Abstract
Objective
The 2019 novel coronavirus disease (COVID-19) outbreak progressed rapidly from a public health (PH) emergency of international concern (World Health Organization [WHO], 30 January 2020) to a pandemic (WHO, 11 March 2020). The declaration of a national emergency in the United States (13 March 2020) necessitated the addition and modification of terminology related to COVID-19 and development of the disease’s case definition. During this period, the Centers for Disease Control and Prevention (CDC) and standard development organizations released guidance on data standards for reporting COVID-19 clinical encounters, laboratory results, cause-of-death certifications, and other surveillance processes for COVID-19 PH emergency operations. The CDC COVID-19 Information Management Repository was created to address the need for PH and health-care stakeholders at local and national levels to easily obtain access to comprehensive and up-to-date information management resources.
Materials and Methods
We introduce the clinical and health-care informatics community to the CDC COVID-19 Information Management Repository: a new, national COVID-19 information management tool. We provide a description of COVID-19 informatics resources, including data requirements for COVID-19 data reporting.
Results
We demonstrate the CDC COVID-19 Information Management Repository’s categorization and management of critical COVID-19 informatics documentation and standards. We also describe COVID-19 data exchange standards, forms, and specifications.
Conclusions
This information will be valuable to clinical and PH informaticians, epidemiologists, data analysts, standards developers and implementers, and information technology managers involved in the development of COVID-19 situational awareness and response reporting and analytics.
Keywords: COVID-19, pandemic, emergency preparedness and response, data standards, electronic data exchange
INTRODUCTION
Timely and accurate data and information gathering for a disease requires the implementation of a unified case definition; the creation of standardized disease nomenclature; the codification of disease-related reporting, including data on illness incidence, patient clinical encounters, deaths, and laboratory test orders and results; the implementation of public health (PH) response operations; and the exchange of electronic data using semantically interoperable information.
The first US case of 2019 novel coronavirus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was reported on 21 January 2020; shortly thereafter, the Centers for Disease Control and Prevention (CDC) Emergency Operations Center (EOC) and Incident Management System (IMS) were activated. One of the first COVID-19 information management tasks initiated by the EOC and IMS was the assessment of existing data flows, regulatory guidance on information exchange, and underlying technical documentation on semantic data interoperability.
As a result of this assessment, the CDC developed the CDC COVID-19 Information Management Repository. This centralized Repository serves as a “1-stop shop” for COVID-19 information management resources, providing quick shared access to the latest reference sources to CDC and state, territorial, tribal, and local health-care and PH partners.
Objective
The primary objective of our review is to introduce the clinical and health-care informatics community to the Repository. We provide an overview of COVID-19 informatics resources, including data specifications for COVID-19 clinical encounters, laboratory results, cause-of-death certification, PH surveillance, and emergency operations. The scope of the review is (1) to demonstrate the CDC COVID-19 Information Management Repository’s ability to categorize and manage critical COVID-19 informatics documentation and standards; and (2) to describe COVID-19 data exchange standards, forms, and specifications.
MATERIALS AND METHODS
The Repository (Figure 1) is the product of collaboration among the CDC COVID-19 IMS; CDC EOC’s Situational Awareness Team; CDC’s Center for Surveillance, Epidemiology, and Laboratory Services; and other CDC centers participating in the COVID-19 response.
Figure 1.
The CDC COVID-19 information management resources repository (https://phinvads.cdc.gov/vads/SearchVocab.action). Abbreviations: CDC, Centers for Disease Control and Prevention; COVID-19, 2019 novel coronavirus disease.
The Repository is publicly available, distributed through the CDC’s Public Health Information Network Vocabulary Access and Distribution System (PHIN VADS; see Figure 1).1 PHIN VADS is a web-based enterprise vocabulary and information management system for accessing, searching, and distributing vocabularies, terminology, and data standards used in PH and clinical care practice. PHIN VADS promotes the use of semantically interoperable information sources to support consistent communication among PH partners.2 The Repository can be accessed via the PHIN VADS homepage or the “CDC COVID-19 Surveillance and Data Analytics”3 page within Additional Information Resources on the primary CDC “Coronavirus (COVID-19)” web page. Within the Repository, essential COVID-19 data management and data definition sources are divided into 6 Sections (Table 1).
Table 1.
Categorization of COVID-19 data interoperability information
| Section number | Section name | Section description | Examples of information sourcesa |
|---|---|---|---|
| 1.1 | General Resources | Lists important regulatory guidance documents, data sources, and foundational semantic interoperability standards |
|
| 1.2 | Representing Healthcare Data for Emergency Medical Services | Lists information sources for documenting COVID-19 EMS |
|
| 1.3 | Patient's Clinical Encounter | Links to COVID-19 reference sources for documenting and codifying patient's clinical encounter, including diagnosis, clinical procedures, billing codes for Medicare patients, certifying death, and accessing standards for certifying death |
|
| 1.4 | COVID-19 Public Health Reporting | Lists references related to a submitting a case reporting form for COVID-19, patient under investigation, patient impact and hospital capacity module, and other references for COVID-19 public health reporting |
|
| 1.5 | Laboratory Data Exchange and Laboratory Surveillance | Lists reference sources with standardized codes for COVID-19 specimen submission, test orders, and test results |
|
| 1.6 | Geospatial Data Sets and Reference Sources | Lists guiding documents and data sets for geospatial COVID-19 representation |
|
Note: Data are within the CDC COVID-19 Information Management Resources Repository.1
CDC: Centers for Disease Control and Prevention; COVID-19: 2019 novel coronavirus disease; CPT: Current Procedural Terminology; EMS: emergency medical services; ICD-10-CM: International Classification of Diseases, Tenth Revision, Clinical Modification; LOINC: Logical Observation Identifiers Names and Codes; NEMSIS: National Emergency Medical Services Information System; ONC: Office of the National Coordinator for Health Information Technology; SNOMED: Systematized Nomenclature of Medicine; WHO: World Health Organization.
Information sources’ URLs are provided in COVID-19 information management resources.1
In this review, we describe 5 sections (1.2-1.6) within the Repository that address specific COVID-19 information management use cases. Additionally, we provide references to documents included in the General Resources (Section 1.1) that address information exchange needs across specific use cases.
Primary standard development and standard implementation organizations that collaborate with the CDC and contribute to the CDC COVID-19 Repository and data interoperability tasks are presented in Table 2.
Table 2.
Organizations contributing to development of the CDC COVID-19 Information Management Resources Repository
| Name of organization | Examples of COVID-19 information management standards and data harmonization products |
|---|---|
| WHO | Case definition, case classification, ICD-10 |
| US Department of Health and Human Services, ONC | ONC 2020 ISA that harmonizes standards for Health Information technology (ie, documenting encounter diagnosis, EMS) |
| CDC/CMS ICD-10-CM | ICD-10-CM provides diagnostic codes and diseases classification for US health-care settings, including those related to COVID-19 |
| CSTE | National COVID-19 case definition and case classification |
| AMA | CPT codes for reporting medical procedures (ie, for COVID-19 testing) |
| LOINC | COVID-19 laboratory test codes |
| HL7 | COVID-19 laboratory test codes |
| SNOMED International | SNOMED codes for a diagnosis and laboratory tests results |
Note: AMA: American Medical Association; CDC: Centers for Disease Control and Prevention; CMS: Centers for Medicare and Medicaid Services; COVID-19: 2019 novel coronavirus disease; CPT: current procedural terminology; CSTE: Council of State and Territorial Epidemiologists; EMS: emergency medical services; HL7: Health Level 7; ICD-10-CM: International Classification of Diseases, Tenth Revision, Clinical Modification; ISA: Interoperability Standards Advisory; LOINC: Logical Observation Identifiers Names and Codes; ONC: Office of the National Coordinator for Health Information Technology; SNOMED: Systematized Nomenclature of Medicine; WHO: World Health Organization.
The CDC’s COVID-19 IMS Data Team and EOC Situational Awareness informaticians developed ongoing communications with these organizations to assure that (1) essential elements of COVID-19 information exchange are included in the data harmonization and standardization process; and (2) these organizations provide the most recent data standard and specifications to the Repository management team. The Repository management team conducts a daily review of incoming documents, posts new reference sources, and updates/deletes outdated documents. As of 2 May 2020, the Repository contained 53 reference sources, all included in the present review.
RESULTS
Applying the NEMSIS standard for collection and analysis of pre-hospital emergency care information (repository section 1.2)
The National Emergency Medical Services Information System (NEMSIS) provides a framework for collecting, storing, and sharing standardized EMS data from states nationwide.4 NEMSIS develops and manages the national universal standard for the collection and transmission of EMS operations and patient care data. NEMSIS uses Extensible Markup Language (XML) for data exchange. The Office of the National Coordinator for Health Information Technology (ONC) 2020 Interoperability Standards Advisory (ISA) recommends the “NEMSIS data standard version 3.4”5 as a primary national standard for representing health-care data for emergency medical services.6
Analyzing and reporting a COVID-19 clinical encounter (repository section 1.3)
Section 1.3 provides reference sources pertaining to COVID-19 clinical encounter information management. These clinical encounter information sources could be included in 1 of the following categories.
CSTE COVID-19 case definition and national notification
On 5 May 2020, the Council of State and Territorial Epidemiologists (CSTE) released the “standardized surveillance case definition and national notification for 2019 novel coronavirus disease (COVID-19) Interim-20-ID-01.”7 This provides criteria for case ascertainment (clinical, laboratory, epidemiologic, vital records, and other criteria for case reporting), as well as case classifications (confirmed, probable) for the COVID-19 case definition (Figure 2).
Figure 2.
Infographic for the CSTE standardized surveillance case definition and national notification for COVID-19. Interim-20-ID-01. Abbreviations: COVID-19, 2019 novel coronavirus disease; CSTE, Council of State and Territorial Epidemiologists; FDA, Food and Drug Administration; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
This COVID-19 case definition and case classification serves as a foundation for COVID-19 cases’ codification via the data standards described above.
ICD-10-CM COVID-19 codes
The International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) is a coding system based on the World Health Organization’s (WHO’s) ICD-10, modified for US clinical use by the Department of Health and Human Services (spearheaded by the CDC’s National Center for Health Statistics and Centers for Medicare and Medicaid Services [CMS]) and used by physicians and other health-care providers to classify and code all diagnoses, symptoms, and procedures recorded in conjunction with hospital care in the United States.8 Epidemiologists and data analysts use ICD-10-CM codes for tracking and analyzing COVID-19 morbidity. Since 20 February 2020, following WHO COVID-19 recommendations, the CDC and CMS twice released COVID-19 ICD-10-CM guidance:
“ICD-10-CM official coding guidelines—supplement: coding encounters related to COVID-19 coronavirus outbreak.”9 These guidelines should be used while analyzing historic COVID-19 clinical encounters from 20 February 2020 to 31 March 2020.
“ICD-10-CM official coding and reporting guidelines. April 1, 2020 through September 30, 2020.”10
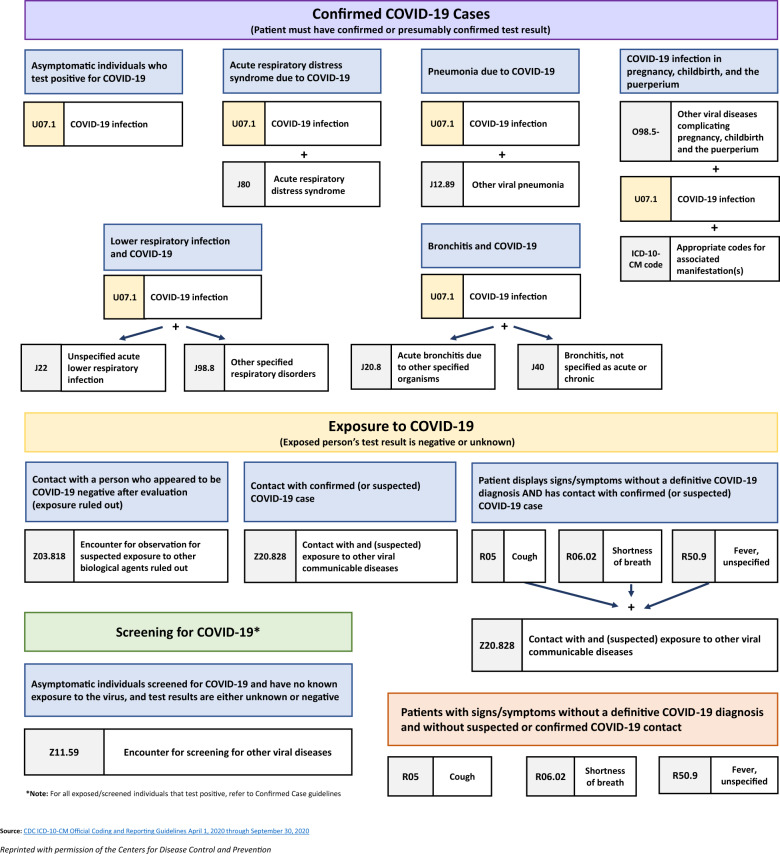
The infographic presented in Figure 311 demonstrates algorithms for COVID-19 ICD-10-CM codification for 4 types of clinical encounters: confirmed COVID-19 cases; exposure to COVID-19; screening for COVID-19; and patients with signs/symptoms without a definitive COVID-19 diagnosis and without suspected or confirmed COVID-19 contact.
Figure 3.
Infographic for COVID-19 ICD-10-CM coding and reporting guidelines, 1 April–30 September 2020.11 Abbreviations: COVID-19, 2019 novel coronavirus disease; ICD-10-CM, International Classification of Diseases, Tenth Revision, Clinical Modification.
Healthcare common procedure coding systems for Medicare billing
The Healthcare Common Procedure Coding System (HCPCS) is a standardized coding system used to submit insurance claims to Medicare. On 23 March 2020, CMS issued a PH news alert12 about new HCPCS codes: U0001 and U0002. The purpose of HCPCS code U0001 is to bill Medicare for the ordered CDC 2019 Novel Coronavirus Real-Time RT-PCR Diagnostic Test Panel at any CDC-approved laboratory; HCPCS code U0002 is used to bill Medicare for any non-CDC SARS-CoV-2 laboratory technique, multiple types, or subtypes (includes all targets) performed by laboratories and health-care facilities.
Utilization of the new billing codes will allow testing laboratories to bill for the specific test instead of using an unspecified code. These codes could be used as an additional source for data analysis of COVID-19 testing for Medicare patients.
American Medical Association current procedural terminology COVID-19 codes for testing
The American Medical Association (AMA) is the oldest and largest professional group of American physicians.13 Managed by the AMA, the Current Procedural Terminology (CPT) coding system is the most widely accepted medical nomenclature used to report medical procedures and services under public and private health insurance programs. CPT is also used for administrative management purposes, such as claims processing and developing guidelines for medical care review.14 Epidemiologists and data analysts analyze CPT codes for tracking COVID-19–related medical procedures. On 13 March 2020, the AMA released CPT code 8736515 for reporting and tracking testing services related to SARS-CoV-2 that utilize molecular pathology. Also, effective 10 April 2020, 2 more CPT codes (86328 and 86769) were published16 to allow reporting for antibody tests (Table 3). For additional guidance on appropriate usage of these CPT codes, download the CPT Assistant articles for codes 87365, 86328, and 86769.17
Table 3.
CPT Category I pathology and laboratory codes for COVID-19
| CPT codes | Code descriptor |
|---|---|
| 86 328 | Immunoassay for infectious agent antibody(ies), qualitative or semiquantitative, single step method (eg, reagent strip); SARS-CoV-2 (COVID-19) |
| 86 769 | Antibody; SARS-CoV-2 (COVID-19) |
| 87 635 | Infectious agent detection by nucleic acid (DNA or RNA); SARS-CoV-2 (COVID-19), amplified probe technique |
Data are as Note: of 13 March 2020.16
COVID-19: 2019 novel coronavirus disease; CPT: current procedural terminology; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
AMA resources include authoritative guidance for using AMA CPT codes regarding evaluation and management services stemming from COVID-19 testing;18 demonstrating how codes differ depending on whether patient assessment was done in the office, via telehealth, or a virtual visit; and determining whether the service was provided to a new or established patient. Another AMA resource outlines a comprehensive set of scenarios19 that physicians may encounter during the pandemic and provides advice on relevant codes for services.
SNOMED international concepts for reporting a COVID-19 diagnosis through electronic data exchange
Systematized Nomenclature of Medicine (SNOMED) Clinical Terms (CT) is a brand name of SNOMED International’s systematically organized, computer-processable collection of medical terms, synonyms, and definitions used in clinical documentation and reporting. SNOMED CT supports standardized coding of clinical and laboratory data.20 According to ONC, in addition to ICD-10-CM, SNOMED-CT codes (United States Edition) can be used for representing a patient medical diagnosis in electronic health records.21 On 11 March 2020, SNOMED International published an Interim Release that includes the following SNOMED-CT codes: SNOMED ID 840539006, for reporting a confirmed COVID-19 case; SNOMED ID 840544004, for a reporting a patient with suspected COVID-19; and SNOMED ID 840546002, for reporting exposure to COVID-19.22
Utilizing ICD for capturing mortality data for COVID-19 on death certificates
Monitoring the emergence of COVID-19 in the United States and guiding the PH response require accurate and timely death reporting.23,24 On 24 March 2020, the CDC National Vital Statistics System released “guidance for certifying death due to coronavirus disease 2019, COVID-19,”25 announcing a new ICD code, U07.1, for COVID-19. This code is consistent with WHO ICD mortality coding guidelines released in March 202026 and April 2020.27 The “ICD-10-CM official coding and reporting guidelines”25 provide details on ICD implementation, noting that existing ICD-10-CM mortality codes will expire on 30 September 2020.
Reference sources for COVID-19 PH surveillance and reporting (repository section 1.4)
COVID-19 information management resources for PH surveillance and reporting are addressed through all Repository Sections, most specifically in Section 1.4. Repository Section 1.1 includes guidance on “interoperability for COVID-19 novel coronavirus pandemic,”28 published by ONC’s ISA.29 The ISA recommends the utilization of 5 vocabulary standards and related vocabulary specifications: Logical Observation Identifiers Names and Codes (LOINC), SNOMED-CT, CPT, ICD-10-CM, and Healthcare Common Procedure Coding System (Figure 4). The document also notes the CDC Repository as a primary COVID-19 information source. In addition, it introduces a draft of the “Health Level 7 (HL7) COVID-19 Fast Healthcare Interoperability Resources (FHIR) Implementation Guide,” developed by Logica.30 This Guide provides open-source resources for areas such as demographics, organizational data, vital signs, exposure histories, symptoms, diagnostics tests, and diagnoses.
Figure 4.
ONC ISA interoperability for COVID-19 novel coronavirus pandemic: vocabulary standards, artifacts, and value sets. Adopted from HealthIT.gov.28 Accessed 6 May 2020. Abbreviations: COVID-19, 2019 novel coronavirus disease; ISA, Interoperability Standards Advisory; ONC, Office of the National Coordinator for Health Information Technology.
Another document included in Repository Section 1.1 and important for COVID-19 electronic data exchange is the “2020 interoperability standards advisory,”31 developed by ONC’s ISA. This document groups information technology standards in 4 categories/sections that provide guidance in establishing interoperable communication with existing electronic health-care systems:
Vocabulary/code set/terminology standards and implementation specifications:32 that is, vocabulary/value sets related to demographics,33 encounter diagnosis,34 assessment and plan of treatment,35 patient medication,36 and pregnancy status.37
Content/structure standards and implementation specifications: that is, specifications for exchanging in vitro diagnostics (IVD) test orders & results38 and implementation specifications and standards for case reporting to public health agencies.39
Standards and implementation specifications for services/transport/exchange: that is, specifications for an unsolicited “push” of clinical health information to a known destination and information system user40 and an unsolicited “push” of clinical health information to a known destination between systems.41
Administrative standards and implementation specifications: that is, specifications for administrative transaction acknowledgements42 and standards and implementation specifications for health-care eligibility benefit inquiry and response for retail pharmacy coverage.43
Section 1.1 also refers to LOINC panel 89724-9, “minimum data set (MDS) for public health emergency operations centers.”44 This LOINC panel was collaboratively developed by the CDC EOC and LOINC to assist PH agencies and emergency management organizations in defining lists of standardized data elements for collecting and reporting PH situational awareness, including patient- and population-level data. Examples of data sets and definitions provided in this panel are “LOINC ID 89737-1, partners list” (for collecting information about organizations participating in PH emergency responses, including COVID-19) and LOINC ID 89740-5, “inter-agency communication” (inter-agency communication documents).
Repository Section 1.4 provides COVID-19 reporting forms and other documents directly related to COVID-19 PH surveillance and reporting.
“Human infection with 2019 novel coronavirus”:45 a standardized reporting form is used to collect data on individuals with confirmed cases of COVID-19. This form includes sections describing case classification and hospitalization, death data, case demographics, health-care worker information, exposure information, clinical course, symptoms, past medical history, and social history. A CDC- or state-generated 2019 novel coronavirus (2019-nCOV) ID is used to track the patient status and match associated records. This form includes patient identifier information, such as first and last name and birthdate, that are not transmitted to the CDC and are for local use only.
- “The CDC National Healthcare Safety Network (NHSN) COVID-19 Module.”46 NHSN introduced the COVID-19 Module, which consists of 3 pathways within NHSN’s Patient Safety Component. Data for each pathway can be submitted via manual entry or through comma-separated value (CSV) file imports. In addition, NHSN group users (eg, state and local health departments, state hospital associations) can batch import data on behalf of facilities in their NHSN Group/jurisdiction. The pathways and associated forms are:
- “The COVID-19 Patient Impact and Hospital Capacity Pathway Form:”47 NHSN48 created this pathway and form to enable hospitals to report daily counts of patients with suspected or confirmed COVID-19 diagnoses, and current use and availability of hospital beds and mechanical ventilators. The calendar-based form allows for the daily entry of COVID-19 summary data for 13 data elements tied to patient impact and hospital capacity categories. For better comparison, this information should reflect the same time of each day. Data can be submitted via manual entry or through CSV file imports.
- “The COVID-19 Healthcare Worker Staffing Pathway Form:”49 this pathway reports data tied to critical staffing shortages among health-care worker staff groups (eg, physicians, nurses, pharmacists, technicians) within an organization. For each health-care personnel group, the organization reports whether it is currently experiencing an urgent shortage of workers for that day or whether it is likely to have an impending shortage within a week. While it is recommended that this form be submitted at least twice a week, it can be submitted daily.
- “COVID-19 Healthcare Supply Pathway Form:”50 this pathway reports critical information regarding the availability of supplies within a health-care organization. For each of 7 listed supply items, the metrics collected include the days of on-hand supply remaining, whether the organization has a policy in place to currently reuse or extend the use of the supply item, and whether the organization will be able to obtain the supply item in the future. Like the Healthcare Worker Staffing pathway, this form can be submitted daily or at least twice a week.
“COVID-19 queries for Electronic Surveillance System for the Early Notification of Community-based Epidemics (ESSENCE),”51 developed by the CDC’s National Syndromic Surveillance Program (NSSP):52 2 COVID-19 queries have been added to ESSENCE, the primary syndromic surveillance tool of the NSSP’s BioSense platform. These queries assist in identifying syndromes that could be caused by COVID-19. Specifically, “Fever and Cough-SOB-DiffBR v1” is a free-text query that can be used to identify and monitor visits of persons who display symptoms commonly associated with patients who test positive for COVID-19. This query allows health scientists to further track hospital visits for individuals with coronavirus-like symptoms while controlling for cases of confirmed influenza.
Information sources for laboratory data exchange and laboratory surveillance (repository section 1.5)
Collecting and exchanging patient-level laboratory data requires the utilization of general categories of data that are defined by ONC ISA,29 representing patient contact information for telecommunications,53 patient medical encounter diagnosis,54 laboratory tests,55 laboratory values/results,56 and other general standards described in Repository Section 1.1. Additional specific COVID-19 reference sources are included in Section 1.5.
CDC division of laboratory systems LOINC in vitro diagnostic test code mapping for SARS-CoV-2 tests
As described by the CDC’s Division of Laboratory Systems (DLS), using LOINC and SNOMED-CT code mapping for LOINC in-vitro diagnostics (LIVD) to identify and report SARS-CoV-2 test results in electronic reporting systems will facilitate timely and quality data reporting to state and federal PH agencies.57
In collaboration with partners, the DLS maintains the “mapping tool: LIVD SARS-CoV-2 test codes,” which provides LOINC and SNOMED mappings for SARS-CoV-2 diagnostic tests available in the United States.58 In addition to providing a vendor analyte description, vendor specimen description (via SNOMED), vendor result description (also via SNOMED), and LOINC test codification, the tool also provides suggested implementation for HL7 messaging versions 2.3.x, 2.5.1, and higher, as well as HL7 FHIR.59
Recommendations of the association of PH laboratories for reporting COVID-19 test results utilizing HL7 messaging standards
The Repository contains the following 2 Association of Public Health Laboratories (APHL) documents that specify HL7 versions, messaging segments, and encoding guidelines for LOINC and SNOMED:60
Message validation guidance, updated COVID-19 terminology and encoding guidelines, and updated HL7 sample messages are presented in the “APHL COVID-19 HL7 data messaging FAQs.”61
Data definitions and value sets for COVID-19 orders and observations used in HL7 messages from the LOINC and SNOMED-CT code systems are presented in the “APHL SARS-CoV-2 encoding guidelines.”62
Additional information sources for laboratory data exchange and laboratory surveillance
In addition to CDC DLS and APHL recommendations, Section 1.5 refers to the “CDC Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019,”63 which include the “CDC Specimen Submission Form 50.34,”64“guidelines for submitting specimens to CDC for laboratory testing for SARS-CoV-2,”65 and other reference documents. Section 1.5 also provides direct links to COVID-19–specific LOINC and SNOMED-CT codes.
The “LOINC COVID-19 special use pre-release terms”66 registry defines codes and terminology for laboratory tests. For example, LOINC issued code 94720-0 for reporting SARS coronavirus 2 immunoglobin A Ab (units/volume) in serum or plasma by immunoassay. LOINC has also published codes for commercial IVD test kits.67
SNOMED International has published the “March 2020 COVID-19 international interim edition of SNOMED,”68 which provides standardized values for reporting laboratory observations: that is, SNOMED-CT code 840535000, for reporting an antibody to severe acute respiratory syndrome coronavirus.
Geospatial reference sources (repository section 1.6)
Geospatial representation of COVID-19 data informs the management of PH COVID-19 operations. Repository Section 1.6 provides an example (developed by the CDC’s EOC) of the “COVID-19 Esri Geographic Information System data specification”69 for presenting a number of new COVID-19 cases, a cumulative number of cases, and a cumulative number of deaths. Repository Section 1.6 also refers to the “CDC Coronavirus Disease 2019 (COVID-19) World Map,”70 developed based on the “WHO Coronavirus Disease (COVID-19) Situational Reports.”71
The “number of COVID-19 cases in the US, by state or territory,”72 is available for download through the CDC COVID-19 web page. The CSV file provides a COVID-19 mapping layer for presenting US COVID-19 cases by state. It includes the following data elements: jurisdiction, range of cases, number of reported cases, presence in a jurisdiction of community transmission, and URL to reference sources by jurisdiction.
Figure 5 demonstrates the recently developed “CDC COVID-19 data tracker,”73 added to Repository Section 1.6. This tool presents mapping layers for US cases, US cases and deaths by county, social impact, and school closures.
Figure 5.
Representing COVID-19 mapping layers through the CDC COVID-19 data tracker.73 Data are as of 10 June 2020. Abbreviations: CDC, Centers for Disease Control and Prevention; COVID-19, 2019 novel coronavirus disease.
DISCUSSION
Understanding currently available COVID-19 information management resources and utilization of the CDC COVID-19 Information Management Repository will facilitate prompt implementation of the most recent solutions for data interoperability, in turn improving timelines and the accuracy of reported information.
In this review, we describe the rapid pace of the COVID-19 information management tasks, spurred not only by the progression of the pandemic, but also the involvement of many standards development and standards implementation organizations in COVID-19 semantic interoperability tasks. For example, at the time of writing, COVID-19–related ICD-10-CM and CPT codes had already been updated twice. The ICD-10-CM committee stated that current ICD-10-CM codes are valid only until 30 September 2020, so users may expect additional updates. Following the development of new SARS-CoV-2 diagnostic tests, LOINC released numerous code updates, listing them in the LOINC Prerelease Terms.59
In this context, the CDC COVID-19 Information Management Repository plays a critical role in supplying informaticians with current information management resources. The Repository has also had a critical impact on the development of COVID-19 semantic interoperability; its development has increased participation in COVID-19 information governance through the ongoing assessment of situational awareness information needs. Development activities have included bringing partners together to identify standardization gaps and resolve potential discrepancies between data standards. For example, the Repository’s management works with APHL to make sure that APHL laboratory coding guidelines reflect changes caused by the introduction of new LOINC codes. Another example is a collaboration with AMA CPT editors on the introduction of new COVID-19–related CPT codes.
CONCLUSION
The use of COVID-19 information resources can yield benefits for interoperability, data availability and analysis, and data-driven response activities at all jurisdictional levels. An up-to-date information management Repository is integral for keeping pace with rapidly evolving specifications and guidance documents. The Repository serves as a nexus for connecting data standards developers, implementors, and users with solutions for making COVID-19 data interoperable and improving communication for the PH emergency response.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
AUTHOR CONTRIBUTORS
Each of the authors contributed substantially to the article by providing substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; providing final approval of the version to be published; and agreeing to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
The authors thank the partners collaborating in development and management of the Centers for Disease Control and Prevention (CDC) 2019 novel coronavirus disease (COVID-19) Information Management Repository. From the CDC, they thank Adi V. Gundlapalli, MS, MD, PhD; Paula Yoon, ScD, MPH; Joanne Andreadis, PhD; Meeyoung Park, MPH; Robert C. Neurath, MS; Roger T. Harlan, BS; Jaqueline Burkholder, MS, PhD; Amanda Evanson; Kevin Chatham-Stephens, MD; Maribeth C. Gagnon, MS; Margaret Dudeck, MPH; Sandra W. Roush, MT, MPH; and Donna Pickett, MPH. From Johns Hopkins University, they thank Harold P. Lehmann, MD, PhD. From the Association of Public Health Laboratories, they thank Riki Merrick, MPH; Patina Zarkone, MPH; and Michelle Meigs, MBA. From the American Medical Association/Current Procedural Terminology, they thank Jay Ahlman, MS; Zachary Hochstetler, MBA; and Leslie Prellwitz, MBA. From the Regenstrief Institute/Logical Observation Identifiers Names and Codes, they thank Jamalynne Deckard, MS; and Swapna Abhyankar, MD.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1. Centers for Disease Control and Prevention. Public Health Information Network Vocabulary Access and Distribution System (PHIN VADS). https://phinvadsstg.cdc.gov/vads/SearchHome.action Accessed 5 April 2020.
- 2. Centers for Disease Control and Prevention. PHIN vocabulary and access distribution system. https://www.cdc.gov/phin/tools/phinvads/ Accessed 10 April 2020.
- 3. Centers for Disease Control and Prevention. COVID-19 surveillance and data analytics. https://www.cdc.gov/coronavirus/2019-ncov/php/open-america/surveillance-data-analytics.html Accessed 10 April 2020.
- 4. EMS.gov. NEMSIS. https://www.ems.gov/projects/nemsis.html Accessed 10 April 2020.
- 5. National Emergency Medical Services Information System. V3 data dictionaries and XSD. https://nemsis.org/technical-resources/version-3/version-3-data-dictionaries/ Accessed 10 April 2020.
- 6. Office of the National Coordinator for Health Information Technology 2020 Interoperability Standards Advisory. Representing health-care data for emergency medical services. https://www.healthit.gov/isa/representing-health-care-data-emergency-medical-services Accessed 10 April 2020.
- 7.Council of State and Territorial Epidemiologists. Standardized surveillance case definition and national notification for 2019 novel coronavirus disease (COVID-19). Interim-20-ID-01. https://cdn.ymaws.com/www.cste.org/resource/resmgr/2020ps/Interim-20-ID-01_COVID-19.pdf Accessed 10 April 2020.
- 8. Centers for Disease Control and Prevention. National Center for Health Statistics. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). https://www.cdc.gov/nchs/icd/icd10cm.htm Accessed 10 April 2020.
- 9. Centers for Disease Control and Prevention. ICD-10-CM official coding guidelines—supplement coding encounters related to COVID-19 coronavirus outbreak, February 20, 2020–March 31, 2020. https://www.cdc.gov/nchs/data/icd/interim-coding-advice-coronavirus-March-2020-final.pdf Accessed 10 April 2020.
- 10. Centers for Disease Control and Prevention. ICD-10-CM official coding and reporting guidelines. April 1, 2020 through September 30, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf Accessed 10 April 2020.
- 11. Centers for Disease Control and Prevention Public Health Information Network Vocabulary Access and Distribution System. Infographic for the ICD-10-CM official coding and reporting guidelines. April 1, 2020 through September 30, 2020. https://phinvadsstg.cdc.gov/vads/DownloadHotTopicDetailFile.action? filename=8959038F-BF79-EA11-818E-005056AB7AAC Accessed 10 April 2020.
- 12. Centers for Medicare and Medicaid Services. Coverage and payment related to COVID-19 Medicare. https://www.cms.gov/files/document/03052020-medicare-COVID-19-fact-sheet.pdf Accessed 23 March 2020.
- 13. American Medical Association. About. https://www.ama-assn.org/about Accessed 10 April 2020.
- 14. American Medical Association. CPT editorial panel. CPT purpose & mission. https://www.ama-assn.org/about/cpt-editorial-panel/cpt-purpose-mission Accessed 10 April 2020.
- 15. American Medical Association. March 13, 2020 CPT assistant guide. https://www.ama-assn.org/system/files/2020-03/cpt-assistant-guide-coronavirus.pdf Accessed 10 April 2020.
- 16. American Medical Association. CPT COVID-19 coding and guidance. https://www.ama-assn.org/practice-management/cpt/COVID-19-coding-and-guidance Accessed 10 April 2020.
- 17. American Medical Association. CPT assistance guide. https://www.ama-assn.org/system/files/2020-04/cpt-assistant-guide-coronavirus-april-2020.pdf Accessed 10 April 2020.
- 18. American Medical Association. CPT reporting for COVID-19 testing. https://www.ama-assn.org/system/files/2020-04/cpt-reporting-COVID-19-testing.pdf Accessed 10 April 2020.
- 19. American Medical Association. Special coding advice during COVID-19 public health emergency. https://www.ama-assn.org/system/files/2020-04/COVID-19-coding-advice.pdf Accessed 10 April 2020.
- 20. Systematized Nomenclature of Medicine International. Why SNOMED CT? http://www.snomed.org/snomed-ct/why-snomed-ct Accessed 10 April 2020.
- 21. HealthIT.gov. Representing patient medical encounter diagnosis. https://www.healthit.gov/isa/representing-patient-medical-encounter-diagnosis Accessed 10 April 2020.
- 22. Systematized Nomenclature of Medicine International. March 2020 SNOMED CT international edition interim release: up to date COVID-19 content available. http://www.snomed.org/news-and-events/articles/march-2020-interim-snomedct-release-COVID-19 Accessed 11 March 2020.
- 23. Centers for Disease Control and Prevention. National Center for Health Statistics. National vital statistics system. https://www.cdc.gov/nchs/nvss/index.htm Accessed 10 April 2020.
- 24. Centers for Disease Control and Prevention. National vital statistics system (NVSS). COVID-19 Alert No 2. March 24, 2020. https://www.cdc.gov/nchs/data/nvss/coronavirus/Alert-2-New-ICD-code-introduced-for-COVID-19-deaths.pdf Accessed 10 April 2020.
- 25. Centers for Disease Control and Prevention. National vital statistics system (NVSS). Vital statistics reporting guidance. Report no. guidance for certifying death due to coronavirus disease 2019 (COVID-19). https://www.cdc.gov/nchs/data/nvss/vsrg/vsrg03-508.pdf Accessed 3 April 2020.
- 26. World Health Organization. COVID-19 coding in ICD-10. https://www.who.int/classifications/icd/COVID-19-coding-icd10.pdf?ua=1 Accessed 25 March 2020.
- 27. World Health Organization. International guidelines for certification and classification (coding) of COVID-19 as cause of death. Based on ICD. https://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19.pdf?ua=1 Accessed 16 April 2020.
- 28. HealthIT.gov. Interoperability for COVID-19 novel coronavirus pandemic. 2020. https://www.healthit.gov/isa/COVID-19 Accessed 10 April 2020.
- 29. HealthIT.gov. The interoperability standards advisory (ISA). https://www.healthit.gov/isa/about-isa Accessed 10 April 2020.
- 30. Logica Health. Logica implementation guide: COVID-19. https://COVID-19-ig.logicahealth.org/?mc_cid=15d567de77&mc_eid=e1040c4572 Accessed 10 April 2020.
- 31. Office of the National Coordinator for Health Information Technology. 2020 interoperability standards advisory. Reference edition. https://www.healthit.gov/isa/sites/isa/files/inline-files/2020-ISA-Reference-Edition.pdf Accessed 10 April 2020.
- 32. HealthIT.gov. ISA document table of contents. Vocabulary/code set/terminology standards and implementation specifications. https://www.healthit.gov/isa/isa-document-table-contents Accessed 10 April 2020.
- 33. HealthIT.gov. ISA. demographics. https://www.healthit.gov/isa/section/demographics Accessed 10 April 2020.
- 34. HealthIT.gov. ISA. Representing patient encounter diagnosis. https://www.healthit.gov/isa/representing-patient-medical-encounter-diagnosis Accessed 10 April 2020.
- 35. HealthIT.gov. ISA. Representing assessment and plan of treatment. https://www.healthit.gov/isa/representing-assessment-and-plan-treatment Accessed 10 April 2020.
- 36. HealthIT.gov. ISA. Representing patient medications. https://www.healthit.gov/isa/representing-patient-medications Accessed 10 April 2020.
- 37. HealthIT.gov. ISA. Representing patient pregnancy status. https://www.healthit.gov/isa/representing-patient-pregnancy-status Accessed 10 April 2020.
- 38. HealthIT.gov. ISA. Exchanging in vitro diagnostics (IVD) test orders & results. https://www.healthit.gov/isa/exchanging-invitro-diagnostics-ivd-test-orders-results Accessed 10 April 2020.
- 39. HealthIT.gov. ISA. Case reporting to public health agencies. https://www.healthit.gov/isa/case-reporting-public-health-agencies Accessed 10 April 2020.
- 40. HealthIT.gov. ISA. An unsolicited “push” of clinical health information to a known destination and information system user. https://www.healthit.gov/isa/unsolicited-push-clinical-health-information-a-known-destination-and-information-system-user Accessed 10 April 2020.
- 41. HealthIT.gov. ISA. An unsolicited “push” of clinical health information to a known destination between systems. https://www.healthit.gov/isa/unsolicited-push-clinical-health-information-a-known-destination-between-systems Accessed 10 April 2020.
- 42. HealthIT.gov. ISA. Administrative transaction acknowledgements. https://www.healthit.gov/isa/administrative-transaction-acknowledgements Accessed 10 April 2020.
- 43. HealthIT.gov. ISA. Health care eligibility benefit inquiry and response for retail pharmacy coverage. https://www.healthit.gov/isa/health-care-eligibility-benefit-inquiry-and-response-retail-pharmacy-coverage Accessed 10 April 2020.
- 44. Logical Observation Identifiers Names and Codes. Panel 89724-9, minimum data set (MDS) for public health emergency operations centers (EOC) [CDC Emergency Operations Centers]. https://loinc.org/89724-9/ Accessed 10 April 2020.
- 45. Centers for Disease Control and Prevention. Human infection with 2019 novel coronavirus case report form. https://www.cdc.gov/coronavirus/2019-ncov/downloads/pui-form.pdf Accessed 10 April 2020.
- 46. Centers for Disease Control and Prevention. National Health Safety Network (NHSN). COVID-19 module. https://www.cdc.gov/nhsn/acute-care-hospital/covid19/index.html Accessed 10 April 2020.
- 47. Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN). COVID-19 module patient impact and hospital capacity pathway. OMB No. 0920-1290. https://www.cdc.gov/nhsn/pdfs/covid19/57.130-covid19-pimhc-blank-p.pdf Accessed 10 April 2020.
- 48. Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN). https://www.cdc.gov/nhsn/ Accessed 10 April 2020.
- 49. Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN). COVID-19 healthcare worker form. OMB No. 0920-1290. https://www.cdc.gov/nhsn/pdfs/covid19/57.131-covid19-hwp-blank-p.pdf Accessed 10 April 2020.
- 50. Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN). COVID-19 healthcare worker form. OMB No. 0920-1290. https://www.cdc.gov/nhsn/pdfs/covid19/57.132-covid19-sup-blank-p.pdf Accessed 10 April 2020.
- 51. Centers for Disease Control and Prevention. National Syndromic Surveillance Program (NVSS). COVID-19 queries added to ESSENCE. https://www.cdc.gov/nssp/news.html?deliveryName=USCDC_1133-DM25504#COVID_Slack Accessed 10 April 2020.
- 52. Centers for Disease Control and Prevention. National Syndromic Surveillance Program (NVSS). https://www.cdc.gov/nssp/index.html Accessed 10 April 2020.
- 53. Office of the National Coordinator for Health Information Technology. Interoperability Standards Advisory (ISA). Representing patient contact information for telecommunications. https://www.healthit.gov/isa/representing-patient-contact-information-telecommunications Accessed 10 April 2020.
- 54. Office of the National Coordinator for Health Information Technology. Interoperability Standards Advisory (ISA). Representing patient medical encounter diagnosis. https://www.healthit.gov/isa/representing-patient-medical-encounter-diagnosis Accessed 10 April 2020.
- 55. Office of the National Coordinator for Health Information Technology. Interoperability Standards Advisory (ISA). Representing laboratory tests. https://www.healthit.gov/isa/representing-laboratory-tests Accessed 10 April 2020.
- 56. Office of the National Coordinator for Health Information Technology. Interoperability Standards Advisory (ISA). Representing laboratory values/results. https://www.healthit.gov/isa/representing-laboratory-valuesresults Accessed 10 April 2020.
- 57. Centers for Disease Control and Prevention. Division of Laboratory Systems (DLS). LOINC in vitro diagnostic (LIVD) test code mapping for SARS-CoV-2 tests. https://www.cdc.gov/csels/dls/documents/livd_test_code_mapping/LIVD-SARS-CoV-2-2020-05-12.xlsx Accessed 10 April 2020.
- 58. Centers for Disease Control and Prevention. Division of Laboratory Systems (DLS). Mapping tool. LIVD SARS-CoV-2 test codes. https://www.cdc.gov/csels/dls/documents/livd_test_code_mapping/LIVD_SARS-CoV-2_2020-04-28c.xlsx Accessed 10 April 2020.
- 59.Health Level 7 Fast Healthcare Interoperability Resources. Release 4. 2.17. Introducing HL7 FHIR. https://www.hl7.org/fhir/summary.html Accessed 10 April 2020.
- 60. Association of Public Health Laboratories. APHL. https://www.aphl.org/Pages/default.aspx Accessed 10 April 2020.
- 61. Association of Public Health Laboratories. APHL COVID-19 HL7 data messaging FAQs. https://www.aphl.org/programs/preparedness/Crisis-Management/Documents/03.03.2020_Updated_Informatics_FAQs_Guidance_(002).pdf Accessed 10 April 2020.
- 62. Association of Public Health Laboratories. APHL SARS-CoV-2 encoding guidelines. https://www.aphl.org/programs/preparedness/Crisis-Management/_layouts/15/WopiFrame.aspx? sourcedoc=/programs/preparedness/Crisis-Management/Documents/2019-nCoV_Encoding%20Guidelines_FINAL_r1.xlsx&action=default Accessed 10 April 2020.
- 63. Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019. https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html Accessed 10 April 2020.
- 64. Centers for Disease Control and Prevention. Specimen submission form: specimens of human origin, v.3.3.1. https://www.cdc.gov/laboratory/specimen-submission/pdf/form-50-34.pdf Accessed 10 April 2020.
- 65. Centers for Disease Control and Prevention. Guidelines for submitting specimens to CDC for laboratory testing for SARS-CoV-2. https://www.cdc.gov/coronavirus/2019-ncov/downloads/COVID-19-Specimen-Submission-Guidance.pdf Accessed 10 April 2020.
- 66.Logical Observation Identifiers Names and Codes. LOINC prerelease terms. SARS-CoV-2 information and frequently asked questions. https://loinc.org/prerelease/ Accessed 10 April 2020.
- 67.Logical Observation Identifiers Names and Codes. Codes for commercial in vitro diagnostics (IVD) test kits. https://loinc.org/sars-coronavirus-2/ Accessed 10 April 2020.
- 68. Systematized Nomenclature of Medicine International. SNOMED CT international edition interim release: up-to-date COVID-19 content available. http://www.snomed.org/news-and-events/articles/march-2020-interim-snomedct-release-COVID-19 AccessedMarch 2020.
- 69. Centers for Disease Control and Prevention. PHIN VADS. COVID-19 information management resources. https://phinvads.cdc.gov/vads/DownloadHotTopicDetailFile.action? filename=820918F3-5F73-EA11-818B-005056ABE2F0 Accessed 10 April 2020.
- 70. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). World map. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/world-map.html? CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Flocations-confirmed-cases.html Accessed 10 April 2020.
- 71. World Health Organization. Coronavirus disease (COVID-19) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ Accessed 10 April 2020.
- 72. Centers for Disease Control and Prevention. Cases of coronavirus disease (COVID-19) in the US. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Accessed 10 April 2020.
- 73. Centers for Disease Control and Prevention. CDC COVID data tracker. https://www.cdc.gov/covid-data-tracker/index.html Accessed 10 April 2020.