Abstract
Background
The global coronavirus disease 2019 (COVID-19) pandemic offers the opportunity to assess how hospitals manage the care of hospitalized patients with varying demographics and clinical presentations. The goal of this study was to demonstrate the impact of densely populated residential areas on hospitalization and to identify predictors of length of stay and mortality in hospitalized patients with COVID-19 in one of the hardest hit counties internationally.
Methods
This was a single-center cohort study of 1325 sequentially hospitalized patients with COVID-19 in New York between March 2, 2020, to May 11, 2020. Geospatial distribution of study patients’ residences relative to population density in the region were mapped, and data analysis included hospital length of stay, need and duration of invasive mechanical ventilation (IMV), and mortality. Logistic regression models were constructed to predict discharge dispositions in the remaining active study patients.
Results
The median age of the study cohort (interquartile range [IQR]) was 62 (49–75) years, and more than half were male (57%) with history of hypertension (60%), obesity (41%), and diabetes (42%). Geographic residence of the study patients was disproportionately associated with areas of higher population density (rs = 0.235; P = .004), with noted “hot spots” in the region. Study patients were predominantly hypertensive (MAP > 90 mmHg; 670, 51%) on presentation with lymphopenia (590, 55%), hyponatremia (411, 31%), and kidney dysfunction (estimated glomerular filtration rate < 60 mL/min/1.73 m2; 381, 29%). Of the patients with a disposition (1188/1325), 15% (182/1188) required IMV and 21% (250/1188) developed acute kidney injury. In patients on IMV, the median (IQR) hospital length of stay in survivors (22 [16.5–29.5] days) was significantly longer than that of nonsurvivors (15 [10–23.75] days), but this was not due to prolonged time on the ventilator. The overall mortality in all hospitalized patients was 15%, and in patients receiving IMV it was 48%, which is predicted to minimally rise from 48% to 49% based on logistic regression models constructed to project disposition in the remaining patients on ventilators. Acute kidney injury during hospitalization (odds ratioE, 3.23) was the strongest predictor of mortality in patients requiring IMV.
Conclusions
This is the first study to collectively utilize the demographics, clinical characteristics, and hospital course of COVID-19 patients to identify predictors of poor outcomes that can be used for resource allocation in future waves of the pandemic.
Keywords: COVID-19, SARS-CoV-2, geospatial distribution, ventilation
Since the first reported cases of coronavirus disease 2019 (COVID-19) in early December 2019 in Wuhan, China, devastating morbidity and mortality coupled with disastrous economic and societal ramifications have characterized this global pandemic [1]. As of May 11, 2020, New York State far exceeds any other state for the number of individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with Suffolk County in the top 6 counties in the entire United States at the time of this report [2].
The global COVID-19 pandemic offered the opportunity to assess how different geographies manage the care of patients with a predominantly devastating respiratory illness with significant inflammatory, thrombotic, kidney, and cardiovascular morbidities. Suffolk County lies directly east of Nassau County and greater New York City and was chronologically later affected by SARS-CoV-2 after its appearance in the 5 boroughs of New York (Manhattan, Queens, Brooklyn, Staten Island, and the Bronx) and Nassau County. Together Nassau and Suffolk counties constitute the Long Island region and have approximately similar populations (1 358 564 vs 14 817 901 people, respectively), albeit with varying population demographics [3]. Recent case series of hospitalized patients in the New York metropolitan area have highlighted the presenting clinical characteristics, morbidity, and mortality at medical centers in the region [4, 5]. To allow for comparison of these factors with patient outcomes in one of the most highly affected regions in the world, we report our clinical end points based on patient demographics, population density, and ethnicity, presenting clinical characteristics and therapeutic interventions for >1300 hospitalized patients with confirmed SARS-CoV-2 infection.
METHODS
Data Sources
The study was conducted at the Renaissance School of Medicine at Stony Brook University, the largest academic medical center in Suffolk County, New York, which provided care for the greatest number of patients with COVID-19 of the 11 hospitals in the county. Patients hospitalized between March 6, 2020, and May 11, 2020, with COVID-19, as confirmed by at least 1 positive result for SARS-CoV-2 on polymerase chain reaction (PCR) testing of nasopharyngeal samples, were included in this study. Data were extracted for hospitalized patients from the electronic health record (EHR; Cerner Millennium, Kansas City, MO, USA) and mapped to an Observational Health Data Sciences and Informatics Common Data Model (OHDSI CDM), version 5.3 [6]. Medications were mapped to OHDSI based RxNorm codes and to World Health Organization Anatomical Therapeutic Chemical drug classifications.
Definition of Variables
Data collection included baseline patient demographic information, comorbidities based on ICD10 codes mapped to Clinical Classification Software (CCS) groups occurring 30 days before admission beginning January 1, 2017, admission vital signs, and initial laboratory tests. Race and ethnicity were based on self-reporting at the time of registration and mapped to broader categories using relationships in the OHDSI controlled vocabulary. Patient locations were geolocated to latitude and longitude using the most recent patient addresses in the EHR with Easy Geocoder [7]. Total population at the Census Tract level was based on the American Community Survey (ACS) 2018 5-year estimates [3]. Population density was calculated using the Tiger shape file estimates for land area in a Census tract.
Initial laboratory testing was defined as the first test results available within 48 hours of admission. Total patients with available laboratory values are described after exclusion of outlier values. For each of the laboratory values and vital signs provided, we reviewed histograms and defined a range of presumed “valid” measurements (eg, 2 patients had recorded respiratory rates >150 breaths per minute [bpm], and 2 had values <6 bpm), and values outside of these ranges were replaced as “missing” for all subsequent analysis. Acute kidney injury during hospitalization was defined as an increase in serum creatinine of 0.3 mg/dL within 48 hours [8]. Estimated glomerular filtration (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [9]. Kidney replacement therapy was defined as onset of hemodialysis (HD) and/or continuous renal replacement therapy (CRRT). Invasive mechanical ventilation (IMV) was based on the order for an invasive mechanical ventilator and the presence of at least 2 progress notes documenting IMV in the EHR. The number of days on mechanical ventilation was based on the first and last documented date and time of IMV. In analyses, interventions were classified according to intent to treat. Hence, patients who received an intervention once were classified in the intervention arm.
Clinical outcomes, including IMV, kidney replacement therapy, discharge disposition, overall hospital length of stay, duration of IMV, readmission, and mortality, are provided. For patients with a readmission during the study period, data from the first admission are presented. For patients who remained actively hospitalized without a disposition at the end of the study period, interim data are provided for outcomes of IMV, kidney replacement therapy, and readmission. Superimposed on histograms are lines showing an empirical estimation of the continuous probability density function, which is calculated by summation of Gaussian kernels via the stats package in R. Patient density is defined as probability of counts per length of stay (days).
Statistical Analysis
A Student t test was used to compare continuous data between the 2 groups. Chi-square or Fisher exact testing, as appropriate, was used to compare the significance between categorical variables. For continuous variables, data are expressed as interquartile range (IQR; 25th and 75th percentiles) and/or the number and percentage of patients for categorical variables. Statistical significance was defined as P < .05. All analyses were performed using the R programming language (R Project for Statistical Computing, R Foundation).
Logistic regression models were constructed to predict discharge dispositions in our patient population (excluding patients who remained actively hospitalized at the end of the study period)—1 model used only variables available at the beginning of an encounter to predict overall outcomes, and a second model included more variables in an attempt to find associations with outcomes in mechanically ventilated patients. For each model, we constructed a parsimonious logistic regression with a penalty on the sum of coefficients, that is, LASSO, using the glmnet package in R. Models were cross-validated and trained on the set of cases with complete, nonmissing values for all variables considered. Our models were tested on the remaining patient data after missing values were replaced using Multivariate Imputation by Chained Equations, as implemented in the mice package in R.
RESULTS
Demographic and Clinical Characteristics
A total of 2918 SARS-CoV-2-infected patients were seen in our emergency department, with 1580 patients discharged to home quarantine for recovery and 1338 patients admitted for hospitalization. Demographics of real-time PCR-confirmed SARS-CoV-2 hospitalized patients ≥16 years of age (1325/1338) are presented in Table 1. Among the 767 patients with comorbidities identified during a prior visit, 60% had >1 comorbidity, with a predominance of hypertension (464/767, 60%), obesity (434/1065, 41%), and diabetes (324/767, 42%).
Table 1.
Baseline Characteristics of Study Patients ≥16 Years of Age
| No. | % | |
|---|---|---|
| Total cases | 1325 | |
| Age, median (IQR), y | 62 (49–75) | |
| Gender | 1325 | |
| Female | 565 | 43 |
| Male | 760 | 57 |
| Race | 1325 | |
| White | 739 | 56 |
| Other race/unknown | 447 | 34 |
| Black or African American | 92 | 7 |
| Asian | 47 | 4 |
| Ethnicity | 1325 | |
| Hispanic or Latino | 343 | 26 |
| Not Hispanic | 982 | 74 |
| Insurance | 1319 | |
| Medicare | 527 | 40 |
| Commercial | 421 | 32 |
| Medicaid | 254 | 19 |
| Self-pay | 69 | 5 |
| Other | 48 | 4 |
| Former/current smoking status | 1295 | |
| Yes | 486 | 38 |
| Comorbidities | 767 | |
| None | 187 | 24 |
| 1 | 120 | 16 |
| >1 | 460 | 60 |
| Type | ||
| Diabetes | 324 | 42 |
| Hypertension | 464 | 60 |
| Chronic kidney disease | 194 | 25 |
| Coronary artery disease | 254 | 33 |
| Heart failure | 188 | 25 |
| COPD | 172 | 22 |
| Asthma | 84 | 11 |
| Liver disease | 121 | 16 |
| Cancer | 188 | 25 |
| HIV | 5 | 1 |
| Body mass index | 1065 | |
| Median (IQR), kg/m2 | 29 (25–33) | |
| >30 kg/m2 | 434 | 41 |
| >35 kg/m2 | 208 | 20 |
Abbreviations: COPD, chronic obstructive pulmonary disease; IQR, interquartile range.
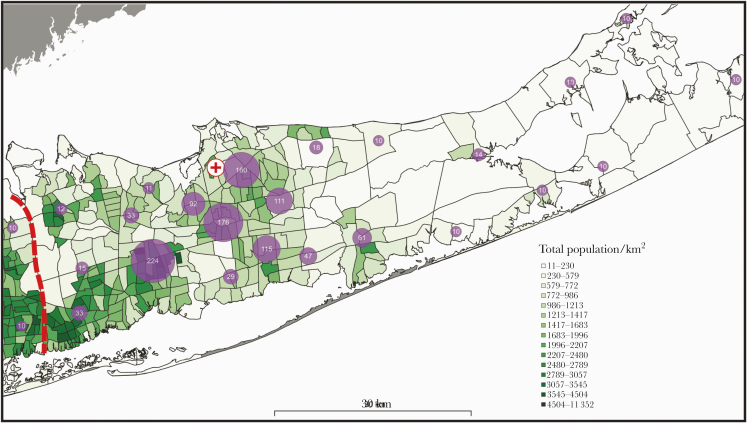
Distribution of residence is presented in Figure 1, demonstrating the geographic residence, the number of hospitalized COVID-19 patient cases, and the population density per census tract (persons per km2 area). Furthermore, the number of hospitalized COVID-19 patient cases per 10 000 persons was associated with higher population density per kilometer area (rs = 0.235; P = .004) when census tracts centroids were restricted to a 20-km2 distance (catchment area) from the medical center.
Figure 1.
Cluster map of hospitalized severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–positive patients in Suffolk County with population density. Geographical distribution of SARS-CoV-2-positive patients per km2 area in Suffolk County, Long Island, New York. Purple circles indicate an aggregate of clusters of geographic residence of hospitalized patients in the study cohort (numerical value indicates the number of patients in the cluster). (+) indicates the location of Stony Brook University Medical Center. Patient cluster sizes ≤10 are noted as 10 to meet the standard for Health Information Privacy. The red dashed line demarcates Suffolk County from Nassau County. In total, the geocoder software matched 93% of addresses in the database.
Vital signs on presentation are shown in Supplementary Table 1 and are noteworthy for diastolic hypertension, as evidenced by an MAP >90 mmHg (670/1322, 51%) and SBP >139 mmHg (362/1322, 27%).
Laboratory data were available for >90% of the laboratory investigations performed in the study cohort, with exceptions noted in Supplementary Table 1. A significant proportion of the cohort was lymphopenic, as defined by absolute lymphocyte count <1000 cells/μL (55%, 590/1081), and hyponatremic, as defined by serum sodium <135 mEq/L (411/1310, 31%). End-organ dysfunction was noted on admission, with ~30% (381/1287) of the study cohort having evidence of kidney dysfunction (eGFR ≤60 mL/min/1.73m2), myocardial injury as measured by elevation in Troponin T (257/1107, 23%), or hepatic injury as measured by elevation in transaminases (aspartate transaminase 632/1287, 51%, and alanine transaminase 239/1287, 19%). A majority of the cohort also had procalcitonin levels ≤0.25 (795/1228, 65%) on presentation, suggesting the absence of concomitant superimposed bacterial infection at the start of the disease (Supplementary Table 1).
Age and Gender as Predictive of Mortality
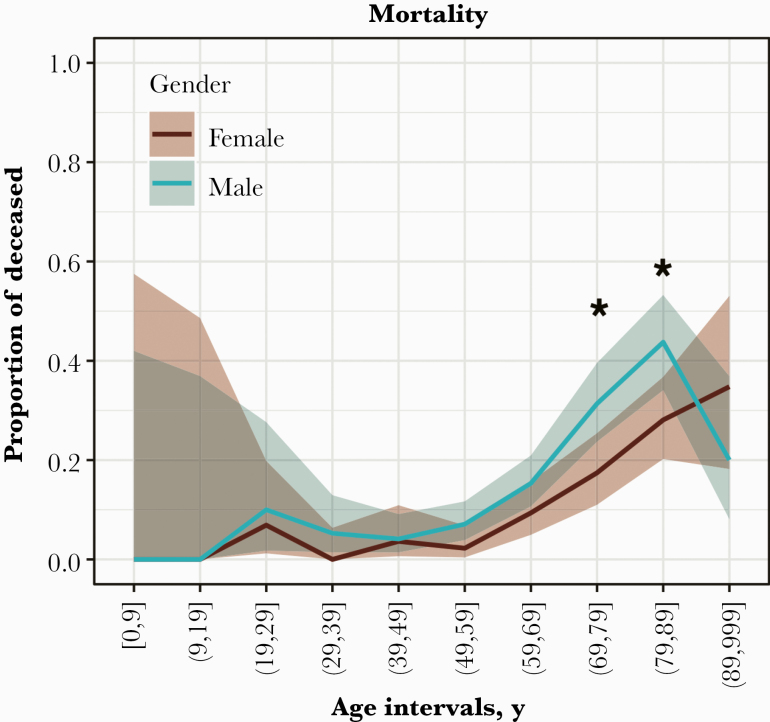
Of the 1325 patients studied, 1011 patients were discharged alive, 177 patients died, and 137 patients remain hospitalized. Discharge disposition and length of stay for those who died or were discharged alive (1188/1325) are presented in Figure 2 and Supplementary Table 2. Among these 1188 patients with a disposition, the overall mortality was significantly higher in males (P = .016). This was most prominent in the age groups 70–79 and 80–89 years, and that difference confers an advantage to females, in whom the death rate does not exceed 10% until age 70, whereas in males that threshold was crossed at age 60.
Figure 2.
Mortality by gender and age interval. Figure 2 shows the mortality rate by gender (male: blue; female: red) per 10-year age intervals (inclusive of the range). Blue and red lines indicate the proportion of deceased with 90% CIs (age intervals 70–79 years and 80–89 years; *P < .01, 1-tailed Fisher exact test).
Predictors of Hospital Length of Stay
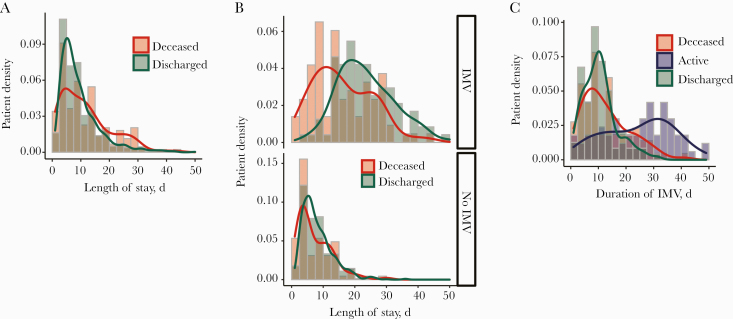
Overall, the median (IQR) hospital length of stay (LOS) was significantly longer in patients who died (10 [4–16] days) as compared with patients discharged alive (8 [5–12] days; P = .0024) (Figure 3A; Supplementary Table 3). No such difference in hospital length of stay was noted for patients who did not receive IMV (Figure 3B, bottom panel; Supplementary Figure 1). Interestingly, the overall hospital LOS in patients on IMV (IQR) was significantly longer in those discharged alive (22 [16.5–29.5] days) as compared with those who died (15 [10–23.75] days; P = 3.84e-07) (Figure 3B, top panel). However, length of time on a ventilator (IQR) in patients discharged alive (10 [6–13] days) was slightly shorter than that of those who died (10 [6.5–17.5] days; P = .04) due to skewing of the interquartile range in the deceased population (Figure 3C). These data suggest that the longer hospital length of stay in patients discharged alive after being on IMV is attributable to a greater number of nonventilator hospital days. It is noteworthy that in the remaining active patients in the hospital by the end of the study period, the duration of invasive mechanical ventilatory support was significantly prolonged as compared with the patients with a disposition by the study end point (Figure 3C).
Figure 3.
Hospital length of stay in study patients. A, Histogram shows the patient density with respect to hospital length of stay (days) in the patients with a disposition (median [IQR] length of stay for deceased, 10 [4–16] days) and discharged alive (median [IQR] length of stay, 8 [5–12] days; P = .003, Welch 2-sample t test). B, Histogram shows the patient density (IQR) with respect to hospital length of stay (days) in the patients with a disposition (deceased and discharged alive) on invasive mechanical ventilation (IMV; top panel); IMV: deceased (15 [10–23.75] days), discharged alive (22 [16.5–29.5] days; P = 3.8e-07, Welch 2-sample t test), and no IMV (bottom panel); no IMV: deceased (5 [3–11] days), discharged alive (7 [5–10.25] days; P = .216, Welch 2-sample t test). C, Histogram shows the patient density (IQR) with respect to total days on IMV (days) in the deceased, discharged alive, and active patients without a disposition. Deceased (10 [6.5–17.5] days), active (26 [14–34] days), discharged alive (10 [6–13] days; P = .04, Welch 2-sample t test between deceased and discharged alive).
Clinical Outcomes for Patients With a Disposition
Of 1188 patients with a disposition, 182 (15%) patients required IMV (Supplementary Table 4), with a majority of these patients (52%) eventually discharged alive. Acute kidney injury developed in 21% (250/1188) of the patients, with ~26% (64/250) of these patients requiring kidney replacement therapy (CRRT or HD) (Supplementary Table 4). Fifty-three percent (34/64) of patients who received kidney replacement therapy were eventually discharged alive (Supplementary Table 4).
Approximately 7.5% (89/1188) of our encounters represented readmissions, and the majority of these readmitted patients were eventually discharged alive (83/89, 91%). Furthermore, of 1011 individuals who were discharged alive, ~77% (773/1011) were discharged home, as compared with a rehabilitation or skilled nursing facility (Supplementary Table 4). Finally, overall mortality was 15% (177/1188) in all hospitalized patients and 48% (87/182) in patients on IMV with a disposition.
Predictors of Survival
A univariate analysis performed with parameters assessed on admission demonstrated that age ≥65 years and male gender as well as several comorbidities were associated with mortality (Supplementary Table 5). Clinical measures such as the need for IMV and kidney replacement therapy were associated with mortality, with acute kidney injury during hospitalization having the highest odds ratio (Supplementary Table 5). Multivariate analysis showed that older age, presenting vital signs of tachypnea and/or tachycardia, initial laboratory values indicating lymphopenia, low eGFR, and elevated D-dimer, ferritin, and C-reactive protein, along with select comorbidities (ie, diabetes, heart failure, and cancer), predicted lower survival in these patients if found on initial presentation (Supplementary Table 6).
In patients who received IMV, only age ≥65 years, male gender, comorbidities (hypertension, heart failure), need for kidney replacement therapy, and acute kidney injury during hospitalization were significantly associated with increased mortality in the univariate analysis (Supplementary Table 5). Subsequent multivariate modeling in these patients demonstrated that, of the variables individually associated with outcomes, only male gender, older age, history of heart failure, and acute kidney injury during hospitalization were predictive of mortality, with acute kidney injury remaining the most important predictor in this analysis (Table 2).
Table 2.
Multivariate Analysis of Factors Associated With Mortality in Patients on Invasive Mechanical Ventilation in the Study Cohort
| Predictors | Coefficient of the Logistic Model |
|---|---|
| Demographics | |
| Age | –0.02 |
| Male | –0.21 |
| Comorbidities | |
| Hypertension | 0 |
| Heart failure | –0.92 |
| Clinical measures | |
| Kidney replacement therapy | 0 |
| Clinical Outcomes | |
| Acute kidney injury (during hospitalization) | –1.69 |
Based on selected variables that were significant in the univariate analysis (age, male, hypertension, heart failure, kidney replacement therapy, acute kidney injury [during hospitalization]).
Despite multiple therapeutic interventions, hydroxychloroquine, azithromycin, therapeutic anticoagulation, tocilizumab, zinc, thiamine, and ascorbic acid were not associated with improved survival in the patients on IMV (Supplementary Table 5). However, we observed a trend toward improved survival in patients on IMV who received therapeutic enoxaparin as compared with intravenous unfractionated heparin.
A logistic regression model was constructed to predict discharge disposition in the remaining active 67 patients who received IMV, with noted characteristics of the predictive performance (Supplementary Figure 2). The mortality in these patients is projected to rise from 48% to 49%, based on the prediction that 36 patients are likely to expire as compared with 31 patients who are likely to be discharged alive in the remaining patients on mechanical ventilation.
DISCUSSION
To date, studies on COVID-19 from single- and multicenter medical centers nationally and internationally have primarily focused on clinical characteristics and early outcomes [1, 4, 5, 10]. Distinct from these previous reports, we demonstrate several novel observations: (1) geographic residence of hospitalized COVID-19 patients was disproportionately associated with higher population density, (2) while the duration of overall hospital length of stay in patients on IMV was longer in the survivors as compared with nonsurvivors, this was not driven by prolonged length of time on the ventilator, and (3) in addition to known risk factors of older age and male gender, mortality in COVID-19 mechanically ventilated patients increased with preexisting heart failure and the development of acute kidney injury during hospitalization.
The observation that the geographic residence of hospitalized COVID-19 patients was disproportionately associated with higher population density is not surprising, in light of the imperative to maintain physical distancing to reduce transmission and mitigate the peak intensity of this pandemic. While our medical center catchment area overlaps with several medical centers in the county, the identification of these “hot-spots” will be essential for future resource allocation planning. Investigating potential cofounders, including comorbidities, socioeconomic status, occupational exposure, education level, and ethnically based differences contained within these geographic spaces is also critical for future responses.
Over 75% of hospitalized patients (with a documented previous visit to our system) had at least 1 comorbidity, with 60% of individuals reporting more than 1 comorbidity. While our findings support those of studies in the New York City area [1, 4], we observed much higher rates of these comorbidities than the original Wuhan study [1] and a meta-analysis of ~3500 patients from China [1, 4, 11]. This likely represents our older patient cohort, but may also represent underlying differences in the prevalence of these diseases in different countries as well as the detection and definition of disease during previous visits to our system.
While the history of cancer, preexisting diabetes, and heart failure were independent predictors of mortality on presentation, the presence of preexisting heart failure alone predicted mortality in patients requiring IMV. Our model revealed that other strong predictors for mortality in mechanically ventilated COVID-19 patients were older age, male gender, and acute kidney injury during hospitalization. Still, these features remain correlative, making it difficult to assign relative importance to any 1 factor. However, our model of mortality on presentation used only basic data available in the first 48 hours of admission and achieved a 91% positive predictive value for survival (AUROC 83%, sensitivity 90%, specificity 54%). Additional studies are required to determine if these observations can be validated in cohorts with differing demographics.
The lack of an overwhelming clinical response to the evolving treatment strategies employed during this period of the pandemic is not unanticipated. The progressive nature of the disease skewing our outcomes is mitigated by limiting the analysis to mechanically ventilated patients, where none of these treatments were associated with improved survival. Similarly, although the use of tocilizumab was not associated with improved survival in the mechanically ventilated patients, randomized clinical trials are ongoing to determine the true efficacy of these therapies in patients with COVID-19. This affirmed our academic medical center’s strategy to be one of the hospitals that continued to stress randomization into clinical trials during this critical time period. For instance, we began the use of remdesivir under the compassionate and expanded use protocol for critically ill COVID-19 patients. To date, the number of patients who received the treatment was low (50 patients) due to external delays in implementation of the protocol and availability of expanded access to the drug. In addition, study protocols involving initiation of hemoperfusion in the critically ill, initiation of angiotensin II type 1 receptor blockade, and convalescent plasma have been recently implemented at our medical center, with active patient recruitment underway. Finally, the use of hydroxychloroquine (HCQ) alone and azithromycin in tandem did not identify a significant risk for increased mortality, perhaps reflective of our early discontinuation of the combination based on reports of QTc prolongation and limited efficacy and in compliance with recommendations from the Centers for Disease Control and Prevention [12, 13].
Unsurprisingly, the overall hospital length of stay was longer in individuals who died as compared with those who survived. However, in patients who required IMV, we observed the opposite; the overall hospital length of stay was significantly longer in those who survived as compared with those who died. Interestingly, this prolonged hospital course was not driven by the duration of mechanical ventilation, but rather by hospitalization events predating or postdating mechanical ventilation. Further studies are required to determine the factors that mediate duration of nonventilator days in COVID-19 patients requiring mechanical ventilation. We also recognize the need for future studies to assess the long-term outcomes of the study cohort and the clinical measures and interventions employed during their hospitalization.
Excluding patients who remain active in the hospital at the end of the study period, we observed that 48% of hospitalized COVID-19 patients who received IMV died. To our knowledge, this represents the largest and most complete data set yet reported, even though disposition in 67 patients remains unknown. However, our models project a minimal rise in mortality from 48% to 49% after accounting for the predicted disposition in these remaining active patients receiving mechanical ventilation. Several factors could have contributed to our outcomes in mechanically ventilated patients as compared with previous studies: (1) variability in patient demographics, (2) single-center vs multicenter studies, and (3) advanced notice that facilitated hospital preparedness and strategic planning due to the slight delay in the onset of the pandemic in Suffolk County as compared with New York City.
CONCLUSIONS
Collectively, the impact of the slight delay in the onset of the pandemic in Suffolk County, as compared with New York City, allowing for enhanced hospital preparedness, strategic planning, and resource allocation, coupled with unprecedented communication at the local, regional, and global health care community levels, likely contributed to the outcomes experienced at our academic medical center. Future delineation of delivering comprehensive clinical management from global integration and communication of best practices, as well as conducting effective therapeutic trials with investigational interventions, is of critical importance as we prepare for future waves in this and other pandemics.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
COVID-19 Writing Group. Mallipattu, SK., Jawa, R., Moffitt, R., Hajagos, J., Fries, B., Nachman, S., Gan, T. J., Saltz, M., Saltz, J., Kaushansky, K., and Skopicki H.
COVID-19 Data Analysis Group. Moffitt R., Hajagos J., Abell-Hart K., Chaudhri I., Deng J., Garcia V., Gayen S., Kurc T., Bolotova O., Yoo J., Dhaliwal S., Nataraj N., Sun S., Tsai C., Wang Y., Saltz M., and Saltz J.
COVID-19 Clinical Coordinating Group (alphabetical order). Abbasi, S., Abdullah, R., Ahmad, S., Bai, K., Bennett-Guerrero, E., Chua, A., Fries, B., Gomes, C., Griffel, M., Jawa, R., Kalogeropoulos, A., Kiamanesh, D., Kim N., Koraishy, F., Lingham, V., Mallipattu, S.K., Mansour, M., Marcos, L., Miller, J., Poovathor S., Rubano, J., Rutigliano, D., Sands, M., Santora, C., Schwartz, J., Shroyer K., Skopicki, H., Spitzer, S., Stopeck, A., Talamini M., Tharakan, M., Vosswinkel, J., Wertheim, W.
Author contributions. Stony Brook Covid-19 Research Consortium: The Stony Brook Research Network contributed collectively to this study. Coordinating clinical care and implementation of clinical and research protocols during the COVID-19 pandemic was under the direction of the Clinical Contributing Group. Implementation of the COVID-19 Data Registry, data extraction, and analysis were performed by the Data Analysis Group. Manuscript writing and preparation were conducted by the Writing Group.
Patient consent. The design of the study was approved by the Stony Brook University Institutional Review Board, which deemed that the study does not include factors necessitating patient consent.
Financial support. None.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Stony Brook COVID-19 Research Consortium:
S K Mallipattu, R Jawa, R Moffitt, J Hajagos, B Fries, S Nachman, T J Gan, M Saltz, J Saltz, K Kaushansky, H Skopicki, K Abell-Hart, I Chaudhri, J Deng, V Garcia, S Gayen, T Kurc, O Bolotova, J Yoo, S Dhaliwal, N Nataraj, S Sun, C Tsai, Y Wang, S Abbasi, R Abdullah, S Ahmad, K Bai, E Bennett-Guerrero, A Chua, C Gomes, M Griffel, A Kalogeropoulos, D Kiamanesh, N Kim, F Koraishy, V Lingham, M Mansour, L Marcos, J Miller, S Poovathor, J Rubano, D Rutigliano, M Sands, C Santora, J Schwartz, K Shroyer, S Spitzer, A Stopeck, M Talamini, M Tharakan, J Vosswinkel, and W Wertheim
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Coronavirus COVID-19 global cases. Available at: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Accessed 11 May 2020. [Google Scholar]
- 3.US Census Bureau. American Community Survey, 2018-2013. Detailed tables; generated by Janos Hajagos. Available at: https://systems.jhu.edu/research/public-health/ncov/
- 4. Richardson S, Hirsch JS, Narasimhan M, et al. ; and the Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 2020; 323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wadhera RK, Wadhera P, Gaba P, et al. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA 2020; 323:2192–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Voss EA, Makadia R, Matcho A, et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc 2015; 22:553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rashidian S, Dong XY, Avadhani A, et al. Effective Scalable and Integrative Geocoding for Massive Address Datasets. 25th ACM Sigspatial International Conference on Advances in Geographic Information Systems (ACM Sigspatial Gis 2017). 2017. [Google Scholar]
- 8. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120:c179–84. [DOI] [PubMed] [Google Scholar]
- 9. Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020; 323:1612–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med 2020; 8:e35. [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of Health, COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. Available at: https://covid19treatmentguidelines.nih.gov/. Accessed 19 May 2020. [PubMed] [Google Scholar]
- 13. Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA 2020; 323:2493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.