages, COVID-19, SARS, SARS-CoV-2, Heart, Myocardium, Autopsy
Introduction
Coronavirus disease 2019 (COVID-19) due to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is primarily a respiratory disease,1 but systemic and cardiovascular involvement can occur. Acute cardiac injury with elevation of serum troponins is the most commonly described cardiac abnormality in COVID-19, reported in ∼8–12% of patients, and elevated troponin levels have been associated with increased mortality in patients with COVID-19.2–4 In addition, a small fraction of patients develop acute heart failure with reduced ejection fraction, raising the clinical concern for myocarditis.5–7
SARS-CoV-2 can elicit intense release of cytokines and chemokines, possibly leading not only to vascular inflammation and atherosclerotic plaque instability but also to myocardial inflammation. Therefore, possible mechanisms for elevated troponin levels in these patients include demand ischaemia, stress cardiomyopathy, microvascular thrombosis, and secondary effects of systemic inflammation. Direct viral infection of the myocardium is another possible causal pathway of myocardial damage. The unique affinity of SARS-CoV-2 for the host angiotensin-converting enzyme 2 receptor raises the possibility of direct viral infection of vascular endothelium and myocardium, such that in some patients, COVID-19-associated myocardial injury could represent viral myocarditis.8 Any of these mechanisms may exacerbate underlying cardiovascular conditions.9
There have been a few recent reports describing the cardiac histology from a small number of patients with COVID-19, mostly entailing endomyocardial biopsies and limited tissue sampling at autopsy, but these have not yielded a very clear picture of the overall cardiac pathological changes in these patients.10–15 The current study aimed to assess the pathological spectrum of cardiac involvement in COVID-19 patients and to determine the frequency and type of myocarditis as well as other forms of acute cardiac injury.
Methods
The COVID-19 Working Group was established through the leadership of the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology. Cardiovascular pathologists within these societies were solicited to provide information relating to the cardiac pathology obtained from consecutive autopsies performed at their institutions on patients with COVID-19. The inclusion criteria were a positive nasopharyngeal swab for SARS-CoV-2, a clinical diagnosis of COVID-19, and an autopsy with cardiac examination by a cardiac pathologist from the working group, including sampling of the myocardium (left ventricle, septum, and right ventricle) and epicardial coronary arteries. All sequential autopsies that met these criteria were included. Information provided included the presence of myocarditis and other patterns of inflammation and injury identified on haematoxylin and eosin- (H&E) stained slides of the heart. The degree of coronary artery stenoses and the presence of disrupted plaques were also provided. The assessment of the myocardium by electron microscopy and evaluation of immunohistochemical stains for CD68, CD3, CD4, and CD8 were provided if available. For immunohistochemical stains, the number of cells staining in the ×400 high-power field with the most inflammation were counted. All immunohistochemical stains were performed on routine automated diagnostic immunohistochemical staining devices. The presence of fibrosis in the left ventricle was assessed on a semi-quantitative scale: none; mild (<10% of myocardial area); moderate (10–25% of myocardial area); or severe (>25% of myocardial area).
For the purposes of this study, myocarditis was defined as the presence of an inflammatory infiltrate associated with myocyte injury not due to some other cause, which was present in multiple foci. The opinions put forth here do not necessarily represent the opinions of all members of the Society for Cardiovascular Pathology or the Association for European Cardiovascular Pathology.
Serum troponin levels were obtained at the individual institutions using the following tests: high-sensitivity troponin T, normal <15 ng/L (n = 6), high-sensitivity troponin I, normal <35 ng/L (n = 6), high-sensitivity troponin I, normal <19.8 ng/L (n = 4). Electrocardiographic changes were considered new when not present on previous electrocardiograms during either the current or previous admissions. All electrocardiographic changes were from 12-lead ECGs, except where specified in the text, and were identified as part of the clinical care and documented in the medical records.
Categorical variables were compared using Fisher’s exact test, and continuous variables were compared using Wilcoxon test. Inflammatory cell counts were compared using Kruskal–Wallis with post-test by Wilcoxon. All tests were two sided. P-values <0.05 were considered significant in terms of secondary analyses for hypothesis generation.
Results
Twenty-one cases met the study criteria. These autopsies were performed in March or April 2020 at the Azienda Ospedaliera-University of Padua, the Sant’Orsola-Malpighi University Hospital of Bologna, the Massachusetts General Hospital in Boston, the University of Amsterdam, and the Mayo Clinic in Rochester, Minnesota. For all 21 patients, the underlying cause of death was COVID-19. The mechanisms of death were acute respiratory distress syndrome (ARDS, n = 15), viral pneumonia (n = 4), cardiogenic shock (n = 1), and cardiac arrest (n = 1). The mechanisms of death associated with specific clinical presentations are shown in Supplementary material online, Table S1. Eighteen of the patients died in the intensive care unit. Two of the patients were treated with veno-venous extracorporeal membrane oxygenation. No mechanical circulatory support was employed.
The patient characteristics are summarized in Table 1. All but one of the patients had known underlying medical conditions including hypertension (n = 16), diabetes mellitus (n = 7), haematological malignancies (n = 4), chronic renal failure (n = 2), chronic obstructive pulmonary disease (n = 1), and Crohn’s disease (n = 1). Two patients were former smokers, and four patients were immunosuppressed. Thirteen patients were obese. Seven patients had a prior history of cardiovascular disease(s) including atrial fibrillation (n = 4), coronary artery disease/ischaemic heart disease (n = 3), left ventricular hypertrophy (n = 1), and prior valve replacement (n = 1).
Table 1.
Patient characteristics
| All cases | Without myocarditis | With myocarditis | P-value | |
|---|---|---|---|---|
| n | 21 | 18 | 3 | |
| Age | 69 (44–86) | 70 (44–82) | 64 (59–86) | 1.00 |
| Male sex | 15 (71) | 12 (67) | 3 (100) | 0.53 |
| Duration of symptoms (days)a | 15 (5–31) | 15 (5–31) | 15 (9–30) | 0.65 |
| Duration of hospitalization (days) | 7 (2–24) | 6 (2–23) | 9 (7–24) | 0.19 |
| History of hypertension | 16 (76) | 14 (78) | 2 (67) | 1.00 |
| History of diabetes mellitus | 7 (33) | 6 (33) | 1 (33) | 1.00 |
| Previous immunosuppression | 4 (19) | 3 (17) | 1 (33) | 0.49 |
| History of smoking | 2 (10) | 2 (11) | 0 (0) | 1.00 |
| Prior cardiovascular disease | 7 (33) | 6 (33) | 1 (33) | 1.00 |
| Prior atrial fibrillation | 4 (19) | 3 (17) | 1 (33) | 0.49 |
| New-onset atrial fibrillation | 5 (24) | 3 (17) | 2 (67) | 0.13 |
| Any new ECG changes | 12 (62) | 9 (50) | 3 (100) | 0.23 |
| Peak troponin (ng/L)b | 56 (2.8–2494), n = 16 | 44 (2.8–702), n = 13 | 66 (60–2494), n = 3 | 0.24 |
| Time of peak troponin (days before death) | 3.5 (0.5–22) | 4 (3–9) | 3 (0.5–22) | 0.54 |
Data are expressed as median (range) or n (%).
From onset of symptoms to death.
Highest troponin value if multiple troponin values were available.
From the terminal hospital admission, serum high-sensitivity troponin T or I levels were available for 16 of the patients, with a median peak value of 56 ng/L, range 2.8–2494 ng/L. The troponin levels were abnormal for 11 of these 16 patients. Selected laboratory values at the time of the peak troponin measurement are shown in Supplementary material online, Table S2. Selected initial and final laboratory values for these 16 patients are shown in Supplementary material online, Table S3. Five of these 16 patients received renal replacement therapy.
New electrocardiographic changes were identified in 12 patients, including new-onset atrial fibrillation (n = 5), partial right bundle branch block (n = 2), non-specific ST-segment or T-wave changes (n = 3), transient ST-segment elevation (n = 1), premature ventricular beats (n = 1), and ST-segment depression (n = 1). The atrial fibrillation was documented only on rhythm strips in one patient. The duration of atrial fibrillation was 12 h in one patient, 2 days in three patients, and 3 days in one patient. Limited bedside transthoracic echocardiography was performed on five patients without myocarditis, and the observations are summarized in Supplementary material online, Table 4. Sixteen patients received one or more medications for COVID-19 including hydroxychloroquine/chloroquine (n = 15), azithromycin (n = 8), atorvastatin (n = 4), inhaled nitric oxide (n = 2), lopinavir/ritonavir (n = 6), oseltamivir (n = 2), remdesivir (n = 1), tocilizumab (n = 3), and sarilumab (n = 1).
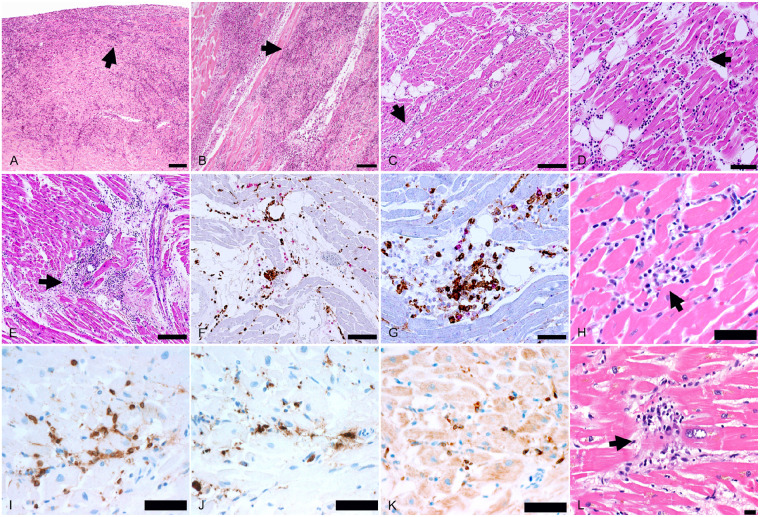
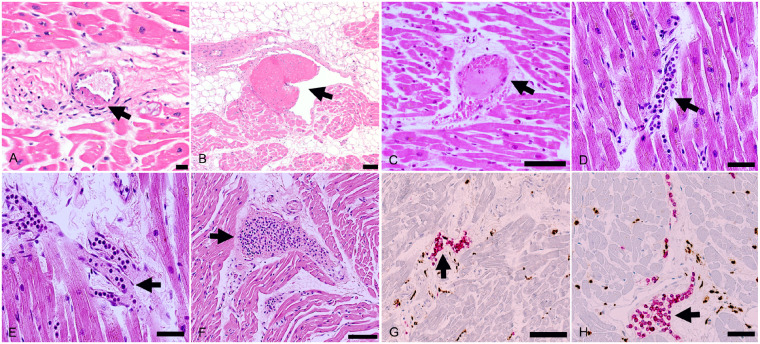
At autopsy, pulmonary thrombo-embolism of the main pulmonary arteries was not found in any of the cases. A median of 20 full-thickness blocks of myocardium were examined histologically (range 5–29 blocks), and immunohistochemical stains for inflammatory cell markers were evaluated in all cases. Myocarditis was identified in three cases (Figure 1). The myocarditis was multifocal in all three cases, involving the left and right ventricles, but was with right ventricular predominance in one case. In all three cases, the myocarditis was classified as lymphocytic, containing substantial CD3+ T lymphocytes and a significant proportion of CD68+ macrophages, without eosinophils, giant cells, or granulomas. In two cases, the lymphocytes were CD4+ predominant and in one case the lymphocytes were CD8+ predominant. In addition to the three cases of multifocal myocarditis, six cases had focally increased interstitial T lymphocytes within the myocardium, with or without focal myocyte injury, in which the number of T cells focally ranged from 22 to 65 per ×400 high-power field.
Figure 1.
Spectrum of myocarditis in COVID-19 patients. (A and B) Biventricular multifocal/diffuse lymphocytic myocarditis (arrows) with extensive myocyte injury in an 86-year-old man with previously undiagnosed cardiac amyloidosis (H&E ×50). (C–G) Biventricular multifocal lymphocytic myocarditis (arrows) with myocyte injury in a 64-year-old man, who developed atrial fibrillation 2 days before death (C, H&E ×100; D, H&E ×200; E, H&E ×100; F, double immunostaining CD68 brown/CD3 red, ×200; G, double immunostaining CD4 brown/CD8 red, ×400). (H–K) Biventricular multifocal lymphocytic myocarditis (arrow) in a 59-year-old man (H, H&E ×400; I, CD3 immunostaining brown, ×400; J, CD68 immunostaining brown, ×400; K, CD4 immunostaining brown, ×400). (L) Focal myocardial lymphocytic infiltration with myocyte injury (arrow) in a 70-year-old man (H&E ×400). Scale bars represent 500 μm (A, B), 200 μm (C, E), 100 μm (D, F), 50 μm (G–K), and 20 μm (L).
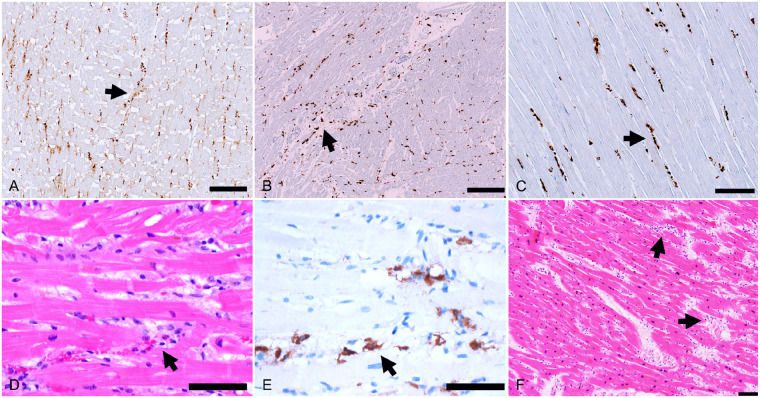
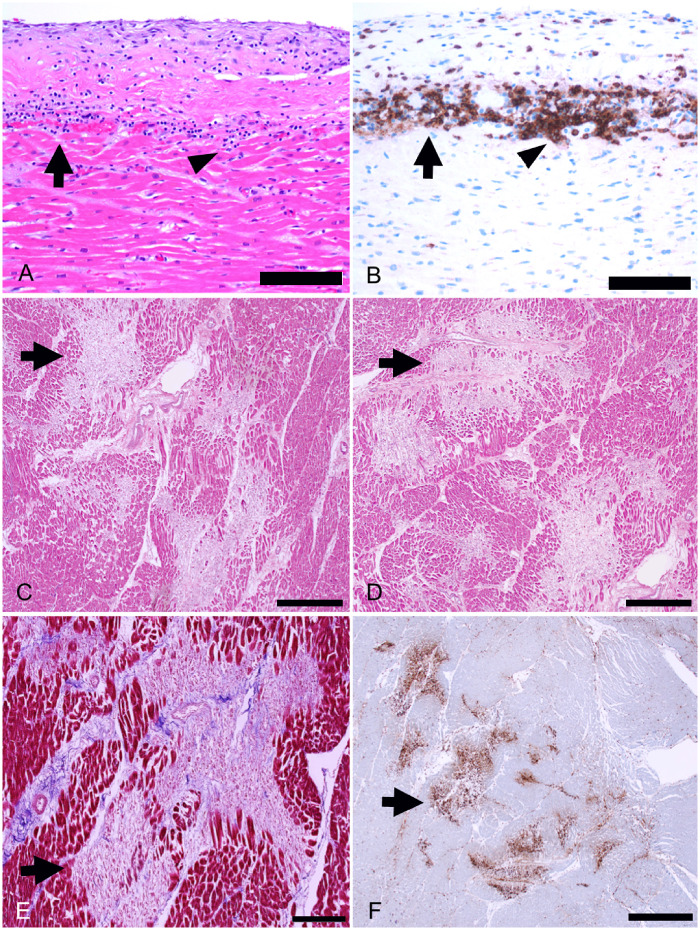
In 18 (86%) of the cases, there was relatively widespread increased interstitial macrophage infiltration in the myocardium without clearly associated myocyte injury involving both the left and right ventricles (Figure 2). These diffuse macrophage infiltrates were seen in two of the three patients with myocarditis and in 16 of the 18 patients without myocarditis. For the third patient with myocarditis, the inflammatory infiltrates were relatively extensive, making identification of a distinctive separate macrophage infiltrate difficult. The median macrophage density was 44 cells per high-power field (range 20–177) in these cases. A mild pericarditis of the epicardium (visceral pericardium) was present in four cases (Figure 3). Acute myocyte injury in the right ventricle most probably due to strain/overload was present in four cases (Figure 4), and was characterized by acute myocyte coagulative necrosis primarily in the subendocardial region, with the necrotic myocytes staining for the complement component C4d.
Figure 2.
Increased interstitial macrophages. The majority of patients showed increased interstitial macrophages without associated myocyte injury (arrows). (A) A 50-year-old man, (CD68 immunostaining, ×100). (B) A 44-year-old man, (double CD68 brown/CD3 red immunostaining, ×100). (C) A 64-year-old man (CD68 immunostaining, ×200). (D and E) A 60-year-old man (D, H&E, ×400; (E) CD68 immunostaining, ×400). (F) A 73-year-old woman with increased cells within the myocardial interstitium (H&E, ×100). Scale bars represent 200 μm (A, B), 100 μm (C, F), and 50 μm (D, E).
Figure 3.
Other myocardial inflammatory changes in patients with COVID-19. (A and B) Focal lymphocytic pericarditis (arrows), mainly composed of CD8+ lymphocytes, associated with focal myocardial inflammation without myocyte injury (arrowhead) in a 66-year-old man (A, H&E, ×400; B, CD8 immunostaining, ×400). (C–F) Healing myocardial injury (arrows) in the subendocardium of the left ventricle in a 50-year-old man (C and D, H&E, ×50; E, Azan Mallory trichrome, ×100; F, CD68 immunostaining, ×25). Scale bars represent 50 μm (A, B), 500 μm (C, D), 200 μm (E) and 1000 μm (F).
Figure 4.
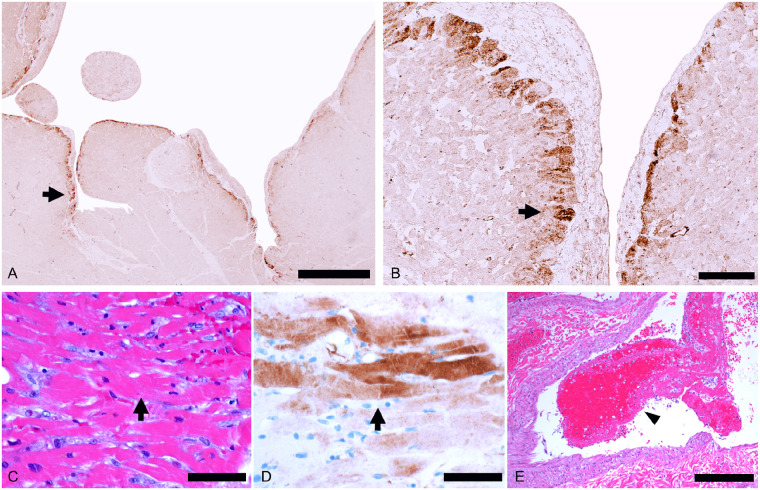
Right ventricle myocardial injury consistent with strain. (A and B) Right ventricle myocardium showing subendocardial C4d+ necrotic myocytes (arrows) in a 50-year-old man (C4d immunostaining: A ×25; B ×200). (C–E) C4d + necrotic myocytes (arrows) in the right ventricle in a 60-year-old woman with microvascular pulmonary arterial thrombi (E, arrowhead) (C, H&E, ×400; D, C4d immunostaining 4 × 00; E, H&E ×100). Scale bars represent 1000 μm (A), 100 μm (B), 50 μm (C, D), and 200 μm (E).
Only two of the patients had severe coronary artery atherosclerosis with maximal cross-sectional area stenosis of ≥75% (Supplementary material online, Figure S1). None of the patients showed features of coronary artery plaque disruption with thrombus formation. There was no vasculitis or intimalitis. Thrombi composed of fibrin and platelets were present in the small vessels of the myocardium in four cases, involving <10% of the vessels. There was recent myocardial infarction dating 2–3 weeks in age, present in multiple foci, in one patient who had both stable severe epicardial coronary artery disease and small vessel microthrombi. There were thrombi involving the endocardial surface in three cases (Figure 5). There were aggregates of leucocytes within small vessels in the myocardium in 11 cases. Electron microscopy performed on three of the cases without myocarditis was negative for virions. Fibrosis was present in the left ventricle of 19 of the 21 cases, consistent with the underlying comorbidities. The fibrosis was moderate and diffuse in 5 patients, mild and diffuse in 12 patients, and mild and limited to the subendocardial region in 2 patients.
Figure 5.
Myocardial small vessel changes. (A and B) Organizing microthrombus (arrow) in a small myocardial artery (A, H&E, ×400) and organizing venous thrombus (arrow) (B, H&E, ×100) in a 70-year-old man. (C) Thrombus (arrow) in a small myocardial vein (H&E, ×200) in a 71-year-old man. (D and E) Leucocyte aggregates (mainly neutrophils and mononuclear cells, arrows) in capillaries and small veins in a 64-year-old man (H&E, ×400). (F–H) A 44-year-old man with eosinophils and mononuclear cells (arrow) filling the lumen of a small dilated vein (F, H&E, ×200) and intravascular T lymphocytes (arrows) in myocardial small veins (double immunostaning CD68 brown/CD3 red: G ×200; H ×400). Scale bars represent 20 μm (A), 100 μm (B, C, F, G), and 50 μm (D, E, H).
In comparing the cases with myocarditis with those without myocarditis, the densities of CD3+ lymphocytes and CD68+ macrophages were both higher in the myocarditis patients than in the non-myocarditis patients, but there were no differences in the inflammatory cell densities between the left and right ventricles for either group (Figure 6). There were no differences between the two groups in the duration of symptoms, duration of hospitalization, age, history of hypertension, history of diabetes, history of prior immunosuppression, history of smoking, or history of prior cardiovascular disease (Table 1). There was also no discernible difference between the two groups in the COVID-19-related treatments (not shown). New-onset atrial fibrillation was seen in two of the three patients with myocarditis, and the third had a pre-existing history of atrial fibrillation and also demonstrated ST-segment depression during the final hospital admission. In contrast, new-onset atrial fibrillation was only seen in 21% of the patients without myocarditis and without a prior history of atrial fibrillation. For the 16 patients with documented troponin levels, there was no significant difference in the serum troponin levels between the patients with and without myocarditis (Table 1). While the patient with the highest troponin level (2494 ng/L) did have myocarditis, the patient with the second highest troponin level (702 ng/mL) did not have myocarditis, but had acute right ventricular myocardial injury most probably resulting from strain. All three of the patients with myocarditis had troponins levels ≥60 ng/L compared with only 38% of the patients without myocarditis (P = 0.20). In addition, all three patients with myocarditis had serum troponin levels ≥60 ng/mL in the setting of new electrocardiography changes, compared with only 2 (15%) of the 13 patients without myocarditis (P = 0.02). Eleven patients had more than one troponin measurement, for whom plots of the troponin values are shown in Supplementary material online, Figure S2.
Figure 6.
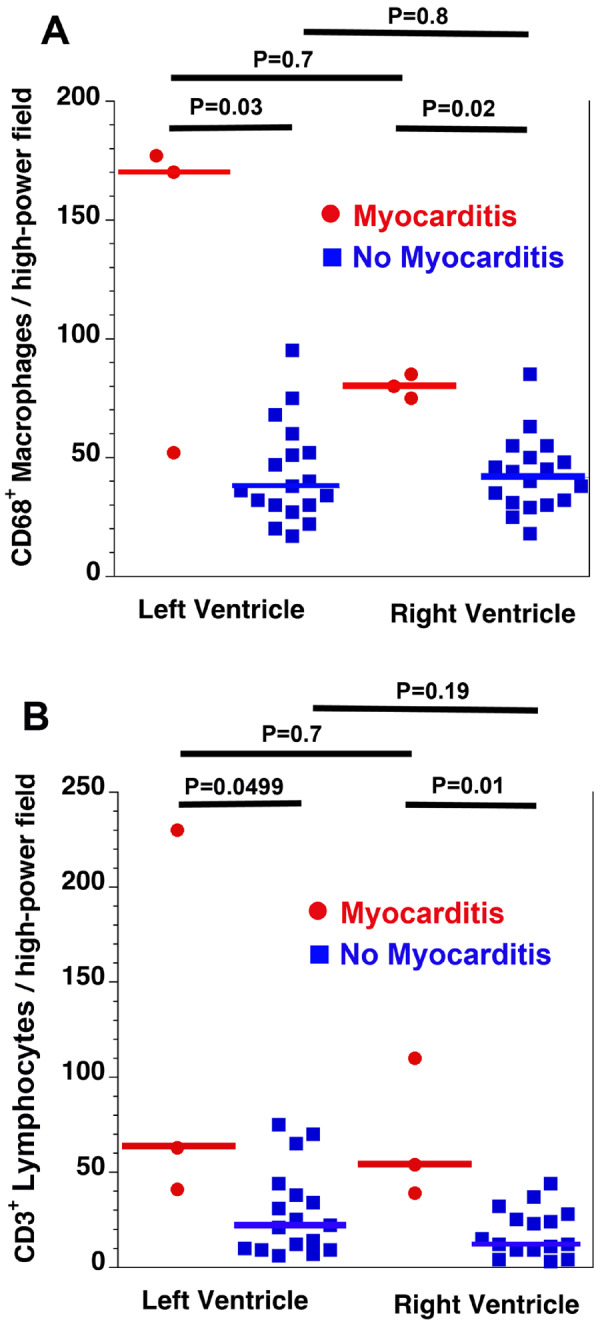
Quantification of inflammatory cells. There were more CD68+ macrophages (A) and CD3+ lymphocytes (B) in the myocarditis group compared with the no myocarditis group. There were no differences in inflammatory cell density between the left and right ventricles for either group. Horizontal bars indicate the median values. Overall P = 0.01 (A) and P = 0.005 (B).
Discussion
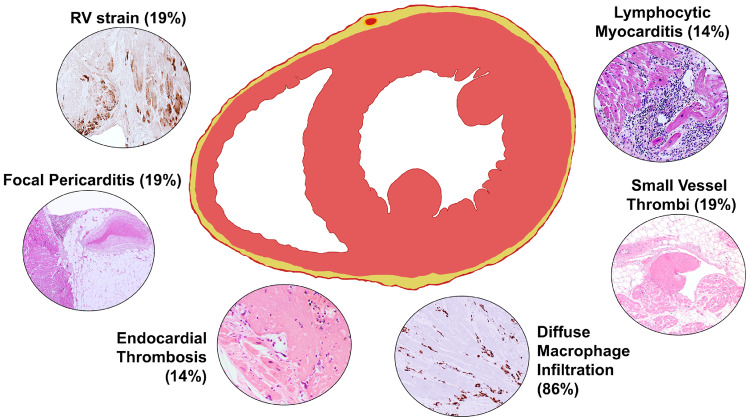
One of the key pathological findings in this series is that in patients dying with COVID-19, there is frequently present a myocardial interstitial macrophage infiltration, with a median of 44 cells per ×400 high-power field, without myocyte injury affecting 86% of the patients (Figure 7). In a smaller number of cases, there is true multifocal lymphocytic myocarditis affecting 14% of the patients. Compared with the prior SARS-CoV virus, the inflammatory cardiac changes seen in COVID-19 appear overall more severe. Previously, autopsies of patients dying from SARS showed that in 35% of patients, SARS-CoV could be detected in myocardial tissue by PCR, and in that subset of patients there was a degree of myocardial macrophage infiltrate comparable with that present in 86% of the COVID-19 cases in this series.16 The mean age of the patients in the prior SARS study was 68 years with 45% men, compared with a mean age of 69 years with 71% men for the COVID-19 patients in this study. Underlying diseases and comorbidities were not reported for the SARS group. The mechanisms underlying these macrophage infiltrations remain uncertain at this time, but in the study by Oudit et al., it was suggested that SARS-CoV-induced myocardial inflammation is mediated predominantly by macrophages. Also in that study, SARS-CoV was not shown to be associated with increased lymphocytic infiltrates or multifocal myocarditis as is present with SARS-CoV-2 infection.
Figure 7.
Cardiac pathological changes associated with COVID-19.
In normal human hearts, the macrophage density is typically <3 cells per ×400 high-power field.17 In elderly patients and in the setting of chronic cardiac diseases such as ischaemic heart disease, heart failure, and amyloidosis, the macrophage density increases, but is still typically <10 cells per ×400 high-power field.18,19 The high degree of macrophage infiltration seen in these patients with COVID-19 (20–177 cells per ×400 high-power field) and previously with SARS,16 is uncommon, but not unique to coronavirus infection. Patients dying from bacterial sepsis have macrophage densities in the myocardium of the order of 26 cells per ×400 high-power field,20 similar to those of the COVID-19 patients. Thus, the high levels of myocardial macrophages in these COVID-19 patients may largely result from the elevated systemic levels of proinflammatory cytokines. Patients with severe COVID-19 have been shown to have systemic elevations of the proinflammatory cytokines interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α).3,21 The macrophage infiltration may reflect underlying diseases rather than COVID-19.
While there was a non-significant trend toward higher troponin levels in the patients with myocarditis in this study, myocarditis does not fully explain the elevated troponin levels seen in patients with COVID-19. Other forms of myocardial injury such as right ventricular strain are clearly contributing to elevated troponin levels in these patients. All of the patients with multifocal myocarditis in this series had new electrocardiography changes including atrial fibrillation in two cases, and new ST-segment depression in the setting of chronic atrial fibrillation in the third case. Given that the majority of patients with COVID-19 have increased macrophages in the heart, it may also be challenging to discern which of these patients have actual lymphocytic myocarditis by imaging studies.
While the presence of virus in cardiac macrophages on electron microscopy has been reported, we were unable to verify this finding.13 However, so far, the electron microscopy in this patient series has only been completed in three cases without actual myocarditis.
Preliminary observations in the literature, together with the observations in this series, suggest that myocardial injury with or without depressed cardiac function in these patients may result from aetiologies other than viral myocarditis. Acute myocardial tissue injury might be related to elevated cytokines, hypoxaemia, right ventricular strain, and thrombotic complications. Both myocardial microvascular thrombi and right ventricular strain injury were present in some of the patients in this series. Thus, the term myocarditis should be used cautiously in describing patients with elevated troponin levels in the setting of COVID-19, particularly in the absence of more specific diagnostic testing such as endomyocardial biopsy and/or cardiac magnetic resonance imaging, which may be difficult to obtain in these patients.
Details of the cardiac pathology from autopsies of patients dying with COVID-19 are currently very limited. Despite the high mortality rate worldwide, only a few studies, comprising a small number of patients, have so far reported information concerning the cardiac pathology in these patients.10,12,14,15 Some of these studies employed limited diagnostic approaches such as biopsies. Thus, it is not surprising that in the few studies published to date, no substantial myocardial changes have been identified. Our study represents the first series with extensive histological cardiac examination, with a median of 20 full-thickness blocks of myocardium per case.
This study has several limitations. Molecular analysis for virus in the myocardium was not performed. While this is the largest and most detailed study of the cardiac pathology in the setting of COVID-19 to date, this study is still relatively small, and underpowered for identifying and excluding differences between the groups. Also this series focused on a subset of patients who died from COVID-19, and the observations may not be readily extended to all patients with COVID-19. The definition for diagnosing COVID-19 in patients infected with SARS-CoV-2 may not have been uniform across the institutions of this multicentre study. Only limited bedside echocardiography was performed on a small number of the patients. Electrocardiographic findings were based on documented clinical observations, and were not obtained in a standardized fashion. Likewise, there was not a uniform process for obtaining the autopsies in this series, and the results may not be readily extended to all patients dying with COVID-19. This study was retrospective in nature, and not all cases were sampled for histology in the same manner. For this study, we used the stringent criterion of multiple foci of inflammation associated with myocardial injury for a diagnosis of myocarditis. Thus, there is high confidence that the three cases meeting this criterion have myocarditis. There were six additional cases with lymphocytic infiltrates but no or only focal myocyte injury. In some previous studies not related to COVID-19, particularly involving endomyocardial biopsies, such pathology was regarded as myocarditis.22,23 However, the significance of these lymphocytic infiltrates with no to only focal myocardial injury in the COVID-19 autopsy population is currently unclear and may not be indicative of myocarditis.
In summary, the cardiac pathological changes associated with SARS-CoV-2 infection appear to be more severe than those associated with the previous SARS-CoV outbreak. In the setting of SARS-CoV-2 infection, there is increased interstitial myocardial macrophages in a majority of the cases and multifocal lymphocytic myocarditis in a small fraction of the cases. Other forms of myocardial injury are also present in these patients, such as right ventricular strain injury.
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. A novel coronavirus from patients with pneumonia in China 2019. N Engl J Med 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu H, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis 2020;doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J 2020;41:doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, Wang LF, Gao H, Wang Y, Dong CF, Li YJ,, Xie XJ, Feng C, Liu L. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection 2020;doi: org/10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. COVID-19 and the cardiovascular system. Nature Rev Cardiol 2020;17:259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J 2020;41:1798–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol 2020;33:1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Cappelletti AM, Basso C, Godino C, Esposito A. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J 2020;41:1861–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol 2020;153:725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020;22:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oudit GY, Kassiri Z, Jiang C, Liu PP,, Poutanen SM, Penninger JM, Butany J. SARS coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS . Eur J Clin Invest 2009;39:618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Azzawi M, Hasleton PS, Kan SW, Hillier VF, Quigley A, Hutchinson IV. Distribution of myocardial macrophages in the normal human heart. J Anatomy 1997;191:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Azzawi M, Kan SW, Hillier V, Yonan N, Hutchinson IV, Hasleton PS. The distribution of cardiac macrophages in myocardial ischaemia and cardiomyopathy. Histopathology 2005;46:314–319. [DOI] [PubMed] [Google Scholar]
- 19. Stats MA, Stone JR. Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc Pathol 2016;25:413–417. [DOI] [PubMed] [Google Scholar]
- 20. Rossi MA, Celes MR, Prado CM, Saggioro FP. Myocardial structural changes in long-term human severe sepsis/septic shock may be responsible for cardiac dysfunction. Shock 2007;27:10–18. [DOI] [PubMed] [Google Scholar]
- 21. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leone O, Veinot JP, Angelini A, Baandrup UT, Basso C, Berry G, Bruneval P, Burke M, Butany J, Calabrese F, d’Amati G, Edwards WD, Fallon JT, Fishbein MC, Gallagher PJ, Halushka MK, McManus B, Pucci A, Rodriguez ER, Saffitz JE, Sheppard MN, Steenbergen C, Stone JR, Tan C, Thiene G, van der Wal AC, Winters GL. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol 2012;21:245–274. [DOI] [PubMed] [Google Scholar]
- 23. Champion SN, Stone JR. Immune checkpoint inhibitor associated myocarditis occurs in both high-grade and low-grade forms. Mod Pathol 2020;33:99–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.