Abstract
The pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 infection is a severe complication of coronavirus disease 2019. Since impaired coagulation and thrombosis/endotheliitis are suspected pathomechanisms, we treated 2 patients with defibrotide, a profibrinolytic, antithrombotic, antiinflammatory oligonucleotide. Symptoms resolved during treatment. Moreover, coagulation parameters indicating hypofibrinolysis and complement activation normalized.
The pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 infection is a severe complication of coronavirus disease 2019. Since impaired coagulation and thrombosis/endotheliitis are suspected pathomechanisms, 2 patients received defibrotide, a profibrinolytic, antithrombotic, antiinflammatory oligonucleotide. Symptoms resolved and hypofibrinolysis/complement activation normalized during treatment.
Keywords: children, complement, coronavirus disease 2019, defibrotide, endotheliitis
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in adults can cause respiratory failure [1], impaired coagulations, and thromboembolic complications. Especially high levels of D-dimers seem to be a negative prognostic factor [2]. The reason for this might be endothelial damage restricted not only to the lungs but affecting the whole organ system, resulting in multiorgan failure. Endothelial cell infection associated with an endotheliitis has been described in autopsy cases [3].
More recently, a rare pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 infection (PIMS-TS) has been described. Presenting signs and symptoms are fever, abdominal pain, cardiac involvement, and/or a hyperinflammatory shock [4], which are also suggestive for endothelial damage.
The sinusoidal obstruction syndrome (SOS) or venoocclusive disease is another disease of the endothelial system that occurs in adults and children after intensive chemotherapy/radiotherapy in the context of allogeneic stem cell transplantation. It is a serious complication most likely caused by endothelial injuries and consecutive activation of complement with formation of microthrombi mainly in the liver, although any organ can be affected. The endothelial cell injury promotes a prothrombotic–hypofibrinolytic state with elevated levels of von Willebrand factor (vWF) and plasminogen activator inhibitor as well as reduced levels of thrombomodulin and tissue plasminogen activator (TPA) [5].
The only agent with proven efficacy for the treatment of severe SOS is defibrotide (DF), a polydisperse oligonucleotide with profibrinolytic, antithrombotic, antiischemic, antiinflammatory, and antiadhesive activity [6]. It has been shown that DF protects endothelial cells from toxic, inflammatory, and reperfusion damage and might restore the thrombofibrinolytic balance by increasing TPA and thrombomodulin and by reducing vWF [7]. Given the similarity of the endothelial damage in patients with SOS and the positive action of DF in this disease, we hypothesized that DF can be a treatment for our patients with PIMS-TS, which might also resemble a generalized endotheliitis.
RESULTS
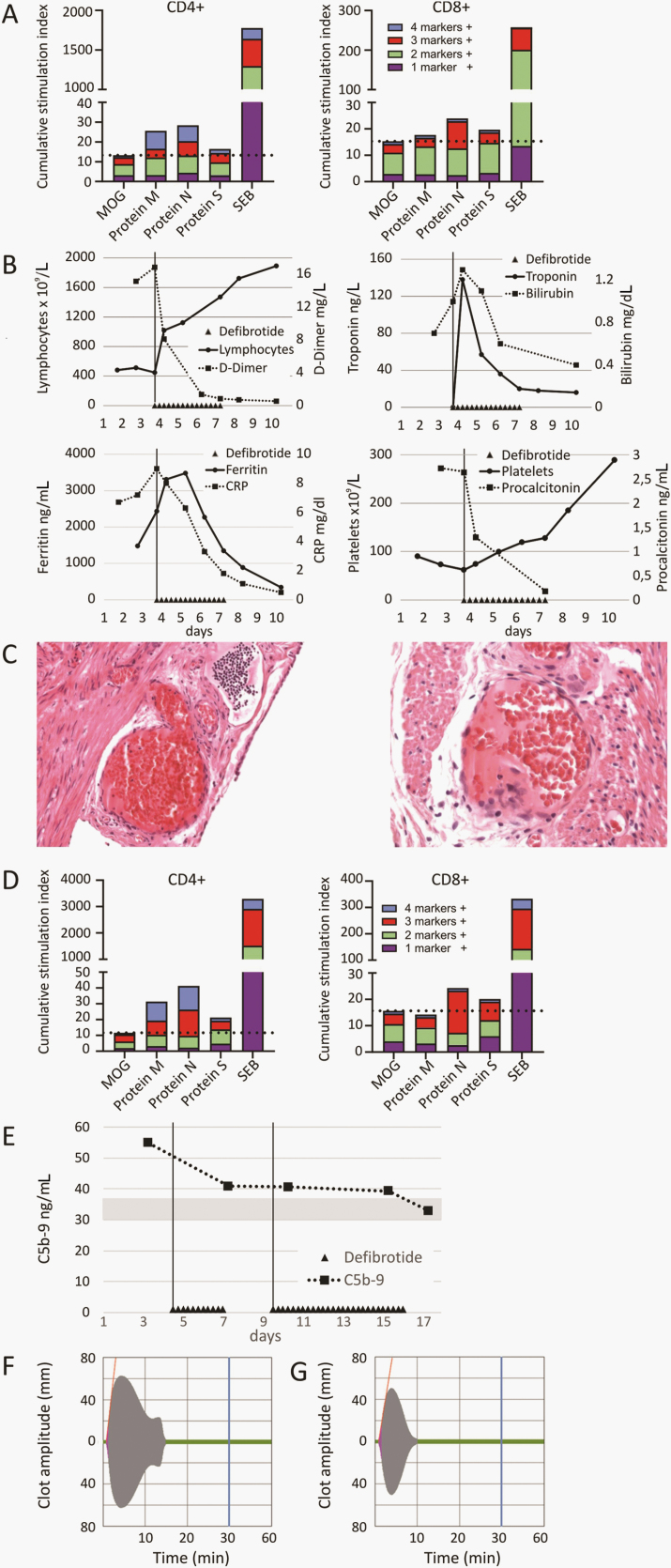
The first patient, a 13-year-old female without any comorbidities, was admitted for a diagnosis of appendicitis. After a laparoscopic appendectomy, the patient was discharged in good clinical condition. Two weeks later, she presented with fever, headache, abdominal pain, nausea, and altered sense of smell. An oropharyngeal swab was positive for SARS-CoV-2 by real time polymerase chain reaction (RT-PCR), and she had immunoglobulin G (IgG) against SARS-CoV-2 spike, receptor binding domaine, and nucleocapsid. IgM and IgA were negative. Restimulation of T cells ex vivo with overlapping peptide pools derived from SARS-CoV-2 antigens and subsequent intracellular cytokine staining revealed specific T-cell responses against viral proteins M and S (CD4+) and protein N (CD4+ and CD8+; Figure 1A). The chest X ray was normal. Laboratory tests revealed lymphopenia, thrombocytopenia, increased troponin, abnormal liver function tests, increased bilirubin, and markedly elevated D-dimers. Blood cultures and virologic workup for other viruses were negative. The patient was admitted to the intensive care unit (ICU) for treatment of arterial hypotension with catecholamines. After informed consent by the legal guardians, DF was started as an off-label treatment at a dose of 4 mg/kg every 6 hours for the first day followed by 6 mg/kg every 6 hours for an additional 3 days together with low-dose heparin (5 units/kg/h). After start of DF, D-dimers, troponin, and bilirubin decreased rapidly, while lymphocyte and platelet numbers increased (Figure 1B). No side effects of DF were seen, and the patient was discharged after 9 days. Since no other antiinflammatory drug was given, the rapid response was attributed to the application of DF.
Figure 1.
Immunological and histological findings and course of laboratory parameters. A, Detection of T-cell response for patient 1. Overlapping peptide pools covering the sequence of major severe acute respiratory syndrome coronavirus 2 proteins (proteins M, N, and S) and irrelevant control peptides (MOG) were used to stimulate T cells independent of human leukocyte antigen types ex vivo for 16 hours followed by intracellular cytokine staining. Cumulative stimulation indexes of CD4+ and CD8+ T-cell responses are shown. Simultaneous expression of cytokines (interferon [IFN]-γ, tumor necrosis factor [TNF]-α, interleukin [IL] 2) and activation markers (CD154) in response to stimuli was calculated using Boolean gating. Stimulation indexes indicate quotients of stimulus and negative control (dimethyl sulfoxide [DMSO]) values for all 16 possible combinations of marker expression. B, Course of laboratory parameters for patient 1. Temporal course of D-dimers, thrombocytes, lymphocytes, bilirubin, troponin, ferritin, C-reactive protein, and procalcitonin during administration of defibrotide is shown. Defibrotide (DF) was started due to clinical deterioration and strongly increasing D-dimers, bilirubin, troponin, and thrombocytopenia. Parameters normalized rapidly after start of DF. No other antiinflammatory agents were used. C, Pathological specimen of the resected appendix from patient 1. A retrospective analysis revealed the presence of subserosal and intramural capillary endotheliitis without signs of bacterial infection 2 weeks prior to clinical deterioration. D, T-cell response for patient 2. Cumulative stimulation indexes of CD4+ and CD8+ T-cell responses are shown. Simultaneous expression of cytokines (IFN-γ, TNF-α, IL2) and activation markers (CD154) in response to stimuli was calculated using Boolean gating. Stimulation indexes indicate quotients of stimulus and negative control (DMSO) values for all 16 possible combinations of marker expression. E, Complement activation for patient 2. The membrane attack complex C5b-9 was measured at admission using enzyme-linked immunosorbent assay. A 2-fold elevated level was found compared with 4 healthy donors (gray bar depicts mean ±1 standard deviation). During DF administration, levels normalized. F and G, Thromboelastographic analysis for patient 2. Blood samples from patient 2 showed increased clot firmness and remarkable resistance to clot lysis after subsequent induction of fibrinolysis by tissue plasmin activator (F). This finding was significantly improved after the initiation of DF treatment (G). Abbreviations: CRP, C-reactive protein; MOG, myelin oligodendrocyte glycoprotein; protein M, membrane glycoprotein; protein N, nucleocapsid phosphoprotein; protein S, spike glycoprotein; SEB, staphylococcus enterotoxin B.
A retrospective analysis of the pathological specimen of the resected appendix 2 weeks earlier revealed the presence of subserosal and intramural capillaritis/endotheliitis without signs of bacterial infection or mucositis. In addition, a high load of SARS-CoV-2 RNA virus was detected by 2 quantitative RT-PCRs of the appendix (RdRp gene: cycle threshold [Ct], value 24.63 and N-gene: Ct value, 23.14; Figure 1C).
The second patient, a 10-year-old female without any preexisting diseases, was admitted with a differential diagnosis of appendicitis with fever (>40°C) and diffuse abdominal pain. A routine pharyngeal swab was positive for SARS-CoV-2 by RT-PCR. The patient was also positive for IgG and IgA against SARS-CoV-2 spike protein and IgG, IgM, and IgA against SARS-CoV-2 RBD. She also had IgG and IgM against SARS-CoV-2 nucleocapsid. Restimulation of T cells ex vivo with overlapping peptide pools derived from SARS-CoV-2 antigens revealed specific T-cell responses against protein M (CD4+) and proteins N and S (CD4+ and CD8+; Figure 1D). A blood culture for bacteria was negative. Abdominal ultrasound revealed a wall-thickened ileum suggestive of ileitis terminalis, while the appendix appeared normal. Laboratory tests at admission showed lymphopenia, increased C-reactive protein, slightly increased interleukin-6, and increased D-dimers (3.3 mg/L). Two days later, the abdominal ultrasound showed a hepatomegaly and ascites, and the ileum wall was still thickened. An X ray of the lungs showed moderate pleural effusions and pulmonary infiltrates.
Over the following days, increasing ascites and pleural and mild pericardial effusions required oxygen supplementation. Troponin and D-dimers increased to 14 ng/mL and 4.7 mg/L, respectively. Extended investigation of the cellular and plasmatic coagulation system revealed slight hyperactivation status of platelets. Most importantly, we observed increased clot firmness in a viscoelastic coagulation assay (maximum clot firmness, 63 mm; Figure 1F). The formed clot showed remarkable resistance toward TPA (lysis time [LT] in TPA test, 520 sec; Figure 1F). Given the laboratory tests and the clinical state, a diagnosis of PIMS-TS was made, and we started treatment with DF at a dose of 25 mg/kg/day divided into 4 doses together with low-dose heparin. After 3 doses of DF, the clot firmness was reduced (MCF, 50 mm; Figure 1G) and TPA-mediated lysis was significantly improved (LT, 360 sec; Figure 1G). Although this is still speculative, our data indicate that the DF treatment might have supported the TPA-mediated clot lysis, probably by increasing plasmin concentration or downregulating the plasminogen activator inhibitor. After 8 days of DF treatment, the patient was afebrile and was discharged 17 days after admission with normal laboratory results and without signs of ascites or pleural effusions.
DISCUSSION
Both patients presented with fever and abdominal pain with an initial differential diagnosis of acute appendicitis and both had SARS-CoV-2 infection documented by positive pharyngeal swabs (PCR), by presence of anti-SARS-CoV-2 antibodies, as well as detection of SARS-CoV-2–specific T cells (Figure 1A and 1D). The inflammatory status and laboratory tests were suggestive for the diagnosis of the recently described PIMS-TS. To date, no specific treatment for this syndrome has been reported. In a recent publication, 48 children with coronavirus disease 2019 (COVID-19) admitted to ICUs were described. Most children were treated with hydroxychloroquine. Other agents, primarily used in combinations, were azithromycin, remdesivir, and tocilizumab [8]. Feldstein et al describe pediatric patients with multisystem inflammatory syndrome who mainly received steroids or intravenous immunoglobulin (IVIG) in case of Kawasaki disease–like features. None of these approaches are primarily directed toward the treatment of assumed endothelial damage [9]. The reasons for the endotheliitis are currently not clear. It might be a direct toxic effect of the virus since expression of angiotensin-converting enzyme 2 on endothelial cells can provide an entry mechanism for the virus. Another possible mechanism might be a complement-mediated injury either by direct complement activation or one that is antibody-mediated [10]. Indeed, significant deposits of C5b-9 have been described in the lungs in autopsy cases [11]. Therefore, we measured the C5b-9 complex in patient 2 and found a 2-fold elevated level at time of admission that normalized during DF treatment (Figure 1E). Furthermore, one could speculate that higher titers of anti–SARS-CoV-2 IgM and/or IgG might induce enhanced complement-mediated endothelial damage since highly specific IgG antibodies correlated with more severe illness and poorer outcome compared with patients with lower titers [12].
We favor the hypothesis of complement-mediated endothelial cell damage, which is further supported by increased numbers of endothelial cells and endothelial colony-forming cells in the peripheral blood of adults with severe illness (R. Handgretinger, unpublished observations). The endothelial damage in turn is associated with an increased risk of microthrombi [13] and hypofibrinolysis, which is also seen in COVID-19 patients (Bakchoul et al, manuscript submitted). Interestingly, we observed a normalization of the hypofibrinolytic state of patient 2 during DF infusions using the TPA test. Heparin possibly supplemented DF treatment but is unlikely to exert the observed effects alone as a single agent.
Another observation was that patient 1 had most likely 2 waves of disease. The first wave was the COVID-19–associated appendicitis with capillaritis/endotheliitis, and the second wave presented symptoms of PIMS-TS. One could speculate that this patient developed an antibody-mediated immune response that then may have led to the second wave of disease by complement-mediated mechanisms. Thus, we monitor complement activation by determination of the C5b-9 membrane attack complex in children with suspected PIMS-TS. In case of elevated levels, monoclonal antibodies represent a potential treatment option. In addition, thromboelastography can be useful to monitor the deranged thrombofibrinolytic balance.
Given the multiple positive activities of DF, it might be an effective drug to treat endothelial damage and to restore the thrombofibrinolytic balance in SARS-CoV-2 infections, potentially supplemented by steroids in case of massive inflammation and/or IVIG in patients with Kawasaki disease–like features. Since DF is already approved in adults and children aged >1 month, a clinical study using this “old” drug is warranted in all age categories. In addition, monitoring the C5b-9 levels and the coagulation status of the patients might provide helpful information to guide this approach.
Notes
Financial support. This work was supported by grants from the excellence cluster iFIT (Image-Guided and Functionally Instructed Tumor Therapies) funded by the German Research Foundation under Germany’s Excellence Strategy - EXC2180 - 390900677 [Gefördert durch die Deutsche Forschungsgemeinschaft (DFG) im Rahmen der Exzellenzstrategie des Bundes und der Länder - EXC 2180 - 390900677] to P. L. and A. R., the Dieter Schwarz Stiftung Neckarsulm, the Reinhold-Beitlich Stiftung Tuebingen, the Deutsche Herzstiftung to K. K., and the Stiftung fuer krebskranke Kinder Tuebingen to P. L. and R. H. We thank Barbara Lang, MD, for reviewing the manuscript and the Foerderverein fuer krebskranke Kinder Tuebingen e.V. for continuous support.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Goh KJ, Choong MC, Cheong EH, et al. Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID-19 infection. Ann Acad Med Singap 2020; 49:108–18. [PubMed] [Google Scholar]
- 2. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395:1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant 2011; 46:1495–502. [DOI] [PubMed] [Google Scholar]
- 6. Richardson PG, Corbacioglu S, Ho VT, et al. Drug safety evaluation of defibrotide. Expert Opin Drug Saf 2013; 12:123–36. [DOI] [PubMed] [Google Scholar]
- 7. Pescador R, Capuzzi L, Mantovani M, et al. Defibrotide: properties and clinical use of an old/new drug. Vascul Pharmacol 2013; 59:1–10. [DOI] [PubMed] [Google Scholar]
- 8. Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr 2020; 174:868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang R, Xiao H, Guo R, et al. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect 2015; 4:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020; 220:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang B, Zhou X, Zhu C, et al. Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci 2020; 7:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; 191:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]