Abstract
Novel coronavirus disease 2019 (COVID-19) has spread to > 10 000 000 individuals in a short time. With no pharmacological agents successfully implemented to control the outbreak, the use of less invasive nonpharmacological agents, such as vitamin D, are increasingly being studied. This purpose of this article is to determine the current knowledge about the risk of COVID-19 development for populations at risk for vitamin D deficiency, including individuals living with overweight and obesity, those of older age, and racial or ethnic minorities. Despite the documented impact of vitamin D on viral disease prevention, many subgroups at risk for contracting COVID-19 are also known to have increased rates of vitamin D deficiency. Because vitamin D is most commonly obtained from sunlight, when interpreted alongside the stay-at-home orders, the importance of identifying safe approaches to obtain sufficient vitamin D is apparent. Furthermore, elucidating the cause-and-effect relationship between vitamin D and COVID-19, including optimal dosing for COVID-19 outcomes, is also warranted for immediate investigation.
Keywords: COVID-19, nutrition, obesity, viral diseases, vitamin D
INTRODUCTION
The novel virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), known colloquially as coronavirus disease 2019 (COVID-19), has rapidly spread from its outbreak location in Wuhan, China, to > 195 counties and 10 000 000 individuals in a short time.1,2 Recent data have shown that the disease disproportionally affects specific subgroups of individuals, such as the elderly, those living with overweight and obesity, racial and ethnic minority groups, and those with various obesity-related comorbidities and other chronic diseases.1 It is also known that many of these at-risk subgroups are also disproportionally affected by poor vitamin D status, likely due to limited sun exposure and reduced ability to synthesize vitamin D in the skin; the prevalence of overall deficiency is estimated to be approximately 40%.3–6 Because of past research supporting the role of vitamin D in immune function, particularly for improving viral-related disease outcomes, the implications of the stay-at-home orders on the ability to achieve sufficient vitamin D, particularly in at risk individuals, are unclear and are discussed in this narrative review article.
COVID-19 BACKGROUND
COVID-19 is largely spread through respiratory droplets exchanged during close person-to-person contact, though recent reports suggest that spread via infected surfaces and community spread are sustainable means of transmission.1,2 Once infected, individuals may experience a range of disease severity signs and symptoms, including being asymptomatic; or having mild flu-like symptoms, including dry cough, fever, and alterations to taste and smell; or development of disease-induced pneumonia and respiratory failure, requiring ventilator-based therapy; and death.1,2,7 The mortality rate reported by the World Health Organization on June 29, 2020, was 5.0%, and the mortality rate in the United States is also 5.0%, though the situation continues to evolve daily around the globe.1
GROUPS AT RISK FOR COVID-19 INFECTION
Individuals considered at higher risk for contracting COVID-19 include individuals age 65 years or older, especially those living in a nursing home or community long-term care facility; individuals with pre-established respiratory conditions such as chronic lung disease or asthma, or those who smoke; and individuals with weakened immune systems, including those undergoing cancer treatment or organ transplant, those with HIV or AIDS, or those who have a prolonged use of systemic corticosteroids or other medications that may affect the immune system.1,2,8 Individuals with excess weight, especially those falling into the severe obesity category (body mass index > 40 kg/m2), as well as those who have been diagnosed with weight-related comorbidities, including diabetes, kidney or liver diseases, or heart conditions, are also at increased risk to contract COVID-19 after exposure.1,2 Last, information on the effect of COVID-19 on different racial and ethnic minority groups suggests there is a disproportionate rate of infection and death compared with the White population.2
Methods to reduce the risk of contracting COVID-19 include limiting exposure to the virus by social distancing, avoiding large crowds, staying inside as much as possible, and avoiding unnecessary travel.1,2,7 In addition, practicing good hygiene can help prevent spread of the virus, including washing hands often, using hand sanitizer if hand washing is not available, cleaning and disinfecting frequently touched surfaces, and avoiding other individuals if feeling sick.1,2,7 Although multiple pharmacological agents are undergoing clinical testing as treatment options, no vaccine has been successfully implemented at present, highlighting the need for testing of nonpharmacological alternative treatments, such as vitamin D, for their impact on COVID-19 risk and disease-related outcomes.1,2,7
VITAMIN D DEFICIENCY AND COVID-19
Vitamin D metabolism
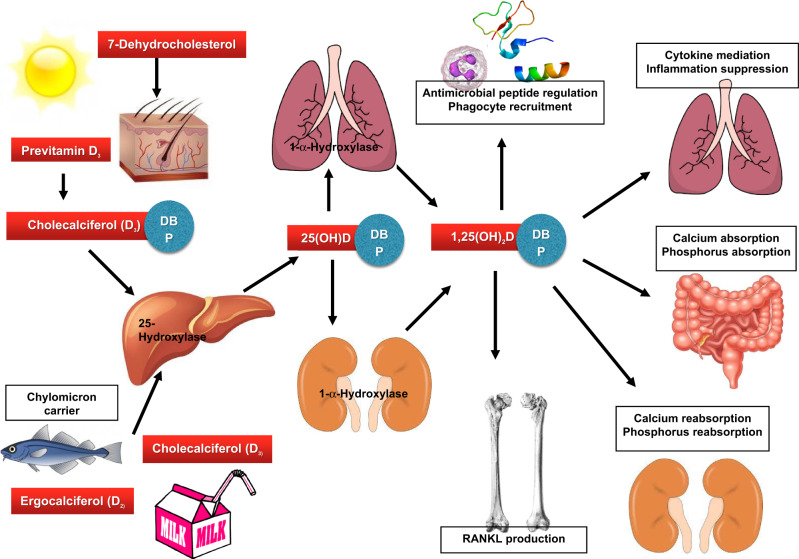
Vitamin D is a secosteroid, primarily functioning to aid in calcium absorption and use in the body.9 It can be obtained through dietary intake and supplements, as well as sunlight exposure, with endogenous synthesis in the body occurring with the latter.9–11 When sun exposure at ultraviolet B radiation of 290–315 nm occurs, the vitamin D precursor 7-dehydrocholestrol, which is found in the skin, is converted to previtamin D3.9–11 Through an isomerization process, previtamin D3 can be converted to vitamin D3, or cholecalciferol, which can then be transported by a carrier protein, vitamin D–binding protein, to the liver.9–12 Once in the liver, vitamin D3 is converted to 25-hydroxyvitamin D (25OHD), or calcidiol, through the addition of a hydroxyl group and remains in circulation in this form for 10–50 days, making it a strong indicator of overall vitamin D status.10–14 When active vitamin D is needed for functioning, 25OHD is again transported by vitamin D–binding protein to the kidneys, where it is converted to the biologically active form 1,25-dihydroxyvitamin D, or calcitriol, which functions through the nuclear receptor vitamin D receptor.9–11,13,15 Both the enzyme that activates 1,25(OH)2D, 1-α-hydroxylase, and vitamin D receptor are expressed in other tissues outside of the kidneys, such as within the respiratory epithelial cells and other immune cells, suggesting other locations for vitamin D activation and subsequent action.11–13,16–20
Vitamin D function in COVID-19
Vitamin D’s functions in the body can be separated into calcemic effects, such as those mitigating the regulation of calcium and phosphorus absorption, and noncalcemic effects (Figure 1).9,10 Researchers have also suggested that vitamin D may act as a nonpharmacological treatment for COVID-19 because vitamin D can beneficially affect immune functioning of the host against other known viral infections, such as the influenza virus (season flu) the rhinovirus (common cold), and HIV.11 In a recent observational study comparing plasma 25-hydroxyvitamin D concentrations in patients testing positive for COVID-19 infection with those testing negative, significantly lower concentrations of 25-hydroxyvitamin D (11.1 ng/mL) were found in those positive for COVID-19 compared with the negative group (24.6 ng/mL), further substantiating this claim.12
Figure 1.
Vitamin D metabolism and functions in relation to viral infections. Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25OHD, 25-hydroxyvitamin D; DBP, Vitamin D Binding Protein; RANKL, receptor activator of nuclear factor-κB ligand.
Vitamin D’s mechanism of effect in relation to these viruses is multifaceted. First, vitamin D can regulate the antimicrobial peptides β-defensin and cathelicidin, which can directly inactivate viral pathogens or inhibit replication.13–17 Second, vitamin D increases recruitment of phagocytes, thus acting as a defense for the respiratory system.14–18 Finally, vitamin D also mediates production of proinflammatory cytokines and T-helper–17 cells, which frequently have been noted in the literature to suppress inflammation and help maintain healthy immune functioning in the lungs and other body systems18–21COVID-19 infection is associated with increased numbers of proinflammatory cytokines and risk of pneumonia, sepsis, and acute respiratory distress.22–24 The combination of the mechanistic effects of vitamin D related to these characteristics of COVID-19 help elucidate how a sufficient vitamin D status of the virus’ host may help support optimal immunoregulatory functioning in response to respiratory-based viral infections such as COVID-19 by improving clearance of the viral species, as well as by decreasing inflammatory responses.15,17,18,22
POPULATIONS AT RISK FOR COVID-19 AND VITAMIN D DEFICIENCY
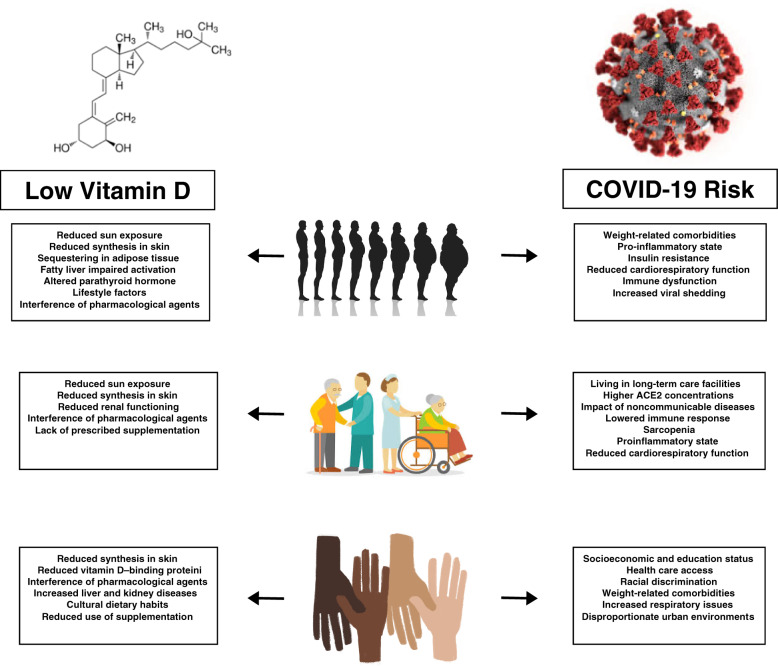
Certain subgroups of individuals are considered at greater risk of COVID-19 infection (Figure 2).2,25 Interestingly, many of these subgroups also have higher risk of vitamin D deficiency, highlighting additional evidence of vitamin D’s potential role in COVID-19 incidence and disease-related outcomes.4–6
Figure 2.
Concurrent low vitamin D levels and COVID-19 risk experienced by individuals with overweight and obesity, the elderly, and ethnic and racial minorities. Abbreviations: ACE2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019.
Overweight and obesity
A disproportionate number of individuals diagnosed with COVID-19 have underlying conditions, including obesity and weight-related comorbidities including hypertension, cardiovascular diseases, and diabetes.25 It also is known that individuals with overweight and obesity exhibit increased shedding of the virus after exposure, which may be related to increased exposure for individuals in close contact.26
It is suspected that excess weight can result in poor COVID-19 outcomes due to the impact of overweight on cardiorespiratory and lung functioning, as well as on immune dysfunction from the overall proinflammatory state that can eventually result in organ failure.26 From a cardiorespiratory standpoint, obesity can also influence the development of hypertension, diabetes, stroke, atrial fibrillation, kidney and liver diseases, and overall heart failure.26 These comorbidities cause physical manifestations in the body such as reduced pulmonary function, functional capacity, and respiratory system compliance, along with reduced β-cell functioning and increased insulin resistance, all of which can limit proper immune responses to viral exposures and increase a patient’s difficulty responding to common COVID-19 therapy such as ventilatory support.26,27
Vitamin D deficiency is also more prevalent in individuals living with overweight and obesity compared with individuals of normal weight, with some research suggesting that deficiency prevalence is 25%–35% with overweight and obesity.5,28 Multiple factors are considered to lead to vitamin D deficiency in individuals with overweight and obesity, including reduced synthesis from sun exposure, sequestering of vitamin D in excess adipocyte cells, impaired activation in the body due to fatty liver, altered parathyroid hormone concentrations and signaling, and lifestyle factors.9,29–31 In addition, pharmacological agents such as antibiotics, anti-inflammatory agents, antihypertensive medications, antiretroviral drugs, endocrine drugs, and herbal medications commonly taken in response to weight-related comorbidities can affect sunlight synthesis of vitamin D3 and reduce serum 25OHD concentrations available for activation in the body.22,32
Age
Older adults are especially at risk for COVID-19, with the greatest incidence of disease spread occurring in nursing homes or community long-term care facilities.2Angiotensin-converting enzyme 2, a receptor for SARS-COV-2, which causes COVID-19, exists in higher concentrations in older adults compared with younger individuals, which may be a cause for the increased disease spread in the former.33 Other factors that also may be related to the increased risk of COVID-19 include the impact of noncommunicable diseases that develop with age, such as heart diseases, cancers, and metabolic diseases, as well as the treatments for these diseases, which may lower immune response, particularly to highly infectious diseases such as COVID-19.33 Finally, the increased incidence of COVID-19 among elderly individuals may also be due to sarcopenia (the decreased muscle mass and increased fat mass common with advanced age), because of its relation to a proinflammatory state, along with the altered cardiorespiratory and lung functioning, as well as the immune dysregulation that is seen with overweight and obesity.26
Vitamin D deficiency is highly prevalent in elderly individuals. In their study of vitamin D concentrations in 125 adults aged 75 years or older, researchers Kweder and Eidi34 found that 85% of the sample had some degree of vitamin D deficiency and 43% had severe or very severe deficiency. Some of the key reasons that may result in vitamin D deficiency in this population include overall lack of sun exposure; reduced capacity to synthesize vitamin D from the sun, due to reduced 7-dehydrocholsterol in the skin; reduced renal functioning; and various surgeries and medications commonly used to control age-related comorbidities.34 It also has been reported that vitamin D supplementation is not necessarily commonly prescribed for the elderly by medical professionals to combat vitamin D deficiency. Kweder and Eidi34 also noted that 78% of the patient sample were not prescribed a vitamin D supplement treatment in the prior 3 years, and 90% were not taking any supplement at the time of study participation. It is possible that this may be due to decreased clinical practice of prescribing vitamin D supplements to elderly individuals because of the known increased risk of falls that may occur with high-dose supplements.35,36
Race and ethnicity
Recent reports suggest that racial and ethnic minorities have experienced both an increased rate of infection as well as a disproportionate amount of negative COVID-19–related outcomes compared with White individuals.37,38 The racial and ethnic minorities considered the most at risk within the United States include Black, Latino, American Indian, Alaska Native, and Pacific Islander minorities.37 The increased risk of COVID-19 transmission continues to highlight the health disparities experienced by these minority groups and can be related to social determinants of health, socioeconomic, and educational input; health care access; racial discrimination; and biological factors.37,38 Biologically, racial and ethnic minorities experience a higher rate of comorbidities that may negatively affect COVID-19 outcomes, including morbid obesity, cardiovascular diseases, diabetes, liver and kidney diseases, HIV, and respiratory issues including asthma and chronic obstructive respiratory disease.37 Socially, racial and ethnic minorities disproportionately live in urban environments where crowded conditions, substandard air quality, and public services such as transportation and essential work limit social distancing.37 In addition, lower socioeconomic status and less education can result in loss of work, loss of health insurance, limited access to adequate health resources, food insecurity, and house insecurity, further exacerbating risk of COVID-19.37,38
Race and ethnicity are considered strong predictors of 25OHD concentrations. In a recent study, authors found that when comparing Black vs White individuals, Black individuals had lower 25OHD concentrations and a higher proportion of Black individuals were considered vitamin D deficient.39 Factors that may cause disproportionate vitamin D deficiency in darker-skinned individuals include reduced ability to synthesize vitamin D from the skin, because of the darker pigmentation, as well as reduced levels of vitamin D–binding protein, which transports vitamin D in the body for activation and functioning.40,41 Racial and ethnic minorities also experience a disproportionate rate of comorbidities, including obesity, cardiovascular diseases, diabetes, and liver and kidney diseases, which can reduce the amount of vitamin D available for active functioning.37 Finally, cultural dietary habits are suggested to reduce vitamin D intake from foods, and reduced use of supplementation in ethnic minorities is also common.41
IMPACT OF STAY-AT-HOME ORDERS ON VITAMIN D STATUS
Despite plentiful research documenting the impact of vitamin D on various facets of health, many subgroups, including elderly individuals, racial and ethnic minorities, individuals with overweight and obesity, and individuals with other chronic diseases such as diabetes, cancer, and heart diseases, have high deficiency prevalences.4–6 The recommended daily allowance for vitamin D to maintain a sufficient status for proper functioning and to potentially affect viral disease outcomes is 600 IU for adults 18–70 years of age and 800 IU for adults > 70 years of age.42 Because vitamin D is not found naturally in many foods, the process of obtaining enough vitamin D through diet alone is challenging. Foods considered good sources of vitamin D, such as cod liver oil, fatty fish, egg yolks, and fortified dairy products, among others, are not often being consumed in high amounts.9,10,42–44
When dietary intake of vitamin D is lacking, synthesis can occur in the body from sunlight exposure.42–44 The optimal time to achieve sufficient vitamin D from sunlight exposure occurs between 10:00 am and 3:00 pm in spring, summer, and fall months, and the limited vitamin D synthesis that occurs in winter months is suggested to be a reason for the peak in viral infections such as influenza at a coinciding time of year.22,43,45 In as short a period as 20 minutes, adults wearing a bathing suit or other attire exposing much of their legs and arms can synthesize approximately 15 000–20 000 IU of vitamin D3, though the subsequent increase in 25OHD concentrations in the blood occurs more gradually than if the same amount of vitamin D was given orally.43,46 One study of Hawaiian surfers found that continuous sunlight exposure over 3 months could increase serum 25OHD concentrations from 11 ng/mL, a deficient status, to 71 ng/mL, a highly sufficient status, but when sunlight exposure does not occur daily, 25OHD concentrations can be expected to decrease according to the half-life of serum 25OHD, which is approximately 10–50 days.43,44,47 And although 25OHD is a marker of exposure to vitamin D via recent sunlight or supplementation, it is not indicative of the total amount of vitamin D stored in adipose tissue, which is difficult to measure, or of the actual bioavailability of stored vitamin D in the case of sequestering.29
The complications of obtaining sufficient vitamin D are further exacerbated in subgroups at risk for COVID-19; the complications include the global quarantine, isolation, and stay-at-home orders that have been given to individuals who have been officially diagnosed with COVID-19, believe they have contracted COVID-19, and who are taking measures to reduce their risk of exposure.1,2 For many, especially older individuals in nursing homes or community long-term care facilities, as well as for ethnic minorities who more commonly live in densely populated areas, these measures include little or no movement outside their home.1,2 Major implications of limited or no time spent outdoors are the reduced synthesis of vitamin D from sunlight exposure and the increased risk of vitamin D deficiency development. There also is a link to increased rates of anxiety and depression, which could exacerbate risk of COVID-19 and slow immune defense.48,49 Unfortunately, sunlight that passes through materials such as glass or plastic, polluted air, sunscreen, or clothing results in little to no vitamin D production in the skin, affecting the ability to synthesize vitamin D from sunlight penetrating indoors through windows.50 When combined with the typical Western diet high in processed foods that lack vitamin D, quarantine, isolation, and stay-at-home orders can impair one’s ability to obtain sufficient vitamin D needed to maintain or boost immune functioning.9,10,42,44,51,52 This, in turn, could reduce the ability to prevent or fight COVID-19 in susceptible individuals.
CONCLUSION
At a time when vaccines or other drug treatments for COVID-19 are not yet developed, the hypothesis that vitamin D supplementation or exposure is a nonpharmacological means to reduce COVID-19 incidence and prevalence is supported in mechanistic and viral-related outcomes literature.13,15,17,22 Furthermore, it is suggested that daily doses of vitamin D vs bolus administration may be more effective for treating viral infections, and vitamin D administration in this way may be an effective approach to addressing COVID-19.53
Because of the limitations of the major form of obtaining vitamin D—sunlight exposure—for many subgroups at risk, such as older people, those with darker skin tones, and those with excess fat mass, the quarantine, isolation, and stay-at-home orders in place globally at this time have made evident the cyclic relationship between decreasing vitamin D status and increasing risk of COVID-19.1,2,22 On a practical level, proposed actions for susceptible individuals trying to reduce their risk for COVID-19 include identifying safe locations to obtain direct sunlight exposure regularly (ie, not through glass, plastic, pollution, or sunscreen), as well as identifying nonsunlight-based approaches to obtaining sufficient vitamin D, such as through modifying dietary intake to include shelf-stable or frozen foods that contain vitamin D, or using vitamin D supplementation.9,10,42,44,50 Because high doses of vitamin D supplementation are associated with negative outcomes, particularly in elderly individuals, the use of lower doses in combination with other dietary components that may enhance their effect (eg, combined with calcium, magnesium, and dietary fats) may be warranted.22,35,36,54–56
From a research perspective, interventional trials are needed to investigate the impact of vitamin D supplementation or adequate sunlight exposure, as well as of other antioxidant micronutrients such as vitamin C, iron, and zinc, on COVID-19 risk and disease-related outcomes in healthy individuals as well as in those subgroups identified to be at a greater risk for COVID-19.1,2 Furthermore, consideration of a more homogenous spread of study designs and of dosing regimens is imperative to be able to fully elucidate the effects of nonpharmacological vitamins and minerals against respiratory viral infections, as well as the ability to improve outcomes of these diseases while research is ongoing to develop a vaccine or other drug agent to reduce COVID-19 incidence and disease-related mortality.
Acknowledgments
Author contributions. All authors helped conceive the article topic and participated in writing and editing the text. They are solely responsible for this manuscript’s content.
Funding. None.
Declaration of Interest. The authors declare no conflicts of interest for this paper.
References
- 1. World Health Organization. Coronavirus disease (COVID-19) pandemic. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200629-covid-19-sitrep-161.pdf?sfvrsn=74fde64e_2. Accessed June 29, 2020.
- 2. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) situation summary. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/summary.html. Accessed June 15, 2020.
- 3. Gallagher JC. Vitamin D and aging. Endocrinol Metab Clin North Am. 2013;42:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roth DE, Abrams SA, Aloia J, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann N Y Acad Sci. 2018;1430:44–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pereira-Santos M, Costa PR, Assis AM, et al. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. 2015;16:341–349. [DOI] [PubMed] [Google Scholar]
- 6. Parva NR, Tadepalli S, Singh P, et al. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus. 2018;10:e2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun P, Lu X, Xu C, et al. Understanding of COVID-19 based on current evidence. J Med Virol. 2020;92:548–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fauci AS, Lane HC, Redfield RR. Covid-19—navigating the uncharted. N Engl J Med. 2020;382:1268–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. [DOI] [PubMed] [Google Scholar]
- 10. Jones G. Vitamin D In: Ross C, Cousins R, Tucker K, Ziegler T, eds. Modern Nutrition in Health and Disease. Baltimore, MD: Lippincott Williams & Wilkins; 2014:278–304. [Google Scholar]
- 11. Yamshchikov AV, Desai NS, Blumberg HM, et al. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 2009;15:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D’Avolio A, Avataneo V, Manca A, et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12:1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cantorna MT. Vitamin D and lung infection. Infect Immun. 2016;84:3094–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Telcian AG, Zdrenghea MT, Edwards MR, et al. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antiviral Res. 2017;137:93–101. [DOI] [PubMed] [Google Scholar]
- 15. Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. [DOI] [PubMed] [Google Scholar]
- 16. Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. [DOI] [PubMed] [Google Scholar]
- 17. Beisswenger C, Bals R. Antimicrobial peptides in lung inflammation. Chem Immunol Allergy. 2005;86:55–71. [DOI] [PubMed] [Google Scholar]
- 18. Cantorna MT, Yu S, Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Mol Aspects Med. 2008;29:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7:4240–4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sassi F, Tamone C, D’Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10:1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun J. Vitamin D and mucosal immune function. Curr Opin Gastroenterol. 2010;26:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Finer N, Garnett SP, Bruun JM. COVID-19 and obesity. Clin Obes. 2020;10:e12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sattar N, McInnes IB, McMurray J. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation 2020;142:4–6. [DOI] [PubMed] [Google Scholar]
- 27. Dietz W, Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity (Silver Spring). 2020;28:1005–1005. [DOI] [PubMed] [Google Scholar]
- 28. Kaidar-Person O, Person B, Szomstein S, et al. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part A: vitamins. Obes Surg. 2008;18:870–876. [DOI] [PubMed] [Google Scholar]
- 29. Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. [DOI] [PubMed] [Google Scholar]
- 30. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Earthman CP, Beckman LM, Masodkar K, et al. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes. 2012;36:387–396. [DOI] [PubMed] [Google Scholar]
- 32. Grober U, Kisters K. Influence of drugs on vitamin D and calcium metabolism. Dermatoendocrinol 2012;4:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koff WC, Williams MA. Covid-19 and immunity in aging populations—a new research agenda. N Engl J Med. 2020;doi:10.1056/NEJMp2006761. [DOI] [PubMed] [Google Scholar]
- 34. Kweder H, Eidi H. Vitamin D deficiency in elderly: risk factors and drugs impact on vitamin D status. Avicenna J Med. 2018;8:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith LM, Gallagher JC, Suiter C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: a randomized clinical trial. J Steroid Biochem. 2017;173:317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176:175–183. [DOI] [PubMed] [Google Scholar]
- 37. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shippee TP, Akosionu O, Ng W. COVID-19 pandemic: exacerbating racial/ethnic disparities in long-term services and supports. J Aging Soc Policy. 2020;32:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chawla D, Daniels JL, Benjamin-Neelon SE, et al. Racial and ethnic differences in predictors of vitamin D among pregnant women in south-eastern USA. J Nutr Sci. 2019;8:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alzaman NS, Dawson-Hughes B, Nelson J, et al. Vitamin D status of black and white Americans and changes in vitamin D metabolites after varied doses of vitamin D supplementation. Am J Clin Nutr. 2016;104:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harris SS. Vitamin D and African Americans. J Nutr. 2006;136:1126–1129. [DOI] [PubMed] [Google Scholar]
- 42. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nair R, Maseeh A. Vitamin D: the “sunshine” vitamin. J Pharmacol Pharmacother. 2012;3:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(suppl 4):1080s–1086s. [DOI] [PubMed] [Google Scholar]
- 45. Abhimanyu A, Coussens AK. The role of UV radiation and vitamin D in the seasonality and outcomes of infectious disease. Photochem Photobiol Sci. 2017;16:314–338. [DOI] [PubMed] [Google Scholar]
- 46. Haddad JG, Matsuoka LY, Hollis BW, et al. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91:2552–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hollis BW, Wagner CL, Drezner MK, et al. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: an important tool to define adequate nutritional vitamin D status. J Steroid Biochem. 2007;103:631–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kent ST, McClure LA, Crosson WL, Arnett DK, et al. Effect of sunlight exposure on cognitive function among depressed and non-depressed participants: a REGARDS cross-sectional study. Environ Health. 2009;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Coughlin SS. Anxiety and depression: linkages with viral diseases. Public Health Rev. 2012;34:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wacker M, Holick MF. Sunlight and Vitamin D: a global perspective for health. Dermatoendocrinol 2013;5:51–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Astrup A, Bugel S. Overfed but undernourished: recognizing nutritional inadequacies/deficiencies in patients with overweight or obesity. Int J Obes .2019;43:219–232. [DOI] [PubMed] [Google Scholar]
- 52. Martinez Steele E, Baraldi LG, Louzada ML, et al. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open. 2016;6:e009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bergman P, Lindh AU, Bjorkhem-Bergman L, et al. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8:e65835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gallagher JC. Vitamin D and falls—the dosage conundrum. Nat Rev Endocrinol. 2016;12:680–684. [DOI] [PubMed] [Google Scholar]
- 55. Dai Q, Zhu X, Manson JE, et al. Magnesium status and supplementation influence vitamin D status and metabolism: results from a randomized trial. Am J Clin Nutr. 2018;108:1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181–189. [DOI] [PubMed] [Google Scholar]