Abstract
Social distancing (SD) measures aimed at curbing the spread of SARS-CoV-2 remain an important public health intervention. Little is known about the collateral impact of reduced mobility on the risk of other communicable diseases. We used differences in dengue case counts pre- and post implementation of SD measures and exploited heterogeneity in SD treatment effects among different age groups in Singapore to identify the spillover effects of SD measures. SD policy caused an increase of over 37.2% in dengue cases from baseline. Additional measures to preemptively mitigate the risk of other communicable diseases must be considered before the implementation/reimplementation of SARS-CoV-2 SD measures.
Keywords: dengue, SARS-CoV-2, interventions, natural experiment
An estimated 105 million dengue infections occur per year [1]. Primarily, the Aedes aegypti and Aedes albopictus vectors transmit the dengue virus. The combination of increasing human population connectivity, climate change, and urbanization within the region creates an ideal environment for dengue transmission and outbreaks to occur [2-4].
The unprecedented response to the ongoing coronavirus disease 2019 (COVID-19) pandemic has led to widespread social distancing (SD) and other nonpharmaceutical interventions [5]. Population-wide implementation of SD has led to near complete lockdowns in most countries. However, despite the extant literature detailing how human mobility, importation, and home working infection patterns are key determinants of dengue epidemic potential, the effects of SD on vector-borne diseases such as dengue are not known. SD may bring hosts and vectors into closer proximity, if it results in social changes to the time spent at home and if the density of the anthropophilic Ae. aegypti is higher peridomestically.
In Singapore, the reduction of movements through workplace and school closures and cessation of mass gatherings were implemented and heavily enforced during the SD policy. This has led to a large decrease in time spent in localities away from home (summary in Supplementary Material) over a period of 2 to 3 months before gradual relaxation of measures. Most individuals of employment age in Singapore work in air-conditioned premises due to the highly developed economy [6]. The Ae. aegypti vector is a day biter with breeding most concentrated around residential areas [7]. Dengue infections at workplaces are rare at baseline, but an increase in dengue exposure risk due to increased time spent in residences due to SD is likely.
The near-complete coverage of SD policy across most age groups, regular and clear whole of government mass communication, and heavy compliance through enforcement of these measures greatly reduced policy leakage and noncompliance across the entire population. This provides a rare opportunity to identify the effects of shifting exposure patterns to residences on dengue transmissions by considering the implementation of SD policy as a natural experiment.
METHODS
We obtained reported weekly case counts of dengue in Singapore by 5-year age bands from 2003 to 2020, where data was available, until the 21st epidemiological week (EW) of 2020. All confirmed cases of dengue are legally required to be notified to the Ministry of Health for surveillance and control.
We controlled for the effects of weather on potential vector breeding, using weather data obtained from the Meteorological Services Singapore. The measurements of each weather station for each day was averaged and temporally aggregated to the weekly frequency. Maximum and mean temperature, together with relative humidity, were used to represent thermal forcing and stress on the vector population.
Intervention data for SD were obtained through the Ministry of Health, Singapore website. SD measures were implemented from 7 April 2020 to 1 June 2020, with varying degrees of strictness (summary in Supplementary Material).
The primary goal of our analysis was to identify the effects of SD policy in Singapore on reported dengue case counts over time. In Singapore, SD policy led to a closure of around 95% of workplaces as well as all schools and recreational facilities [8]. The increase in time spent in residences and reduction in mobility patterns from SD policy would therefore most drastically affect individuals who were attending school or working prior to policy implementation. Treatment effects from SD policy can therefore be ascribed to those who were older than 5 years or younger than 65 years, which is around the retirement age in Singapore [6]. In Singapore, those who fall outside these bands mostly did not have drastic changes in mobility patterns from SD, as less than 50% of individuals younger than 5 years attend child care centers for full-day programs and less than 1% of individuals of retirement age reside in elderly care facilities [6]. A majority of individuals in the control group thus spends most of their time in residences or in the neighborhood even before SD policy implementation.
Identification Strategy
The difference-in-difference identification strategy was used to determine the effects of SD policy on dengue case counts over time. We took observations of reported dengue case counts within the 5 to 65-year-old age bands during EWs when SD policy was implemented as the treatment group. All observations of reported dengue case counts within past EWs when SD policy was not implemented and for those in the younger than 5 years and older than 70 age bands when the SD policy was implemented were taken as the control group:
where denotes the number of reported dengue case counts at time point for the age band . is an indicator variable taking value 1 if SD policy effect was ascribed at that time point for that age band, 0 otherwise. The policy effect size, , is the primary estimand of interest. The error term is normally distributed with constant variance . is a matrix of controlling variables which include: (1) time trend, to control for the temporal dependence of dengue case counts over time, as well as near-term trends in dengue transmission potential; (2) past EWs, to control for seasonality; (3) year fixed effects, to control for year-specific risk; and (4) maximum, mean temperature and relative humidity of up to 4 weeks lag, to control for thermal forcing and stress on vector population growth. The coefficient values for controlling variables are denoted as .
We built our difference-in-difference specification by sequentially adding confounders (1) to (4), to ensure robust identification of the policy effects and to ensure that the policy effect of interest was not an artefact of other phenomena that affect dengue transmissions in the same period when SD policy was implemented. Other robustness checks to regression assumptions are reported in the Supplementary Material. In general, policy effects reported below are robust to relaxation changes in model specification.
No ethical approval was required for this study.
RESULTS
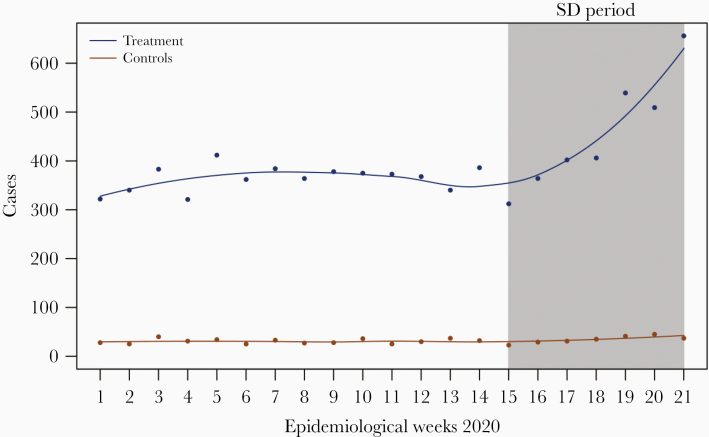
SD causes increased incidence of dengue cases (Figure 1). The treatment effect ranged from 6.612 (95% confidence interval [CI], 4.256–8.967; Table 1) when controlling for EW as factors with year and age-group fixed effects, to 16.512 (95% CI, 12.452–19.703; Table 1) when controlling for EW as a linear trend. Controlling for all confounders, we estimate that there was an increase of 9.873 cases (95% CI, 7.528–12.219; Table 1) per treatment group per week. This corresponds to around a 37.2% increase (95% CI, 19.9%–49.8%) from expected baseline levels attributable to SD policy.
Figure 1.
Reported dengue case counts in 2020 across epidemiological weeks for treatment (5–65 years old) and control (younger than 5 years, older than 65 years) groups. Abbreviation: SD, social distancing.
Table 1.
Differences-in-Differences Specification Estimated Using Ordinary Least Squares
| Dependent Variable: Weekly Reported Cases | ||||||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | |
| Treatment effect, δ | 15.865* | 16.512* | 15.692* | 8.785* | 6.612* | 9.873* |
| Lower bound, 95% CI | 12.761 | 12.452 | 12.452 | 5.456 | 4.256 | 7.528 |
| Upper bound, 95% CI | 18.969 | 19.703 | 18.932 | 12.115 | 8.967 | 12.219 |
| No. of observations | 2776 | 2776 | 2776 | 2776 | 2776 | 2776 |
Abbreviations: CI, confidence interval.
(1) Without controlling for confounders; (2) controlling for epidemiological week as a linear trend; (3) controlling for epidemiological week as factors; (4) controlling for epidemiological week as factors with year fixed effects; (5) controlling for epidemiological week as factors with year and age-group fixed effects; (6) controlling for epidemiological week as factors with 1–4 week lagged climate variables, year and age-group fixed effects.
*P value < .001.
Discussion
Results indicate that implementation of SD is associated with an increase in the number of reported dengue cases in Singapore. One key pathway that may explain this result is the increased time spent at home addresses due to SD policy together with the propensity for dengue infections to surface at home rather than work addresses in Singapore [9]. Although workplace infections may surface, it was found that, in many areas, residences were the most common place of vector-borne disease infection, rather than workplaces [9]. Concentration of vector breeding sites around residential areas further compounds the risk of transmission in these localities as compared to workplaces [7]. In conjunction with shifting work patterns into homes with naturally ventilated spaces, which do not offer protection during the biting period in the day, these SD policy-motivated pathways together converge risk factors of dengue transmission.
Cross-immunity dynamics is well studied as a possible cause of dengue outbreaks in dengue-endemic regions such as Singapore [10]. Virological surveillance in Singapore revealed an emergence of DENV-3, leading to codominance of DENV-2 and DENV-3 in 2020 in the same period that SD policy was implemented. While prior infection of individuals may have provided temporary/partial cross-immunity, which thereby reduces the transmission potential of dengue in the community [11], this may have waned since the last outbreak in 2013. Antibody-dependent enhancement, the posited pathway by which secondary infections of dengue become more severe and likely to be reported clinically, may have occurred in far more individuals, due to the emergence of DENV-3 and prior lack of exposure to this serotype in large proportions of the population.
Previous serotype switches to/from DENV-1 and DENV-2 have been linked to large outbreaks in Singapore and have suggested low population immunity to these strains [10]. Currently, immunity to DENV-3 is even lower as it was not the predominant serotype in Singapore’s dengue history until recently. These effects may have confounded the effect of SD on reported dengue cases in Singapore. The effects of serotype switching and other cross-immunity dynamics are, however, expressed in multiannual cycles, which is unlikely to greatly bias policy effect estimates when policy is implemented only for a period of 2–3 months.
Prior studies have showed that the likelihood of genetic diversity of dengue around home addresses remains fixed in the absence of mobility and importation [12]. In conjunction with life-long immunity conferred after recovery from a specific serotype [11], the herd immunity within localities may increase, which will possibly lead to a smaller rise in dengue cases should SD policy persist.
Conversely, should SD policies ease and with dengue exposure patterns shifting back to the pre-SD policy regime, increases in mixing patterns away from home may lead to an increased risk of locality-specific importation into residences. It is possible that an outbreak may be prolonged due to increased human mobility spatially spreading the high prevalence of virus. The impact of exiting from SD policy on dengue transmissions was, however, not quantified in this study and future work is necessary.
Of concern also are regions where SD policies have been instituted, relaxed, and reinstituted such as Hong Kong, several states in the United States, and China [13]. Similar policy behavior has not been observed in dengue hyperendemic regions such as Singapore and Malaysia, but the effects of multiple entry and exits from SD policy on dengue transmissions are also not known. It is likely that SD policy institution, relaxation, and reinstitution regimes may amplify, mix, then seed and amplify new areas of vector-borne disease transmission and should SD policy be reinstituted, vector control in residential areas should to preemptively employed to mitigate the transmission of dengue.
The efficacy of SD policy as a public health intervention for reducing the onward spread of respiratory diseases is well known as it reduces human contact by increasing time spent in residences. Increase in time spent in residences during the day and thus their exposure to vectors can likewise influence the transmission pattern of vector-borne diseases—but in an opposing direction. Increasing time spent at a locality elevates transmission risk of vector-borne diseases despite of vector density [14].
Prior studies have shown that the transmission risk of vector-borne diseases could be higher at home and in one’s neighborhood [15]. The highly urban environment of Singapore only allows identification of SD policy effects on reported dengue case counts, as other vector-borne diseases are not widespread. It would be of interest to generalize the natural experiment design of SD on other vector-borne diseases as the transmission patterns of malaria are vastly different in terms of vector breeding behavior and viral dynamics. The differences in habitats for the respective vectors may result in differences in exposure risk before and after distancing policies.
The indirect effects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nonpharmaceutical interventions beyond that of SARS-CoV-2 transmissions need to be urgently quantified. This study reveals that SD policy has led to a sharp increase in dengue incidence in Singapore. Additional measures to preemptively mitigate the risk of other communicable diseases such as dengue must be considered during the implementation of SD measures.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. L. J. T. designed the study, conducted the analysis, and validated the analysis. L. J. T., L. C. Z. X., and E. C. S. L. wrote the manuscript. J. O. and J. A. collected the data. J. O., J. A., and N. L. C. conceptualized the study. A. R. C., N. L. C., B. S. D., J. O., and J. A. critically revised the manuscript.
Financial support. This work was supported by the National Medical Research Council through the Singapore Population Health Improvement Centre (grant numbers NMRC/CG/C026/2017 NUHS and COVID19RF-004 to L. J. T., B. S. D., and A. R. C.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Cattarino L, Rodriguez-Barraquer I, Imai N, Cummings DAT, Ferguson NM. Mapping global variation in dengue transmission intensity. Sci Transl Med 2020; 12:eaax4144. [DOI] [PubMed] [Google Scholar]
- 2. Jue Tao L, Dickens BSL, Yinan M, Woon Kwak C, Ching NL, Cook AR. Explicit characterization of human population connectivity reveals long run persistence of interregional dengue shocks. J R Soc Interface 2020; 17:20200340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim JT, Dickens BS, Haoyang S, Ching NL, Cook AR. Inference on dengue epidemics with Bayesian regime switching models. PLoS Comput Biol 2020; 16: e1007839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lim JT, Dickens BS, Cook AR, et al. Modelling the epidemic extremities of dengue transmissions in Thailand. Epidemics 2020; 33:100402. [DOI] [PubMed] [Google Scholar]
- 5. Lim JT, Dickens BS, Choo EL, et al. Revealing regional disparities in the transmission potential of SARS-CoV-2 from interventions in Southeast Asia. Proceedings of the Royal Society B 2020; 287:20201173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singapore Department of Statistics. https://www.singstat.gov.sg/. Accessed 30 January 2020.
- 7. Ong J, Liu X, Rajarethinam J, Yap G, Ho D, Ng LC. A novel entomological index, Aedes aegypti breeding percentage, reveals the geographical spread of the dengue vector in Singapore and serves as a spatial risk indicator for dengue. Parasit Vectors 2019; 12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheong D Coronavirus: Most workplaces to close, schools will move to full home-based learning from next week, says PM Lee The Straits Times, 3. April 2020. https://www.straitstimes.com/singapore/health/most-workplaces-to-close-schools-will-move-to-full-home-based-learning-from-next. Accessed 1 October 2020. [Google Scholar]
- 9. Khalik S Dengue outbreak threatens to be biggest yet The Straits Times, 4. June 2020. https://www.straitstimes.com/singapore/dengue-outbreak-threatens-to-be-biggest-yet. Accessed 1 October 2020. [Google Scholar]
- 10. Rajarethinam J, Ang L- W, Ong J, et al. Dengue in Singapore from 2004 to 2016: cyclical epidemic patterns dominated by serotypes 1 and 2. Am J Trop Med Hyg 2018; 99:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reich NG, Shrestha S, King AA, et al. Interactions between serotypes of dengue highlight epidemiological impact of cross-immunity. J R Soc Interface 2013; 10:20130414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee KS, Lo S, Tan SS, et al. Dengue virus surveillance in Singapore reveals high viral diversity through multiple introductions and in situ evolution. Infect Genet Evol 2012; 12:77–85. [DOI] [PubMed] [Google Scholar]
- 13. Dickens BL, Koo JR, Lim JT, et al. Modelling lockdown and exit strategies for COVID-19 in Singapore. The Lancet Regional Health-Western Pacific 2020; 1:100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stoddard S, Morrison A, Vazquez-Prokopec GM, et al. The role of human movement in the transmission of vector-borne pathogens. PLoS Negl Trop Dis 2009; 3:e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salje H, Lessler J, Maljkovic Berry I, et al. Dengue diversity across spatial and temporal scales: local structure and the effect of host population size. Science 2017; 355:1302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.