Abstract
Background
Interleukin-6 blockade (IL-6) has become a focus of therapeutic investigation for the coronavirus disease 2019 (COVID-19).
Methods
We report a case of a 34-year-old with COVID-19 pneumonia receiving an IL-6 receptor antagonist (IL-6Ra) who developed spontaneous colonic perforation. This perforation occurred despite a benign abdominal exam and in the absence of other known risk factors associated with colonic perforation.
Results
Examination of the colon by electron microscopy revealed numerous intact severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virions abutting the microvilli of the colonic mucosa. Multiplex immunofluorescent staining revealed the presence of the SARS-CoV-2 spike protein on the brush borders of colonic enterocytes that expressed angiotensin-converting enzyme 2. However, no viral particles were observed within the enterocytes to suggest direct viral injury as the cause of colonic perforation.
Conclusions
These data and absence of known risk factors for spontaneous colonic perforation implicate IL-6Ra therapy as the potential mediator of colonic injury in this case. Furthermore, this report provides the first in situ visual evidence of the virus in the colon of a patient presenting with colonic perforation adding to growing evidence that intact infectious virus can be present in the stool.
Keywords: coronavirus, interleukin-6 receptors, electron microscopy, intestinal perforation
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a global threat to public health. Although the pathophysiology of COVID-19 has not been fully elucidated, direct viral injury and imbalanced host immune responses have been implicated in the immunopathogenesis of the disease [1]. In particular, a hyperinflammatory phenotype with elevated serum concentrations of many cytokines including interleukin-6 (IL-6) has been identified, which correlates with adverse clinical outcomes [2]. As a result, IL-6 receptor antagonist (IL-6Ra) therapy has been investigated in COVID-19 patients, with over 80 clinical trials currently underway [3].
Although the lungs are the primary organ targeted by SARS-CoV-2, gastrointestinal manifestations of the disease are common [4–6]. In this study, we report a case of a patient with COVID-19 pneumonia receiving IL-6Ra who developed colonic perforation. We present the first electron microscopy (EM) image of SARS-CoV-2 virions in a human colon. Although the virus particles were not seen in enterocytes, to our knowledge this report is the first in situ visual evidence of the virus abutting the microvilli of the colonic mucosa.
METHODS
Patient Consent
We obtained written consent from the patient to publish this case report. Our paper is compliant with international guidance for human subjects research.
Case
A 34-year-old man presented to an outside hospital with 2 days of fevers, chills, and dyspnea (Supplementary Figure S1). The patient was previously healthy with no medical comorbidities aside from obesity (body mass index of 36). He was diagnosed with COVID-19 pneumonia and admitted on 4 liters of supplemental oxygen. On hospital day (HD) 2, he had progressive hypoxemic respiratory failure requiring intubation. Proning and neuromuscular blockade (NMB) were initiated for severe acute respiratory distress syndrome, and he was transferred to our hospital on HD 6 for consideration of extracorporeal membrane oxygenation.
On arrival, he received an IL-6Ra on HD 7 and HD 13 as part of a clinical trial. Lung protective ventilation, proning, and NMB were continued. The patient’s procalcitonin and C-reactive protein (CRP) levels decreased with time, but he continued to have low-grade fevers on broad-spectrum antibiotic therapy. Given his otherwise negative infectious workup, the fever was attributed to COVID-19. The patient had gradual improvement of his PaO2/FiO2 ratio, and his cardiac, renal, and liver function remained intact. Despite high sedation and analgesia requirements, he tolerated enteral tube feedings with regular daily bowel movements.
RESULTS
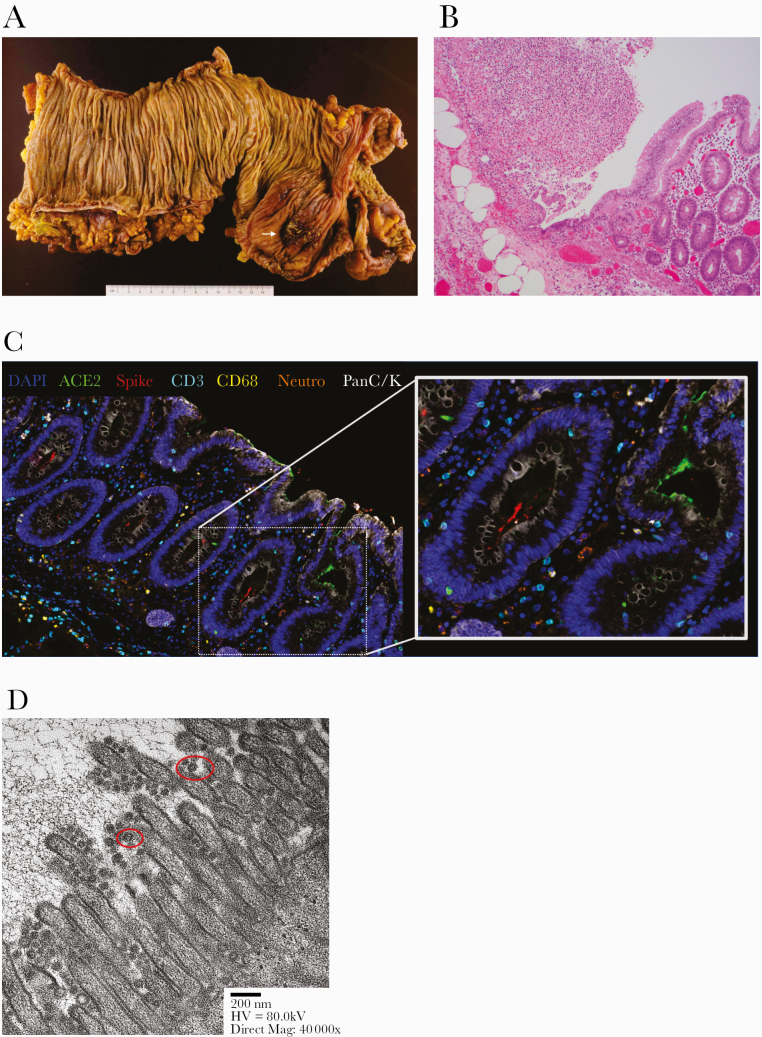
On HD 20, his fever increased to 41.0°C, accompanied by an increasing white blood cell count. Abdominal exam was benign. Procalcitonin and CRP levels were not elevated. A computed tomography scan of the abdomen showed pneumoperitoneum with pneumatosis along the cecum and ascending colon. An urgent exploratory laparotomy revealed a 3-centimeter linear tear and perforation of the cecum (Figure 1A), requiring right hemicolectomy with creation of an end ileostomy and Hartmann’s pouch. Histologic sections confirmed focal ulceration, necrosis, and acute inflammation (Figure 1B). There was no evidence of diverticulitis or tumor. Focal microemboli at the area of perforation were noted; however, there was no definite histological evidence of a thrombotic process in the vasculature of the adjacent uninvolved colon to suggest that thrombosis was the cause of perforation. Multiplex immunofluorescence (MIF) demonstrated minimal expression of angiotensin-converting enzyme 2 (ACE2) on the colonic epithelial cells confined to the brush border [7] but abundance of SARS-CoV-2 spike (S) protein on the surface of the colonic microvilli (Figure 1C). No intracellular staining for SARS-CoV-2 S protein was observed in the colonic epithelial cells. Electron microscopy confirmed the presence of numerous spheroidal viral particles abutting the microvilli of enterocytes (Figure 1D). Intracellular viral particles were not observed.
Figure 1.
Pathologic examination of colonic perforation site. (A) Formalin-fixed resected colon shows a 0.6-cm cecal ulceration of the mucosal surface (white arrow) by gross pathology. This was contiguous to a 3-cm linear serosal tear. (B) Focal ulceration, necrosis, and acute inflammatory exudate on the mucosal lining by histology (×100, hematoxylin and eosin stain). (C) Spheroidal viral particles shown on the microvilli colonic surface by electron microscopy. Representative viral particles outlined with red boxes. (D) Multiplexed immunofluorescence image of perforated colon. Staining was performed with the OPAL system from Akoya Biosciences and imaged using Vectra Polaris using 4’,6-diamidino-2-phenylindole (DAPI) staining for nucleus in blue, angiotensin-converting enzyme 2 (ACE2) staining in green, viral spike protein staining in red, T-cell staining with CD3 marker in cyan, macrophage staining with CD68 in yellow, neutrophil staining with neutrophil elastase in orange, and pan cytokeratin (panC/K) marker in white.
DISCUSSION
We report a case of spontaneous colonic perforation in a patient with COVID-19 receiving IL-6Ra therapy. The differential diagnosis for colonic perforation in this critically ill patient is broad, including colonic pseudo-obstruction, diverticulitis, ischemic colitis, spontaneous stercoral or idiopathic perforation, and virus- or medication-induced colonic injury.
Although our patient required opiates for sedation, he had regular bowel movements with no clinical manifestations of constipation, obstruction, or pseudo-obstruction. In addition, in contrast to stercoral perforations that commonly cause an ovoid pattern of injury in the sigmoid colon or rectum with evidence of impacted stool and ischemic necrosis of the surrounding mucosa [8], our patient had a linear tear in the cecum without feculent material. An ischemic insult was also an unlikely cause of perforation in this patient, because he was on minimal to no vasopressors throughout his hospitalization leading up to the perforation with no signs of hypoperfusion in other organs and no features of ischemia on microscopic examination. Furthermore, the patient did not receive glucocorticoids or nonsteroidal anti-inflammatory medications, and he had no pathological evidence of diverticuli, tumor, or appendicitis. Intestinal infarct or perforation can also occur due to thromboses of the intestinal vascular bed, and COVID-19-associated coagulopathy can result in venous, arterial, and microvascular thrombotic events [9–11]. However, we did not see histopathological evidence of thrombosis in the adjacent colonic vasculature to indicate that this patient’s intestinal perforation occurred due to thrombosis.
With gastroenterological complications of COVID-19 infection becoming increasingly reported [4], we investigated whether this perforation was the result of direct colonic injury by SARS-CoV-2. Severe acute respiratory syndrome coronavirus 2 cell entry depends on binding of the viral S protein to the ACE2 receptor on target cells in concert with activation of the S protein by the host transmembrane serine protease 2 (TMPRSS2) [12]. Although ACE2 expression has been identified in many organs, a recent study revealed that coexpression of ACE2 and TMPRSS2 is highly enriched on type II pneumocytes in the lung, nasal goblet secretory cells, and absorptive enterocytes in the terminal ileum, but not colonic enterocytes [13]. Although previous reports have implicated SARS-CoV-2 as a cause of colitis and enteritis, evidence for natural infection of human colonic enterocytes by SARS-CoV-2 has not been reported [4, 14], although colonic organoids can be infected in vitro [15, 16]. Likewise, high concentrations of viral genome as determined by polymerase chain reaction have been detected in stool samples, but efforts to culture the virus from these samples have been largely unsuccessful [17], except in 1 isolated case [16], thus suggesting viral particles in the gastrointestinal tract may not be intact in most cases. To assess whether direct viral injury contributed to colonic perforation, we evaluated the resected bowel for the presence of SARS-CoV-2 by MIF and EM. Although MIF revealed limited staining of ACE2 on colonic enterocytes, which was confined to the brush borders [7], we did not detect any viral particles within the enterocytes by EM and MIF to suggest direct viral injury to the mucosa. To our surprise, we observed numerous spheroidal viral particles abutting the microvilli of the colonic mucosa on EM, which was corroborated by MIF staining of the viral S protein. We also found numerous viral particles abutting the microvillii of adjacent healthy tissue (data not shown), which is consistent with another recent report in which SARS-CoV-2 virions were detected in healthy rectal tissue of a patient with COVID-19 [18]. To our knowledge, this is the first reported evidence of in situ SARS-CoV-2 virions on colonic enterocytes at the site of intestinal injury.
Although we cannot definitively rule out direct viral injury as the cause, exclusion of other potential etiologies suggests IL-6Ra as a possible cause of spontaneous colonic perforation in this patient. Colonic perforation is a known complication of IL-6 inhibition with tocilizumab and sarilumab [19, 20]. This is partly due to the antiapoptotic and proliferative properties of IL-6 signaling in the colon, which prolongs enterocyte lifespan and stimulates intestinal epithelial regeneration at the onset of colonic injury [21–23]. Interleukin-6 inhibition also results in decreased levels of vascular endothelial growth factor (VEGF), which plays a key role in maintaining vascular integrity via angiogenesis [24]. The importance of VEGF in maintaining mucosal integrity is further suggested by the fact that VEGF inhibitors are associated with gastrointestinal perforation [25].
Of note, the reported IL-6Ra-associated perforations often present silently without abdominal pain [26], as was the case in our patient. Furthermore, although the literature suggests that these perforations occur in patients with other predisposing factors such as diverticulitis, glucocorticoid use, or older age, the increased risk of gastrointestinal perforation with IL-6Ra therapy remains after multivariate adjustment for these factors [26, 27]. This is particularly relevant in our case, because our patient had none of these predisposing factors. These data suggest that the gastrointestinal tract is likely subject to frequent erosions that must be countered by cell proliferation and vascular regeneration, which rely partially on IL-6 signaling. Although the US Food and Drug Administration cautions against using tocilizumab and sarilumab in patients at risk for gastrointestinal perforation, our report suggests that the at-risk population may be potentially larger [28, 29].
CONCLUSIONS
In summary, we report a case of colonic perforation in a patient with COVID-19 who received IL-6Ra therapy. Our patient’s perforation occurred in the absence of known risk factors or clear evidence of direct viral injury to the colon, implicating IL-6Ra therapy as the potential mediator of colonic injury. This case also represents the first reported evidence of in situ SARS-CoV-2 virions in the colon of a patient presenting with colonic perforation. Additional studies in larger cohorts of COVID-19 patients, such as tissue examinations from biopsies or autopsies, are needed to validate our findings and elucidate the role of the colon as a potential target and source of SARS-CoV-2 replication.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure S1. Timeline of disease course. Schematic of clinical events (top) and graphs depicting body temperature and laboratory values (bottom). CRP, C-reactive protein; HCQ, hydryoxychloryoquine; IL6Ra, IL6 receptor antagonist; PCR, polymerase chain reaction (for SARS-CoV-2); Tmax, maximum temperature; WBC, white blood cell count.
Acknowledgments
We thank the UCLA COVID-19 Research Program for research support. We also thank Gray Procop (Department of Pathology at Cleveland Clinic) for assistance with analysis of electron microscopy images. We thank Chris Seet (Department of Hematology and Oncology at University of California, Los Angeles) for fruitful scientific discussions. The following reagent was obtained through BEI Resources, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH): Monoclonal Anti-SARS-CoV S protein (similar to 240C), NR-616.
Financial support. This work was funded by the NIH National Center for Advancing Translational Science UCLA Clinical and Translational Research Center (UL1TR001881), and UCLA David Geffen School of Medicine - Broad Stem Cell Research Center COVID 19 Research Award (OCRC #20-17). J. T. K was funded by the NIH National Institute of Allergy and Infectious Diseases Career Development Award (K08AI155232).
Potential conflicts of interest. S. M. D. is an advisory board member of EarlyDx Inc., T-Cure Bioscience Inc., LungLife AI Inc., and Johnson and Johnson Lung Cancer Initiative. T. S. W. is a consultant for IQVIA. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020; 181:1036–45.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020; 368:473–4. [DOI] [PubMed] [Google Scholar]
- 3. Thorlund K, Dron L, Park J, et al. A real-time dashboard of clinical trials for COVID-19. Lancet Digit Health 2020; 2:e286–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheung KS, Hung IF, Chan PP, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology 2020; 159:81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol 2020; 115:766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chakravartty S, Chang A, Nunoo-Mensah J. A systematic review of stercoral perforation. Colorectal Dis 2013; 15:930–5. [DOI] [PubMed] [Google Scholar]
- 9. Helms J, Tacquard C, Severac F, et al. ; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020; 46:1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; 191:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost 2020; 18:1743–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ziegler CGK, Allon SJ, Nyquist SK, et al. ; HCA Lung Biological Network. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020; 181:1016–35.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020; 158:1831–3.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020; 369:50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med 2020; 26:1077–83. [DOI] [PubMed] [Google Scholar]
- 17. Wolfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465–9. [DOI] [PubMed] [Google Scholar]
- 18. Qian Q, Fan L, Liu W, et al. Direct evidence of active SARS-CoV-2 replication in the intestine [published online ahead of print July 08, 2020]. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gout T, Ostör AJ, Nisar MK. Lower gastrointestinal perforation in rheumatoid arthritis patients treated with conventional DMARDs or tocilizumab: a systematic literature review. Clin Rheumatol 2011; 30:1471–4. [DOI] [PubMed] [Google Scholar]
- 20. Jagpal A, Curtis JR. Gastrointestinal perforations with biologics in patients with rheumatoid arthritis: implications for clinicians. Drug Saf 2018; 41:545–53. [DOI] [PubMed] [Google Scholar]
- 21. Kuhn KA, Manieri NA, Liu TC, Stappenbeck TS. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS One 2014; 9:e114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin X, Zimmers TA, Zhang Z, et al. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut 2010; 59:186–96. [DOI] [PubMed] [Google Scholar]
- 23. Taniguchi K, Wu LW, Grivennikov SI, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature 2015; 519:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakahara H, Song J, Sugimoto M, et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum 2003; 48:1521–9. [DOI] [PubMed] [Google Scholar]
- 25. Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol 2009; 10:559–68. [DOI] [PubMed] [Google Scholar]
- 26. Strangfeld A, Richter A, Siegmund B, et al. Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann Rheum Dis 2017; 76:504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xie F, Yun H, Bernatsky S, Curtis JR. Brief report: Risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol 2016; 68:2612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Genentech. Actemra (tocilizumab) [package insert]. U.S. Food and Drug Administration. 2010. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125276s127,125472s040lbl.pdf. Accessed 24 May 2020.
- 29. Sanofi Synthelabo. Kevzara (sarilumab) [package insert]. U.S. Food and Drug Administration website. 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761037s000lbl.pdf. Accessed 24 May 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.