Abstract
Background
The preventive effect that tenofovir/emtricitabine (FTC) could have against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human immunodeficiency virus-negative people is unknown. The objective of this study was to analyze the seroprevalence and clinical manifestations of COVID-19 among users of pre-exposure prophylaxis (PrEP), disoproxil fumarate/FTC (TDF/FTC), or tenofovir alafenamide (TAF)/FTC and to compare it to that of a control group.
Methods
An observational descriptive study of the seroprevalence of antibodies for SARS-CoV-2 among men who have sex with men and transgender women without use of PrEP (Group 1; n = 250) and PrEP users with TDF/FTC (n = 409) or TAF/FTC (n = 91) (Group 2; n = 500) was conducted from May11, 2020 to June 27, 2020. All participants were provided with a structured questionnaire that collected information on the variables to be analyzed, and testing for immunoglobulin G antibodies to SARS-CoV-2 (chemiluminescent microparticle immunoassay) was then carried out.
Results
The seroprevalence of SARS-CoV-2 was 9.2% (95% confidence interval [CI], 5.9–13.5) in the group without PrEP and 15.0% (95% CI, 12.0–18.4) in the group with PrEP (P = .026). Among users of TDF/FTC it was 14.7% (95% CI, 11.4–18.5), and in users of TAF/FTC it was 16.5% (95% CI, 9.5–25.7) (P = .661). In those who tested positive for SARS-CoV-2 and receiving PrEP, 57.4% manifested symptoms, compared with 78.3% in the control group (P = .070). In users of TDF/FTC the figure was 53.3% and in users of TAF/FTC the figure was 73.3% (P = .100). The duration of symptoms was 11.5 days in the control group, 9.0 days in PrEP users (P = .116), 7.0 days in users of TDF/FTC, and 13.0 days in users of TAF/FTC (P = .100).
Conclusions
Users of PrEP, TDF/FTC, or TAF/FTC presented a higher seroprevalence to SARS-CoV-2 than the control group. No statistically significant differences were found in relation to clinical manifestations. The PrEP users should use the same prevention measures as those indicated for the general population.
Keywords: COVID-19, disoproxil fumarate/emtricitabine (TDF/FTC), pre-exposure prophylaxis (PrEP), SARS-CoV-2, tenofovir alafenamide/emtricitabine (TAF/FTC)
Users of PrEP, TDF/FTC, or TAF/FTC, presented a higher seroprevalence to SARS-CoV-2 than the control group. No statistically significant differences were found in clinical manifestations. PrEP users should use the same prevention measures as those indicated for the general population.
The most common symptoms of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, include fever, cough, dyspnea, anosmia, and ageusia among others, although there may be more serious manifestations, mainly in elderly patients and those with underlying comorbidities [1, 2]. Since the appearance of this infection in China in 2019, there have been multiple articles in relation to the clinical manifestations, diagnosis, treatment, and prevention published [3, 4].
The triphosphates of tenofovir and emtricitabine (FTC) act as chain terminators for the reaction catalyzed by SARS-CoV-2 ribonucleic acid (RNA)-dependent RNA polymerase (RdRp). This fact has been demonstrated by means of molecular analysis, based on the premise that the active site of the SARS-CoV-2 RdRp is highly conserved among the different RNA viruses and shares structural characteristics with them. It presents low specificity when it comes to recognizing nucleotide analogs as substrates, although these have different modifications, which means that many nucleotide and nucleoside analogs can inhibit the reaction catalyzed by RdRp and have a potential use as antivirals against SARS-CoV-2 [5–7]. Other studies describe the immunomodulatory effects of tenofovir, which could also be beneficial against infection [8, 9]. These findings provide a molecular basis to evaluate the possible potential of these drugs in the prevention of COVID-19 infection.
The US Food and Drug Administration (FDA) approved the use of disoproxil fumarate (TDF)/FTC as pre-exposure prophylaxis (PrEP) for human immunodeficiency virus (HIV) in 2012: 1 tablet daily of 300 mg of TDF and 200 mg of FTC [10]. The European Medicines Agency approved it as PrEP in 2016 [11]. In 2019, the FDA approved tenofovir alafenamide (TAF)/FTC as PrEP [12]. In November 2019, the Spanish Ministry of Health included the funding of PrEP as an additional measure of prevention against HIV within the National Health System, aimed at men who have sex with men (MSM) and transgender women (TGW) at risk of acquiring HIV [13]. In the Community of Madrid, the Centro Sanitario Sandoval, Hospital Clínico San Carlos is the reference Center for PrEP, and it carries out the clinical and pharmacological monitoring of approximately 600 users. Among those with proper adherence, there have been no seroconversions to HIV, in keeping with the high preventive efficacy demonstrated in published trials [14].
Until now, the preventive effect that tenofovir/FTC could have against COVID-19 in HIV-negative people is yet to be determined. Knowing this impact could be a finding of great importance in the face of a pandemic caused by an emerging virus. The objective of this study was to analyze the seroprevalence of the SARS-CoV-2 infection among users of PrEP, TDF/FTC, or TAF/FTC, in a reference Center in Madrid and to compare it to that of a control group. In addition, there is a lack of knowledge regarding the possibility of a slower development of the disease in those users of tenofovir/FTC infected with SARS-CoV-2, thus the clinical manifestations were also studied.
METHODS
This was an observational descriptive study of the seroprevalence of antibodies for SARS-CoV-2 in MSM and TGW without the use of PrEP (Group 1) and PrEP users with TDF/FTC or TAF/FTC (Group 2). The study period was 7 weeks, from May 11, 2020 to June 27, 2020.
For Group 2, the PrEP users, the inclusion criteria were as follows: being MSM or TGW, over the age of 18, having received PrEP before March 1, 2020, and having signed the informed consent. For Group 1, the control group, the same inclusion criteria were used except that they received neither PrEP nor antiretroviral treatment (ART).
The study was carried out at a reference HIV/sexually transmitted infection (STI) clinic for PrEP in Madrid, Spain, where more than 600 PrEP users were followed up before confinement due to the COVID-19 pandemic (March 14, 2020). Upon the continuation of PrEP reviews in May 2020, all PrEP users who met the inclusion criteria were consecutively invited to participate in the study (Group 2; n = 500). Patients who came to the Center with a suspicion of STIs/HIV and met the inclusion criteria for the control group were invited to participate (Group 1; n = 250).
Given the lack of bibliography regarding the effect of PrEP on the acquisition of SARS-CoV-2, and based on a number of 500 accessible PrEP users, it was estimated that for a ratio of 2:1, a power of 80%, and a significance level of 5%, a difference of 5.18% in seroprevalence could be detected, starting at an expected value in the control group of 11.3% [15].
All participants who signed the informed consent were provided with a structured questionnaire that collected information on the variables to be analyzed and the serological test for SARS-CoV-2 was carried out. The diagnostic assay used in our study was the SARS-CoV-2 ARCHITECT (Abbott). This assay is a chemiluminescent microparticle immunoassay (CMIA) used for the qualitative detection of immunoglobulin G (IgG) antibodies to SARS-CoV-2 in human serum. The assay is designed to detect IgG antibodies to the nucleocapsid protein of SARS-CoV-2 in serum and plasma, andit has been used as epidemiological marker of previous infection. The specificity and sensitivity values of the test are 99.90% and 100.00%, respectively [16].
Variables
The main outcome variable was the serological result of the presence of IgG antibodies to SARS-CoV-2. The independent variables were as follows: sex (male or TGW), age, comorbidities associated with risk factors for COVID-19 (chronic obstructive pulmonary disease, severe asthma, arterial hypertension, diabetes mellitus, obesity, severe cardiopathy, smoker, immunosuppression, severe obesity, chronic kidney disease on dialysis, liver disease), concomitant treatment associated with COVID-19 (systemic corticosteroids, hydroxychloroquine, methotrexate, finasteride) [17, 18], use and start date of PrEP, drugs for PrEP (TDF/FTC or TAF/FTC), daily regimen (self-report adherence <85% or ≥85%), exposure to coronavirus (community exposure: assigned to the exposure of any citizen of the Community of Madrid during the study period; cohabitant exposure: living with a person with a confirmed diagnosis of COVID-19; occupational exposure: work activity associated with contact with people with a high suspicion of infection), clinical manifestations associated with COVID-19 (asymptomatic, symptomatic), symptoms (fever, cough, dyspnea, myalgia, anosmia, ageusia, loss of appetite, diarrhea, severe asthenia, headaches, skin lesions), duration of symptoms (≤7 days, >7 and a half days), hospital admission and assistance in intensive care unit (ICU), and specific treatment for COVID-19.
Statistical Analysis
The qualitative variables are expressed as their frequency distribution, the quantitative variables are expressed as the mean and standard deviation, or, if they did not follow a normal distribution, as median and interquartile range. A comparison was made between the PrEP users and the control group as well as between the 2 PrEP alternatives, TDF/FTC versus TAF/FTC. The comparison of qualitative variables was analyzed with the χ 2 test (or Fisher’s exact test where appropriate), and the comparison of quantitative variables was analyzed using the Student’s t test for independent samples or the non-parametric Mann-Whitney U test. The seroprevalence data are presented with a confidence interval (CI) of 95%. A significance value of 5% was accepted for all variables. The statistical analysis was done using Stata 15.0.
Patient Consent Statement
The patient’s written consent was obtained. All data derived from structured questionnaire were fully anonymized before access. The study protocol was approved by local ethical committees, institutional review board of Hospital Clínico San Carlos, approval number 20/368-E_COVID.
RESULTS
A total of 750 MSM and TGW were analyzed: 250 without PrEP (Group 1) and 500 with PrEP (Group 2). Of the PrEP users, 409 received TDF/FTC and 91 received TAF/FTC, with an average time in preventive treatment of 3 months up to the date of the serological test for SARS-CoV-2.
Among PrEP users, 99.9% were MSM, with no difference between the 2 groups, and 90.6% were between 20 and 49 years of age. The average age of the group without PrEP was 35.2 years, and the average age of the group with PrEP 37.2 was years (P = .003). In regards to the type of exposure to SARS-CoV-2, occupational activity and cohabiting with confirmed cases were studied, and there were no differences between Groups 1 and 2. However, among the PrEP users, it was 34.2% in those who received TDF/FTC and 5.5% in TAF/FTC (P < .001). In Group 2, 23.5% presented some comorbidities considered as risk factors for SARS-CoV-2, with no significant differences compared with the control group. In the PrEP group, 9.0% received other drugs potentially associated with the treatment or prevention of COVID-19 and the 4.4% in the control group received other drugs potentially associated with the treatment or prevention of COVID-19 (P = .024). There were no significant differences between TDF/FTC and TAF/FTC users. A total of 63.3% had a PrEP adherence greater than 85%, and there were no differences between the 2 combinations (Table 1).
Table 1.
Descriptive Characteristics of the Population Analyzed, According to Use of PrEP and Type of Drug, TDF/FTC and TAF/FTC (n = 750)
| % (n) | Without PrEP n = 250 | PrEP n = 500 | TDF/FTC n = 409 | TAF/FTC n = 91 | P* | P** |
|---|---|---|---|---|---|---|
| Sex | .340 | 1.000 | ||||
| MSM | 98.8 (247) | 99.6 (498) | 99.5 (407) | 100.0 (91) | ||
| TGW | 1.2 (3) | 0.4 (2) | 0.5 (2) | 0.0 (0) | ||
| Age | .002 | .087 | ||||
| 0–19 | 1.2 (3) | 0.2 (1) | 0.2 (1) | 0.0 (0) | ||
| 20–34 | 54.0 (135) | 40.8 (204) | 42.3 (173) | 34.1 (31) | ||
| 35–49 | 36.4 (91) | 49.8 (249) | 47.2 (173) | 61.5 (56) | ||
| 50–64 | 8.0 (20) | 9.0 (45) | 10.0 (41) | 4.4 (4) | ||
| ≥65 | 0.4 (1) | 0.2 (1) | 0.2 (1) | 0.0 (0) | ||
| Average | 35.2 (±9.4) | 37.2 (±7.9) | 37.2 (±8.3) | 37.4 (±6.4) | .003 | .799 |
| Exposure to the Virus SARS-CoV-2 | .166 | <.001 | ||||
| Community | 76.4 (191) | 71.0 (355) | 65.8 (269) | 94.5 (86) | ||
| Occupational | 16.0 (40) | 17.4 (87) | 20.0 (82) | 5.5 (5) | ||
| Cohabitant | 7.6 (19) | 11.6 (58) | 14.2 (58) | 0.0 (0) | ||
| Comorbidities Considered as Risk Factors for COVID-19 | .589 | .211 | ||||
| Yes | 21.8 (54) | 23.5 (117) | 22.4 (91) | 28.6 (26) | ||
| No | 78.2 (194) | 76.5 (380) | 77.6 (315) | 71.4 (65) | ||
| COPD | 0.0 (0) | 0.4 (2) | 0.5 (2) | 0.0 (0) | ||
| Severe asthma | 0.4 (1) | 1.6 (8) | 1.7 (7) | 1.1 (1) | ||
| Severe cardiopathy | 0.0 (0) | 0.4 (2) | 0.5 (2) | 0.0 (0) | ||
| ATH | 2.0 (5) | 2.4 (12) | 2.7 (11) | 1.1 (1) | ||
| Tobacco use | 18.0 (45) | 17.0 (85) | 14.7 (60) | 27.5 (25) | ||
| Immunosuppression | 0.4 (1) | 0.2 (1) | 0.2 (1) | 0.0 (0) | ||
| Severe obesity | 1.2 (3) | 0.6 (3) | 0.7 (3) | 0.0 (0) | ||
| DM | 0.4 (1) | 0.6 (3) | 0.7 (3) | 0.0 (0) | ||
| CKD on dialysis | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | ||
| Liver disease | 0.4 (1) | 0.8 (4) | 1.0 (4) | 0.0 (0) | ||
| Use of Drugs Associated With the Treatment Or Prevention of COVID-19 | .024 | .630 | ||||
| Yes | 4.4 (11) | 9.0 (45) | 9.3 (38) | 7.7 (7) | ||
| No | 95.6 (239) | 91.0 (455) | 90.7 (371) | 92.3 (84) | ||
| Corticosteroids | 0.8 (2) | 1.2 (6) | 1.5 (6) | 0.0 (0) | ||
| Hydroxychloroquine | 0.0 (0) | 0.4 (2) | 0.5 (2) | 0.0 (0) | ||
| Methotrexate | 0.0 (0) | 0.2 (1) | 0.2 (1) | 0.0 (0) | ||
| Finasteride | 3.6 (9) | 7.4 (37) | 7.3 (30) | 7.7 (7) | ||
| Adherence to PrEP | ||||||
| ≥85% | - | 63.6 (318) | 64.8 (265) | 58.2 (53) | .240 | |
| <85% | - | 36.4 (182) | 35.2 (144) | 41.8 (38) |
Abbreviations: ATH, arterial hypertension; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; DM, diabetes mellitus; MSM, men who have sex with men; PrEP, pre-exposure prophylaxis; SARS-CoV-2, severe acute respiratory sydrome coronavirus 2; TAF/FTC, tenofovir alafenamide/emtricitabine; TDF, disoproxil fumarate; TGW, transgender women.
*Without PrEP vs PrEP.
**TDF/FTC vs TAF/FTC.
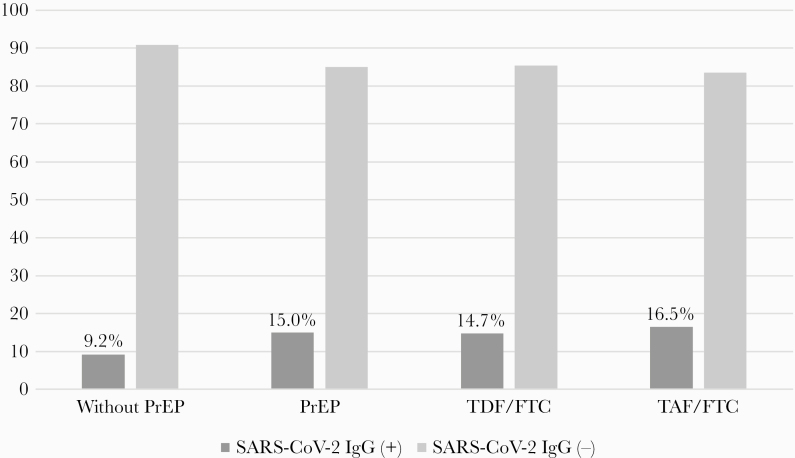
In the population studied, the seroprevalence of SARS-CoV-2, found via an analysis of the presence of IgG antibodies (CMIA), was 9.2% (95% CI, 5.9–13.5) in the group without PrEP and 15.0% (95% CI, 12.0–18.4) in the group with PrEP (P = .026). Among users of TDF/FTC, it was 14.7% (95% CI, 11.4–18.5) and in users of TAF/FTC it was 16.5% (95% CI, 9.5–25.7), with no statistically significant differences (P = .661) (Table 2, Figure 1).
Table 2.
Seroprevalence for SARS-CoV-2 and Associated Clinical Manifestations According to Use of PrEP, TDF/FTC, and TAF/FTC (n = 750)
| % (n) | Without PrEP n = 250 | PrEP n = 500 | TDF/FTC n = 409 | TAF/FTC n = 91 | P* | P** |
|---|---|---|---|---|---|---|
| Imumunoassay for IgG Antibodies for SARS-CoV-2 | .026 | .661 | ||||
| Positive | 9.2 (23) | 15.0 (75) | 14.7 (60) | 16.5 (15) | ||
| Negative | 90.8 (227) | 85.0 (425) | 85.3 (349) | 83.5 (76) | ||
| Clinical Manifestations | .058 | .527 | ||||
| Asymptomatic | 73.2 (183) | 66.4 (332) | 65.8 (269) | 69.2 (63) | ||
| Symptomatic | 26.8 (67) | 33.6 (168) | 34.2 (140) | 30.8 (28) | ||
| Fever | 14.8 (37) | 19.0 (95) | 19.0 (79) | 17.6 (16) | .154 | .703 |
| Cough | 12.4 (31) | 16.2 (81) | 16.4 (67) | 15.4 (14) | .169 | .815 |
| Dyspnea | 4.4 (11) | 7.8 (39) | 6.6 (27) | 13.2 (12) | .078 | .035 |
| Myalgias | 6.4 (16) | 13.2 (66) | 12.2 (50) | 17.6 (16) | .005 | .172 |
| Asthenia | 9.6 (24) | 12.2 (61) | 12.2 (50) | 12.1 (11) | .290 | .971 |
| Loss of appetite | 2.0 (5) | 1.4 (7) | 1.2 (5) | 2.2 (2) | .547 | .616 |
| Anosmia | 7.2 (18) | 9.6 (48) | 37.0 (9.0) | 12.1 (11) | .274 | .430 |
| Ageusia | 4.4 (11) | 6.0 (30) | 5.1 (21) | 9.9 (9) | .364 | .084 |
| Diarrhea | 4.0 (10) | 10.4 (52) | 10.5 (43) | 9.9 (9) | .003 | .860 |
| Cephalgia | 6.0 (15) | 12.0 (60) | 12.5 (51) | 9.9 (9) | .010 | .493 |
| Skin lesions | 0.8 (2) | 1.4 (7) | 1.0 (2) | 3.3 (3) | .725 | 1.117 |
| Duration of the Symptoms | .408 | .270 | ||||
| Median days (IQR) | 7.0 (4.0–14.0) | 7.0 (4.3–13.0) | 7.0 (4.3–12.5) | 10.0 (4.3–14.0) | ||
| Specific Treatment for COVID-19 | .729 | .703 | ||||
| Yes | 2.0 (5) | 2.4 (12) | 2.7 (11) | 1.1 (1) | ||
| No | 98.0 (245) | 97.6 (488) | 97.3 (398) | 98.9 (90) |
Abbreviations: COVID-19, coronavirus disease 2019; IgG, immunoglobulin G; IQR, interquartile range; PrEP, pre-exposure prophylaxis; SARS-CoV-2, severe acute respiratory sydrome coronavirus 2; TAF/FTC, tenofovir alafenamide/emtricitabine; TDF, disoproxil fumarate.
*Without PrEP vs PrEP.
**TDF/FTC vs TAF/FTC.
Figure 1.
Presence of immunoglobulin G antiboideis (chemiluminescent microparticle immunoassay) for severe acute respiratory sydrome coronavirus 2 (SARS-CoV-2) in men who have sex with men and transgender women without the use of pre-exposure prophylaxis (PrEP) (Group 1; n = 250) an PrEP users (Group 2; n = 500) with disoproxil fumarate/emtricitabine (TDF/FTC) (n = 409) and tenofovir alafenamide/emtricitabine (TAF/FTC) (n = 91).
Common symptoms of COVID-19 manifested in 33.6% of PrEP users and 26.8% of people without PrEP (P = .058), and there were no significant differences between TDF/FTC and TAF/FTC. The most mentioned symptoms were fever (17.6%) and cough (14.9%). The manifestations occured for a median 7 days, with no differences related to the use of PrEP. A total of 2.7% received some pharmacological treatment related to COVID-19, with no significant differences between either group (Table 2).
Of people with a positive serological result for SARS-CoV-2 who received PrEP, 57.4% manifested symptoms, whereas this figure was 78.3% among people in the control group (P = .070). The duration of symptoms in PrEP users was shorter, a median of 9.0 days compared with 11.5 days for the other group, with no significant differences. Symptoms associated with COVID-19 were presented by 53.3% of users of TDF/FTC versus 73.3% of TAF/FTC (P = .100). The duration of symptoms among people receiving TAF/FTC was longer (a median of 13.0 days) than in those taking TDF/FTC (7.0 days), without being statistically significant (Table 3). There were 5 patients who were hospitalized, all with a positive serological result: 1 without PrEP, 3 in treatment with TDF/FTC, and 1 with TAF/FTC, who also required care in ICU.
Table 3.
Presence of Symptoms Associated With COVID-19 and Their Duration Among Patients With Positive IgG Serology for SARS-CoV-2, According to PrEP and Drug Use, TDF/FTC and TAF/FTC (n = 75)
| % (n) | Without PrEP n = 23 | PrEP n = 75 | P = .111 | TDF/FTC n = 60 | TAF/FTC n = 15 | P = .100 |
|---|---|---|---|---|---|---|
| Asymptomatic | 21.7 (5) | 42.7 (32) | 46.7 (28) | 26.7 (4) | ||
| ≤7 days | 26.1 (6) | 26.7 (20) | 28.3 (17) | 20.0 (3) | ||
| >7 days | 52.2 (12) | 30.7 (23) | 25.0 (15) | 53.3 (8) | ||
| Median days (IQR) | 11.5 (7.0–19.5) | 9.0 (5.0–14.0) | .116 | 7.0 (4.3–13.0) | 13.0 (5.0–24.0) | .118 |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; PrEP, pre-exposure prophylaxis; SARS-CoV-2, severe acute respiratory sydrome coronavirus 2; TAF/FTC, tenofovir alafenamide/emtricitabine; TDF, disoproxil fumarate.
DISCUSSION
In Spain, the ENE-COVID study found a seroprevalence for SARS-CoV-2, through immunoassay tests, of 4.6% in the general population and more than 10.0% in Madrid [19]. These results concur with the 13.1% found in the total of patients analyzed in our study, most of them being residents in Madrid.
In our work, the seroprevalence of SARS-CoV-2, analyzed through the presence of IgG antibodies, was higher in PrEP users than that found in those who did not take the drug (15.0% vs 9.2%; P = .026). There were some variables that differentiated the 2 groups: people who did not take PrEP were younger (median age, 35.2 vs 37.2 years), and, among PrEP users, there was a greater use of other drugs associated with the treatment and prevention of COVID-19. However, there were no significant differences in the sex, presence of comorbidities, occupational exposure, or exposure by cohabiting with confirmed cases. Therefore, both groups included MSM and TGW with a similar risk profile for the SARS-CoV-2 virus except for the use of PrEP. Despite this, the group with PrEP had the highest seroprevalence.
Several publications have associated the use of PrEP with a greater number of sexual contacts without the use of condoms and a high presence of STIs, mainly of rectal location [20]. In addition, the possibility of oral-fecal transmission of SARS-CoV-2 and the presence of the virus in semen have been evidenced [21, 22]. For these reasons, the community exposure to SARS-CoV-2 among PrEP users was able to be higher than that of the control group, which would explain the higher seroprevalence.
Among the PrEP users analyzed in this study, the prevalence of SARS-CoV-2 in users of TDF/FTC was lower than that found in those of TAF/FTC, without statistically significant differences (14.7% vs 16.5%; P = .661). Adherence to both drugs was similar, and, among TDF/FTC users, there were a higher number of health professionals and cohabitants of confirmed cases, although a lower seroprevalence was found. Even though these results were not statistically significant, they could be likened to the results obtained by Del Amo et al [23] through a study of the prevalence of COVID-19 by polymerase chain reaction conducted in 77 590 people with HIV infection on ART in Spain, in which they describe a lower risk in people receiving TDF/FTC (16.9 per 10 000) when compared with TAF/FTC (39.1 per 10 000).
However, another study that analyzed a cohort of HIV-positive patients with COVID-19 described that the incidence of cases for SARS-CoV-2 was comparable to that found in the general population and that there were no differences between those patients taking TDF/FTC or TAF/FTC versus other ARTs. The use of these drugs was also not associated with differences in the severity of the clinical manifestations [24–26]. Data published in the Annals of Internal Medicine describe that people receiving TDF/FTC had milder symptoms and a lower risk of hospitalization, 10.5 (95% CI, 5.6–17.9) among those receiving TDF/FTC versus 20.3 (95% CI, 15.2–26.7) in those who were taking TAF/FTC [23]. In our study, PrEP users had fewer symptoms and for a shorter duration than the control group, as did those receiving TDF/FTC when compared with TAF/FTC, although no statistically significant differences could be found.
The preventive efficacy of tenofovir/FTC was studied in MSM and TGW and the age range was 18 to 71 years [27]. Thus, these results could not be extrapolated to children, the elderly, or women, in spite of the fact that no significant differences were found in the seroprevalence of COVID-19 according to sex in Spain [15]. In our study, a single diagnostic technique was used to measure the seroprevalence of COVID-19, and this was an immunoassay for the presence of IgG antibodies against SARS-CoV-2, which was considered the most appropriate test bearing in mind the high variability among the different antibody detection tests [28, 29].
This is the first study of the seroprevalence and evaluation of clinical manifestations of COVID-19 in PrEP users with TDF/FTC and TAF/FTC, in which a control group of similar characteristics is also compared, in one of the countries with the highest prevalences of the SARS-CoV-2 virus in Europe [30].
CONCLUSIONS
In conclusion, users of PrEP, TDF/FTC, or TAF/FTC presented a higher seroprevalence to SARS-CoV-2 than the control group, and no statistically significant differences were found in relation to clinical manifestations. In the absence of further studies, PrEP users should use the same prevention measures as those indicated for the general population.
Acknowledgments
Author contributions.J. D. R., C. R., T. P., P. C., M. V., J. B., and O. A. conceived an designed the study. All the menbers of the Sandoval Study Group were responible for the patien inclusion, data collection, clinical follow up, and helped to write the manuscript. M. E. F. and O. A. did the analysis and take responsability for the integrity of the data and the accuracy of the data analysis. All authors critically revised the manuscrip and gave final approval for the final version.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Sandoval Study Group
Jorge Del Romero, Carmen Rodríguez, Teresa Puerta, Petunia Clavo, Mar Vera, Juan Ballesteros, Clara Lejarraga, Nuria Fernández, Estefanía Hurtado, Mónica García, Montserrat González, Natividad Jerez, Florencia Alcudia, Maria Teresa Jiménez, Elisa Torres, Iria de Domingo, Ruth Lázaro, Montserrat Raposo, and Oskar Ayerdi.
Contributor Information
Sandoval Study Group:
Jorge Del Romero, Carmen Rodríguez, Teresa Puerta, Petunia Clavo, Mar Vera, Juan Ballesteros, Clara Lejarraga, Nuria Fernández, Estefanía Hurtado, Mónica García, Montserrat González, Natividad Jerez, Florencia Alcudia, Maria Teresa Jiménez, Elisa Torres, Iria de Domingo, Ruth Lázaro, Montserrat Raposo, and Oskar Ayerdi
References
- 1. The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus disease (COVD-19). China CDC Weekly 2020; 2:113–22. [PMC free article] [PubMed] [Google Scholar]
- 2. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA 2020; 323:709–10. [DOI] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jockusch S, Tao C, Li X, et al. A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19. Antiviral Res 2020; 180:104857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chien M, Anderson TK, Jockusch S, et al. Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase. J Proteome Res 5 August 2020; doi: 10.1101/2020.03.18.997585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elfiky AA. SARS-CoV-2 RNA dependent RNA polymerase (RdRp) targeting: an in silico perspective. J Biomol Struct Dyn 2020; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Melchjorsen J, Risør MW, Søgaard OS, et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. J Acquir Immune Defic Syndr 2011; 57:265–75. [DOI] [PubMed] [Google Scholar]
- 9. Castillo-Mancilla JR, Meditz A, Wilson C, et al. Reduced immune activation during tenofovir-emtricitabine therapy in HIV-negative individuals. J Acquir Immune Defic Syndr 2015; 68:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control, Prevention (CDC). Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR 2012; 61:586–9. [PubMed] [Google Scholar]
- 11. European Medicines Agency. First medicine for HIV pre-exposure prophylaxis recommended for approval in the EU. Truvada to enhance existing HIV prevention strategies Press Office. EMA/CHMP/496941/ 22 July 2016.
- 12. US Food and Drug Administration. FDA approves second drug to prevent HIV infection as part of ongoing efforts to end the HIV epidemic. Rockville, MD: US Food and Drug Administration; FDA. October 3, 2019. [Google Scholar]
- 13. Ministerio de Sanidad, Consumo y Bienestar Social. [Notas de Prensa El Sistema Nacional de Salud (SNS) financia la PrEP desde manana como medida de prevencion del VIH en personas de alto riesgo] 31 October 2019, Madrid.
- 14. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Government of Spain, Ministry of Health. COVID-19 situation in Spain. Available at: www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Actualizacion_104_COVID-19.pdf. Accessed 13 May 2020.
- 16. Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott ARCHITECT SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol 2020; 58:e00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia-Vidal C, Moreno-García E, Hernández-Meneses M, et al. Personalized therapy approach for hospitalized patients with COVID-19. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goren A, Vaño-Galván S, Wambier CG, et al. A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain - A potential clue to the role of androgens in COVID-19 severity. J Cosmet Dermatol 2020; 19:1545–7. [DOI] [PubMed] [Google Scholar]
- 19. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis 2018; 67:676–86. [DOI] [PubMed] [Google Scholar]
- 21. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020; 158:1831–3.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; 323:1843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Del Amo J, Polo R, Moreno S, et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med 2020. doi: 10.7326/M20-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vizcarra P, Pérez-Elías MJ, Quereda C, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV 2020. doi: 10.1016/S2352-3018(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borobia AM, Carcas AJ, Arnalich F, et al. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med 2020; 9:1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Recomendaciones sobre Profilaxis Pre-Exposición en adultos para la Prevención de la Infección por VIH en España AIDS Study Group (GeSIDA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Available at: http://www.cesida.org/wp-content/uploads/2013/09/gesida-guiasclinicas-2016-profilaxis_pre-exposicionVIH.pdf. Accessed 26 December 2018.
- 28. Theel ES, Slev P, Wheeler S, et al. The role of antibody testing for SARS-CoV-2: is there one? J Clin Microbiol 2020; 58:e00797-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jääskeläinen AJ, Kuivanen S, Kekäläinen E, et al. Performance of six SARS-CoV-2 immunoassays in comparison with microneutralisation. J Clin Virol 2020; 129:104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. European Centre for Disease Prevention and Control. HIV/AIDS surveillance in Europe 2019–2018 data. Available at: www.ecdc.europa.eu/en/publications-data/hivaids-surveillance-europe-2019-2018-data. Accessed 10 May 2020.