Abstract
Background
In hospitalized patients with coronavirus disease 2019 (COVID-19) pneumonia, progression to acute respiratory failure requiring invasive mechanical ventilation (MV) is associated with significant morbidity and mortality. Severe dysregulated systemic inflammation is the putative mechanism. We hypothesize that early prolonged methylprednisolone (MP) treatment could accelerate disease resolution, decreasing the need for intensive care unit (ICU) admission and mortality.
Methods
We conducted a multicenter observational study to explore the association between exposure to prolonged, low-dose MP treatment and need for ICU referral, intubation, or death within 28 days (composite primary end point) in patients with severe COVID-19 pneumonia admitted to Italian respiratory high-dependency units. Secondary outcomes were invasive MV-free days and changes in C-reactive protein (CRP) levels.
Results
Findings are reported as MP (n = 83) vs control (n = 90). The composite primary end point was met by 19 vs 40 (adjusted hazard ratio [aHR], 0.41; 95% CI, 0.24–0.72). Transfer to ICU and invasive MV were necessary in 15 vs 27 (P = .07) and 14 vs 26 (P = .10), respectively. By day 28, the MP group had fewer deaths (6 vs 21; aHR, 0.29; 95% CI, 0.12–0.73) and more days off invasive MV (24.0 ± 9.0 vs 17.5 ± 12.8; P = .001). Study treatment was associated with rapid improvement in PaO2:FiO2 and CRP levels. The complication rate was similar for the 2 groups (P = .84).
Conclusion
In patients with severe COVID-19 pneumonia, early administration of prolonged, low dose MP treatment was associated with a significantly lower hazard of death (71%) and decreased ventilator dependence. Treatment was safe and did not impact viral clearance. A large randomized controlled trial (RECOVERY trial) has been performed that validates these findings.
Clinical trial registration. ClinicalTrials.gov NCT04323592.
Keywords: ARDS, COVID-19, methylprednisolone, pneumonia, SARS-CoV-2
This multicenter observational study gave the first evidence that prolonged, low-dose methylprednisolone treatment is associated with a significantly lower hazard of death, reduced ICU burden and decreased ventilator dependence without affecting viral clearance in patients with severe COVID-19 pneumonia/ARDS.
Italy was the first European Country overwhelmed by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, experiencing an unsustainable burden on the health care system. The greatest impact was on intensive care units (ICUs) because 16% of hospitalized cases developed acute respiratory failure (ARF) requiring ICU admission [1]. COVID-19 patients with ARF need weeks of mechanical ventilation (MV) and have an unacceptably high mortality rate [2]. This is an unprecedented global emergency where even countries with advanced health care systems rapidly reach ICU saturation, and intensivists are forced to make difficult ethical decisions that are uncommon outside war zones. Any intervention directed at decreasing dependence on ventilators and mortality in COVID-19 patients is an ethical imperative and would have a significant global impact on public health.
Over the last few decades, Italy has built-up a diffuse network of respiratory high-dependency units (RHDUs), which also treat patients with severe pneumonia-related ARF requiring continuous monitoring and noninvasive positive pressure ventilation (NPPV) [3]. Patients with disease progression who require endotracheal intubation are transferred to the ICU. During the pandemic, RHDUs were pivotal in reducing ICU referral [4].
Indeed, patients with severe COVID-19 have exhausted antiviral defenses and massive tissue and systemic inflammatory response. Corticosteroids are powerful anti-inflammatory drugs that could have a role in promoting the resolution of ARF in patients with severe COVID-19 infection [5]. The rationale for prolonged, low-dose corticosteroid treatment in severe COVID-19 was recently reviewed [6].
We hypothesized that early MP treatment in hypoxemic patients with severe SARS-CoV-2 pneumonia who are at higher risk for ARF progression requiring invasive MV may quicken disease resolution, reducing the need for ICU support and mortality. We investigated the association between early intervention with prolonged MP treatment in this high-risk group and the risk for ICU admission, the need for invasive MV, and all-cause death by day 28.
METHODS
Study Design, Setting, and Participants
We conducted a multicenter, observational, longitudinal study to evaluate the association between MP treatment and outcome in consecutive patients with severe COVID-19 pneumonia admitted to 14 Italian RHDUs between February 27 and April 24, 2020. Follow-up continued through May 21, 2020. The composite primary endpoint included admission to ICU, need for invasive MV, or all-cause death by day 28, while secondary endpoints were MV (combined invasive and noninvasive or invasive alone)-free days by day 28 [7] and changes in C-reactive protein (CRP) levels. The study was carried out in accordance with the Declaration of Helsinki. It was registered on ClinicalTrials.gov (NCT043235929) after approval by the referral Ethics Committee for the Coordinating Centre (University Hospital of Trieste, #CEUR-2020-Os-052).
The study baseline was defined as the time of fulfillment of inclusion criteria after admission to RHDU. Inclusion criteria were the following: (1) SARS-CoV-2 positive (on swab or bronchial wash); (2) age >18 years and <80 years; (3) PaO2:FiO2 <250 mmHg; (4) bilateral infiltrates; (5) CRP >100 mg/L; and/or 6) diagnosis of acute respiratory distress syndrome (ARDS) according to the Berlin definition [8] as an alternative to criteria (4) and (5). Exclusion criteria were heart failure as the main cause of ARF, decompensated liver cirrhosis, immunosuppression (ie, cancer on treatment, post–organ transplantation, HIV-positive, on immunosuppressant therapy), dialysis dependence, on long-term oxygen or home mechanical ventilation, idiopathic pulmonary fibrosis, neuromuscular disorders, dementia or a decompensated psychiatric disorder, severe neurodegenerative conditions, on chronic steroid therapy, pregnancy, a do-not-resuscitate order, and use of tocilizumab or other experimental treatment. Patients in both study groups received standard of care, comprising noninvasive respiratory support, antibiotics, antivirals, vasopressors, and renal replacement therapy as deemed suitable by the health care team.
Exposure to methylprednisolone (nonpatented drug, ATC code H02AB04) complied with the following protocol: a loading dose of 80 mg intravenously (iv) at study entry (baseline), followed by an infusion of 80 mg/d in 240 mL of normal saline at 10 mL/h for at least 8 days, until achieving either a PaO2:FiO2 >350 mmHg or a CRP <20 mg/L; after which, oral administration at 16 mg or 20 mg iv twice daily until CRP reached <20% of the normal range or a PaO2:FiO2 >400 (alternative SatHbO2 ≥95% on room air). The MP protocol was developed by the coordinating center in accordance with the “Recommendation for COVID-19 Clinical Management” by the National Institute for the Infectious Diseases “L. Spallanzani,” Rome, Italy [9]. The decision to apply the protocol to COVID-19 was left to the discretion of the treating team for each individual patient. Unexposed patients (controls) were selected from concurrent consecutive COVID-19 patients with the same inclusion and exclusion criteria.
Data Sources and Variables
Demographic details and laboratory, clinical, and outcome variables were manually extracted from electronic medical records or charts and anonymously coded into a standardized data collection form. Three independent physicians checked the data, and 2 researchers adjudicated any difference in interpretation between the primary reviewers.
Serial measurements included arterial blood gas, CRP, D-dimer, white cell count with differential, hemoglobin, variables for the calculation of the Sequential Organ Failure Assessment (SOFA) score [10], and days free from invasive or noninvasive MV until study day 28. Laboratory methodologies, including SARS-CoV-2 detection by reverse transcription polymerase chain reaction (RT-PCR), and reference values were comparable between centers. Other collected data included date of death, admission to the ICU, dates of discharge from hospital and ICU, intrahospital medications, in-hospital adverse events, and comorbidities. Samples from seriated nasopharyngeal swabs were collected in each group to evaluate viral shedding.
Statistical Methods
Considering a study power (1-beta) of 80% and a probability of type 1 error (alpha) of 0.05, assuming that the proportion of treated patients with the primary end point was 0.7 under the null hypothesis (according to available information from Fang et al. [11]) and 0.42 under the alternative hypothesis, and considering a 5% dropout rate, a minimum study sample of 104 patients was established. Data were described using absolute and relative frequencies (percentage) or position indices (mean or median) and relative dispersion indices (SD or interquartile range), as appropriate according to the type and distribution of the variable analyzed. The differences between study groups (MP-treated and control) in the proportion of patients reaching the primary end point were evaluated using a 2-sided chi-square test. The difference in numerical variables between groups was calculated using the Student t test or Wilcoxon rank-sum test, depending on the distribution of the variables.
Differences between study groups concerning categorical and dichotomous variables were evaluated by means of the chi-square test or Fisher exact test, as appropriate. Time-to-event analyses were performed for both the composite primary end point and death alone. Time at risk for all-cause death was computed from the date of study enrollment up to the date of death, hospital discharge, or 28 days, whichever came first. Event-free probabilities were estimated by the Kaplan-Meier method, and differences between groups were assessed by the log-rank test. Multivariable Cox proportional hazard models estimated the hazard ratio (HR) of both the primary composite end point and all-cause death, with corresponding 95% confidence intervals, taking into account the confounding factors (ie, sex, age, and baseline values of SOFA score, PaO2:FiO2, CRP levels) potentially associated with the outcome. These variables and others with baseline differences (eg, smoke) were tested in univariate survival models, and variables significant at P = .1 were tested in the multivariable models. Proportional hazards assumption was assessed by visual inspection of the log(-log(survival)) plot. There were no missing data with regard either to the composite primary end point and the adjustment factors included in the final Cox models or to MV-free days. Available case analysis was performed for time variation of C-reactive protein (CRP) and PaO2:FiO2 levels. All tests were 2-sided, and a P value of <.05 was considered statistically significant.
Sensitivity analyses were completed as recommended by the STROBE guidelines for reporting observational studies [12]. Although a protocol was used to standardize study measures, we conducted a sensitivity analysis to account for potential variance in medical decision-making that could potentially impact the primary composite outcome. We examined hypothetical scenarios against the hypothesis by varying the number of subjects meeting the primary composite outcome by 3 and 5 subjects to account for potential bias in both groups.
RESULTS
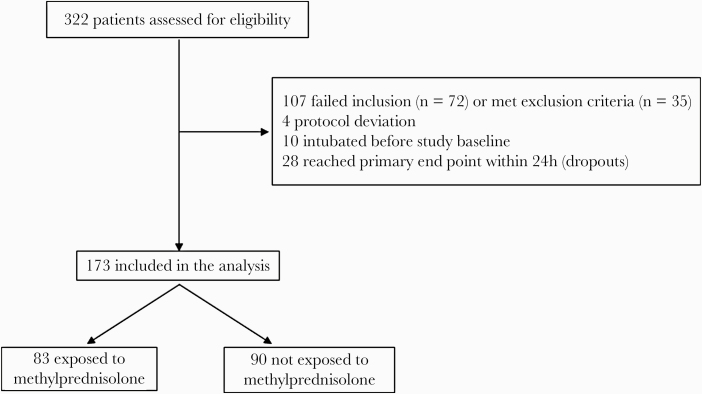
Between February 27 and April 24, 2020, 322 consecutive SARS-CoV-2-positive patients who were admitted to 1 of 14 RHDUs with severe pneumonia were assessed for study eligibility. A total of 173 patients (83 MP-treated exposed and 90 untreated controls) were enrolled, while 149 were excluded, as detailed in Figure 1.
Figure 1.
Flowchart of the study population. Failed to meet inclusion criteria (n = 72): age >80 years (n = 9), criteria for PaO2:FiO2, C-reactive protein level, or acute respiratory distress syndrome (n = 63). Met exclusion criteria (n = 35): heart failure as main cause of acute respiratory failure (n = 2), decompensated liver cirrhosis (n = 3), on long-term oxygen therapy and/or home ventilation (n = 2), dementia or severe neurodegenerative condition (n = 14), active cancer (n = 3), on chronic steroid therapy (n = 4), use of tocilizumab or other experimental treatment (n = 7). Twenty-eight patients who reached the primary end point before admission to a respiratory high-dependency unit (RHDU) or within 24 hours of admission to an RHDU were excluded from the analysis; 20 out of these 28 patients did not start methylprednisolone treatment.
The findings are reported as MP group vs control group. RHDU admission days to study enrollment were comparable (0.83 ± 2.02 vs 0.56 ± 1.50; P = .32). Table 1 shows how the patients’ baseline characteristics did not differ between groups. The mean duration of iv MP treatment was 9.11 ± 2.4 days, while the total duration of MP treatment was 13.7 ± 3.6 days. Table 2 reports the main study outcomes. The composite primary end point was reached by 19 vs 40 (22.9% vs 44.4%; P = .003; adjusted hazard ratio [HR], 0.41; 95% CI, 0.24–0.72), indicating a reduction of 59% in the risk of ICU referral, invasive MV, or death within 28 days. In particular, ICU transfer was necessary in 15 vs 27 (18.1% vs 30.0%; P = .07) and invasive MV in 14 vs 26 (16.9% vs 28.9%; P = .10).
Table 1.
Distribution of 173 Study Patients According to Study Group and Baseline Characteristics
| Methylprednisolone (n = 83) | Control (n = 90) | P Valuea | |
|---|---|---|---|
| Age, mean (SD) | 64.4 (10.7) | 67.1 (8.2) | .07 |
| Male sex, No. (%) | 54 (65.1) | 66 (73.3) | .25 |
| BMI ≥30 kg/m2, No. (%)b | 19 (33.3) | 18 (32.7) | 1.00 |
| Ever smoker, No. (%)c | 22 (29.7) | 29 (45.3) | .05 |
| Presence of major comorbidities, No. (%) | 63 (75.9) | 74 (82.2) | .35 |
| Hypertension, No. (%) | 36 (43.4) | 51 (56.7) | .09 |
| Diabetes, No. (%) | 19 (22.9) | 25 (27.8) | .49 |
| Asthma/COPD, No. (%) | 7 (8.4) | 9 (10.0) | .80 |
| OSAS/OHS, No. (%) | 5 (6.0) | 7 (7.8) | .77 |
| Congestive heart failure, No. (%) | 4 (4.8) | 2 (2.2) | .43 |
| Ischemic cardiovascular disease, No. (%) | 2 (2.4) | 9 (10.0) | .06 |
| Chronic kidney disease, No. (%) | 5 (6.0) | 4 (4.4) | .74 |
| History of malignancy, No. (%) | 7 (8.4) | 4 (4.4) | .36 |
| PaO2:FiO2, mean (SD), mmHg | 152.0 (49.8) | 151.0 (60.3) | .90 |
| Respiratory rate, mean (SD),d breaths/min | 23.7 (5.9) | 25.3 (6.8) | .16 |
| CRP, mg/L, mean (SD) | 136.9 (72.6) | 148.6 (75.6) | .30 |
| D-dimer, median (IQR), μg/FEU/L | 780 (540–1214) | 871 (472–1517) | .82 |
| LDH, mean (SD), U/L | 370.5 (130.9) | 395.3 (169.3) | .34 |
| Lymphocyte count, mean (SD) | 916.2 (657.0) | 954.5 (914.7) | .76 |
| SOFA score, median (IQR) | 3 (2–4) | 3 (2–4) | .96 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; IQR interquartile range; LDH, lactate dehydrogenase; PaO2:FiO2, ratio of partial pressure of arterial oxygen (PaO2 in mmHg) to fractional inspired oxygen (FiO2); OSAS/OHS, obstructive sleep apnea syndrome/obesity-hypoventilation syndrome; SOFA, Sequential Organ Failure Assessment.
a P value of the Fisher exact test for dichotomous variables, unpaired Student t test or Wilcoxon; rank-sum test for numerical variables, as appropriate.
bMissing data: 35 (38.9) methylprednisolone, 26 (31.3) control group.
cMissing data: 26 (28.9) methylprednisolone, 9 (10.8) control group.
dMissing data: 15 (16.7) methylprednisolone, 17 (20.5) control group.
Table 2.
Distribution of 173 Study Patients According to Study Group and Clinical Outcomes at 28 Days
| Methylprednisolone (n = 83) | Control (n = 90) | P Valuea | Crude HRb (95% CI) | Adj. HR (95% CI)c | P Valuec | |
|---|---|---|---|---|---|---|
| Major outcomes | ||||||
| Composite primary end point, No. (%) | 19 (22.9) | 40 (44.4) | .003 | 0.43 (0.25–0.74) | 0.41 (0.24–0.72) | .002 |
| Transfer to intensive care unit, No. (%) | 15 (18.1) | 27 (30.0) | .067 | ·· | ·· | ·· |
| Invasive mechanical ventilation, No. (%) | 14 (16.9) | 26 (28.9) | .095 | ·· | ·· | ·· |
| Death, No. (%) | 6 (7.2) | 21 (23.3) | .005 | 0.28 (0.11–0.68) | 0.29 (0.12–0.73) | .009 |
| Other outcomes | ||||||
| Mechanical ventilation-free days, mean (SD)d | 19.1 (8.7) | 14.3 (11.7) | .003 | |||
| Invasive mechanical ventilation-free days, mean (SD) | 24.0 (9.0) | 17.5 (12.8) | .001 | |||
| Invasive mechanical ventilation, median (IQR),e d | 7 (5.5 to 15.5) | 14.5 (12 to 22) | .031 | |||
| Required tracheotomy, No. (%) | 0 (0.0) | 12 (13.3) | <.001 | |||
| Intrapatient difference between: | ||||||
| CRP at day 3 vs baseline, median (IQR) | –85.0 (–133.0 to –42.5) | –22.0 (–65.0 to 21.3) | <.001 | |||
| CRP at day 7 vs baseline, median (IQR) | –99.4 (–162 to –62.3) | –66.1 (–116 to –0.7) | <.001 | |||
| PaO2:FiO2 at day 3 vs baseline, median (IQR) | 54.0 (7.0 to 155.0) | 6.9 (–41.5 to 77.0) | <.001 | |||
| PaO2:FiO2 at day 7 vs baseline, median (IQR) | 97.5 (42.0 to 162.0) | 68.0 (–5.5 to 139.0) | .09 | |||
| Lymphocytes at day 3 vs baseline, median (IQR) | –45 (–285 to 150) | 0 (–110 to 170) | .18 | |||
| Lymphocytes at day 7 vs baseline, median (IQR) | 110 (–170 to 480) | 130 (–140 to 350) | .88 | |||
| Lymphocytes at day 14 vs baseline, median (IQR) | 590 (–70 to 1390) | 600 (230 to 800) | .68 |
Abbreviations: CRP, C-reactive protein; HR, hazard ratio; IQR, interquartile range; PaO2:FiO2, ratio of partial pressure of arterial oxygen (PaO2 in mmHg) to fractional inspired oxygen (FiO2); SOFA, Sequential Organ Failure Assessment score.
a P value of chi-square or Fisher exact test for dichotomous variables; unpaired T-test or Wilcoxon rank-sum test for numerical variables, as appropriate.
bHR of event among methyprednisolone group vs control group, estimated using Cox regression model. The crude odds ratio (95% CI) for the composite outcomes is 0.37 (0.19–0.71).
cCox regression model was adjusted for sex, age, baseline SOFA score, baseline PaO2:FiO2, and baseline CRP.
dBoth invasive and noninvasive.
eOnly ventilated patients.
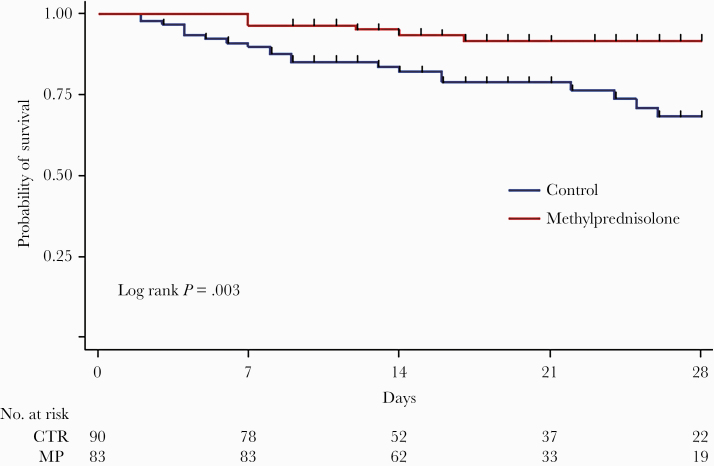
MP-treated patients had a 28-day lower risk of all-cause death than untreated ones (6 deaths, 7.2%, vs 21 deaths, 23.3%; P = .005), with a corresponding adjusted HR equal to 0.29 (95% CI, 0.12–0.73), indicating a 71% reduction in risk of death in MP patients compared with controls. Kaplan-Meier survival curves (Figure 2) showed a statistically significant difference between groups (log-rank test P = .003), with survival probabilities at 28 days of 91.6% (95% CI, 82.2%–96.2%) for MP treated and 68.2% (95% CI, 53.8%–78.9%) for control patients. The HRs did not substantially change when other variables were included in the adjusted Cox models (eg, other allowed treatments, body mass index, smoking, NPPV, and high-flow nasal cannula) (data not shown). The Kaplan-Meier curves shown in Supplementary Figure 1 illustrate timing to removal of mechanical ventilation in both groups.
Figure 2.
Kaplan-Meier estimates of survival probability. Abbreviations: CTR, control; MP, methylprednisolone.
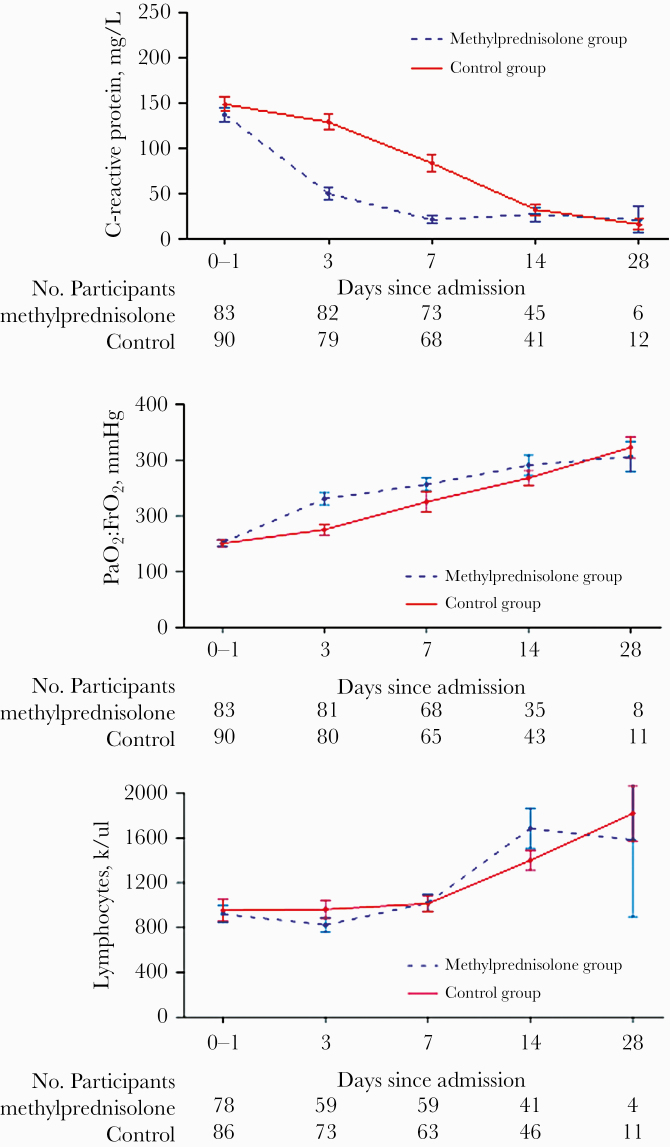
For the secondary end points (Table 2), we observed a significant increment in both MV-free days by day 28 outcomes, combined invasive MV and NPPV (19.1 ± 8.7 vs 14.3 ± 11.7; P = .003), and invasive-MV-free days alone (24 ± 9 vs 17.5 ± 12.8; P = .001). MP exposure was associated with a significant intrapatient median variation in PaO2:FiO2 at day 3 compared with baseline (54.0 [7.0 to 155.0] vs 6.9 [–41.5 to 77.0]; P < .001) but not at days 7, 14, and 28 (Figure 3). Median variation in CRP levels was also prominent in the MP group at day 3 (–85.0 [–133.0 to –42.5] vs –22.0 [–65.0 to 21.3]; P < .001) and at day 7 (–99.4 [–162.0 to –62.3] vs –66.1 [–116.0 to –0.7]; P < .001) compared with baseline, but not at days 14 and 28 (Figure 3). No differences were noted between groups in intrapatient median lymphocyte variation at days 3, 7, and 14 compared with baseline, as detailed in Table 2 and shown in Figure 3. Hospital length of stay did not differ between the groups (P = .38). No tracheostomy was necessary in MP patients vs 12 controls (odds ratio [OR], 0.04; 95% CI, 0.002–0.64; P < .001). Concerning intrahospital adverse events of any type (Supplementary Table 1), only the occurrence of hyperglycemia in nondiabetic patients, or severe glycemic decompensation in diabetic patients, and agitation was significantly higher in the MP group compared with control (8 vs 0; P = .002; and 9 vs 2; P = .03; respectively). No adverse event led to MP discontinuation. Concomitant in-hospital treatments are summarized in Supplementary Table 2.
Figure 3.
Time course of C-reactive protein and PaO2:FiO2 variation. Upper panel: time course of C-reactive protein levels (mean ± SE). The differences between groups were significant at days 3 and 7. Middle panel: time course of mean PaO2:FiO2. The differences between groups were significant at day 3. Lower panel: time course of the mean lymphocyte count showing no significant differences between groups.
There were no relevant differences in viral genome sequencing in the 2 first recruited patients compared with the average sequences reported in open source repositories (Supplementary Figure 2). Nor was any observed in viral shedding, determined as time lapse (days) between hospital admission and the first negative RT-PCR for SARS-CoV-2 nasopharyngeal swabs, in a sample of 41 MP-treated patients compared with 28 untreated ones (19.05 ± 6.11 vs 20.68 ± 7.05; P = .31).
Sensitivity analyses (Supplementary Table 3) show that the primary composite outcome still significantly differs between the MP and control groups in scenarios biased against the original hypothesis.
DISCUSSION
In our multicenter study, patients exposed to MP encountered the primary composite end point of ICU referral, need for invasive MV, or in-hospital all-cause death significantly less compared with the control group (adjusted HR, 0.41). By day 28, MP treatment was associated with a significant reduction in mortality (adjusted HR, 0.29) and an increase in MV-free days. Among patients transferred to the ICU, MP-treated patients had a 7.5-day median reduction (P = .03) in the duration of invasive MV. In line with these data, fewer MP-treated patients required tracheotomy than controls (0 vs 12; P < .001). MP-treated patients had a higher reduction in CRP levels than controls. This was statistically significant on days 3 and 7 from baseline, and there was a quicker improvement in PaO2:FiO2 ratio on day 3 for MP-treated patients. There was no overall increase in adverse events between groups, except for an increase in hyperglycemia and mild agitation in the MP-treated patients; no adverse event necessitated MP discontinuation. No difference was observed in viral shedding, determined as the number of days between hospital referral and the first negative nasopharyngeal swab.
Early interventions aimed at downregulating the SARS-CoV-2-associated hyperimmune response in severe COVID-19 patients may well avoid disease progression and enhance pneumonia resolution. The cytokine profile reported for these patients [13] is within the broad range of regulation provided by corticosteroids [14], particularly MP, which is associated with optimal lung penetration [15]. Our study protocol involved an initial iv bolus to achieve rapid, almost complete glucocorticoid receptor saturation, followed by an infusion to reach a total 160-mg dose over the first 24 hours and to maintain high levels of response throughout the treatment period. After day 7, treatment duration was guided by monitoring the anti-inflammatory response and oxygenation parameters. Our study investigated a dose that was greater than double the one investigated in the RECOVERY RCT and included tapering to minimize the risk of rebound inflammation.
This might explain the rapid reduction observed in inflammatory markers. Treatment duration was guided by monitoring the anti-inflammatory response and oxygenation after at least 8 days. Our MP treatment response is similar to that of randomized controlled studies (RCTs) in COVID-19 [16], nonviral ARDS [17], and severe pneumonia [18], as well as to that of large-scale observational studies in severe pneumonia caused by SARS-CoV (n = 7008) [19–21] and H1N1 influenza (n = 2141) [22]. Additional support for the use of methylprednisolone in COVID-19 originates from transcriptomics data. After matching the expression changes induced by SARS-CoV2 in human lung tissue tissues and A549 lung cell line against the expression changes triggered by 5694 Food and Drug Administration–approved drugs, methylprednisolone was found to be the drug with the greatest potential to revert the changes induced by COVID-19 [23].
This study was carried out before the results of the RECOVERY RCT became available, as visible in ClinicalTrials.gov posting records (results first posted June 4, 2020). In the RECOVERY trial, patients were randomized to receive dexamethasone at a dose of 6 mg/d or standard of care alone, providing evidence of lower 28-day mortality in the dexamethasone group compared with the usual care group only among those who were receiving either invasive mechanical ventilation (29.3% vs 41.4%) or oxygen alone (23.3% vs 26.2%) at randomization, but not among those receiving no respiratory support. In our study, both mortality and mortality reduction in the MP group were better than reported in the RECOVERY trial. Apart from the different study design and setting, we speculate that this difference is possibly due to several factors: First, the RECOVERY trial uses a different drug (dexamethasone) at a lower dose, equivalent to ~32 mg of methylprednisolone [24]. Second, it is likely that MP has pharmacokinetic and pharmacodynamic advantages over dexamethasone, although lung penetration needs further comparison [23]. Third, in the RECOVERY trial, the impact of the study treatment on survival seems to correlate with the need for respiratory support and therefore with illness severity. With this regard, it has been noticed that glucocorticoids are not effective in patients without ARDS and/or sepsis [18]. While permissive inclusion criteria are needed to recruit large populations in RCTs, we designed strict criteria that allowed us to include in the analyses only patients affected by severe pneumonia/ARDS with high levels of systemic inflammation and need for respiratory support. It is worth stressing that inflammatory organ injury with subsequent dysregulated host response is thought to be the main mechanism of damage in COVID-19; as a consequence, the subgroup of patients with markedly elevated levels of inflammatory markers is the one supposed to benefit most from therapeutic interventions aimed at reducing inflammatory organ injury, including corticosteroids.
The safety profile reported in our study is consistent with the findings of multiple RCTs investigating prolonged corticosteroid treatment in thousands of patients with severe sepsis, septic shock, and ARDS [17]. In these RCTs, hyperglycemia was transient in response to the initial loading bolus and did not negatively impact outcome [17]. Viral shedding in both groups of our study was in agreement with the international literature [25, 26]. The World Health Organization quotes a Middle East respiratory syndrome coronavirus study to warn about the risk of reduction in viral clearance with corticosteroid treatment. In the Arabi et al. study [27], however, those who received corticosteroid treatment for >7 days (similar to our study) had a 50% reduction in mortality (adjusted OR, 0.51; 95% CI, 0.26–1.00; P = .05) and no impact on viral clearance (aHR, 0.94; 95% CI, 0.36–2.47; P = .90). Moreover, there is no evidence linking delayed viral clearance to worsened outcome in critically ill COVID-19 patients, and it is unlikely that it would have a greater negative impact than the host’s own cytokine storm [28].
The observational design of our study implies some obvious limitations, namely possible restricted control over data collection and potential inclusion biases. However, internal validity was achieved by (1) the comparability of concurrent groups at baseline, (2) accounting for potential confounders into the multivariable Cox regression analyses, and (3) conducting sensitivity analysis to assess for potential bias in outcome ascertainment potentially influenced by medical decision-making. Our study’s strengths include a prospective evaluation of a predesigned intervention protocol based on established pharmacological principles in patients at high risk of progression to ARF and death. The limitations of the study are that we did not control for center effects and site investigators were not blinded to treatment, as with any observational study. Despite these limitations, we believe that our findings represent valid and generalizable conclusions, which have been further strengthened by the recently published RECOVERY RCT.
Indeed, we observed benefits when MP treatment was started early and prolonged in the hospitalization of hypoxemic patients with COVID-19 pneumonia at high risk of ARF progression. MP treatment was demonstrated to be safe and also allowed for a significant reduction in mortality and immediate improvements in systemic inflammation and oxygenation markers, as well as reducing invasive MV times. We believe our data support the evidence that early low-dose prolonged MP treatment can decrease ICU burden and mortality, thereby contributing to reducing the concern surrounding this therapeutic approach in patients admitted with ARF due to severe SARS-CoV-2 pneumonia in the current state of affairs.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors would like to thank Barbara Wade, contract professor at the University of Torino, for her linguistic advice; Amanda Busby, University of Hertfordshire, for her statistical suggestions; and Dr. Valentina Luzzi and Dr. Marco De Martino for their help with data collection.
Financial support. This material is the result of work supported with the resources of and use of facilities at the University Hospital of Trieste and the Memphis VA Medical Center.
Disclaimer. The contents of this commentary do not represent the views of the US Department of Veterans Affairs or the US or Italian Government.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. F.S., P.C., P.S., S.H., R.S., S.L., V.V., T.O., A.C., V.P., M.T., A.S., M.P., M.V., D.R., S.T., C.R., V.P., A.V., A.T.G., L.G., R.R., P.L., D.L., M.P.F.B., S.C., M.M., M.D., A.F., A.C., M.R., S.G., M.B., B.R., M.C.V., and M.C. collected the clinical data and revised the manuscript. X.C., L.T., A.Z., and M.M. performed the statistical analysis and revised the manuscript. R.U. provided critique, edits, and performed the sensitivity analysis. P.D., A.M., and D.L. performed the experiments related to qualitative viral assessment and genome sequencing and revised the manuscript. F.S., P.C., M.C., and G.U.M. conceptualized the study, analyzed the data, and wrote the manuscript.
Patient consent. All patients signed written consent for this study.
Ethical approval. The design of the study has been approved by the local Ethical Committee (#CEUR-2020-Os-052), and it conforms to the standards currently applied in Italy.
References
- 1. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020; 323:1545–6. [DOI] [PubMed] [Google Scholar]
- 2. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med 2020; 382:2012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Confalonieri M, Gorini M, Ambrosino N, et al. ; Scientific Group on Respiratory Intensive Care of the Italian Association of Hospital Pneumonologists Respiratory intensive care units in Italy: a national census and prospective cohort study. Thorax 2001; 56:373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vitacca M, Nava S, Santus P, Harari S. Early consensus management for non-ICU acute respiratory failure SARS-CoV-2 emergency in Italy: from ward to trenches. Eur Respir J 2020; 55:2000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 2020; 27:992–1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Villar J, Confalonieri M, Pastores SM, Meduri GU. Rationale for prolonged corticosteroid treatment in the acute respiratory distress syndrome caused by coronavirus disease 2019. Crit Care Explor 2020; 2:e0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schoenfeld DA, Bernard GR; ARDS Network Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 2002; 30:1772–7. [DOI] [PubMed] [Google Scholar]
- 8. Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–33. [DOI] [PubMed] [Google Scholar]
- 9. Nicastri E, Petrosillo N, Ascoli Bartoli T, et al. National Institute for the Infectious Diseases “L. Spallanzani”, IRCCS. Recommendations for COVID-19 clinical management. Infect Dis Rep 2020; 12:8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998; 26:1793–800. [DOI] [PubMed] [Google Scholar]
- 11. Fang X, Mei Q, Yang T, et al. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect 2020; 81:147–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370:1453–7. [DOI] [PubMed] [Google Scholar]
- 13. Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med 2020; 58:1021–8. [DOI] [PubMed] [Google Scholar]
- 14. Meduri GU, Chrousos GP. General adaptation in critical illness: glucocorticoid receptor-alpha master regulator of homeostatic corrections. Front Endocrinol (Lausanne) 2020; 11:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vichyanond P, Irvin CG, Larsen GL, et al. Penetration of corticosteroids into the lung: evidence for a difference between methylprednisolone and prednisolone. J Allergy Clin Immunol 1989; 84:867–73. [DOI] [PubMed] [Google Scholar]
- 16. RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print July 17, 2020]. N Engl J Med. 2020; doi: 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 17. Meduri GU, Bridges L, Shih MC, et al. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients’ data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med 2016; 42:829–40. [DOI] [PubMed] [Google Scholar]
- 18. Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med 2005; 171:242–8. [DOI] [PubMed] [Google Scholar]
- 19. Long Y, Xu Y, Wang B, et al. Clinical recommendations from an observational study on MERS: glucocorticoids was benefit in treating SARS patients. Int J Clin Exp Med 2016; 9:8865–73. [Google Scholar]
- 20. Chen RC, Tang XP, Tan SY, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest 2006; 129:1441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yin-Chun Yam L, Chun-Wing Lau A, Yuk-Lin Lai F, et al. Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. J Infect 2007; 54:28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li H, Yang SG, Gu L, et al. ; National Influenza A(H1N1)pdm09 Clinical Investigation Group of China Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza Other Respir Viruses 2017; 11:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Draghici S, Nguyen T-M, Sonna LA, et al. COVID-19: disease pathways and gene expression changes predict methylprednisolone can improve out- come in severe cases [Preprint]. Research Square. 15 May 2020. doi: 10.21203/rs.3.rs-29392/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Czock D, Keller F, Rasche FM, Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 2005; 44:61–98. [DOI] [PubMed] [Google Scholar]
- 25. Wölfel R, Corman VM, Guggemos W et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465–9. [DOI] [PubMed] [Google Scholar]
- 26. Jung J, Oh DK, Ahn JH, et al. Re: Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect 2020; 81:e79–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arabi YM, Mandourah Y, Al-Hameed F, et al. ; Saudi Critical Care Trial Group Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018; 197:757–67. [DOI] [PubMed] [Google Scholar]
- 28. Mehta P, McAuley DF, Brown M, et al. ; HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.