Abstract
Purpose:
To determine the prognostic importance of a positive post-treatment biopsy after prostate radiotherapy.
Materials and Methods:
382 patients underwent a post-treatment biopsy after external beam radiotherapy (EBRT) for clinically localized prostate cancer. Post-treatment biopsies were classified as positive (prostatic adenocarcinoma without typical radiation–induced changes), negative (no evidence of carcinoma) or adenocarcinoma with severe treatment effect (STE). The median follow-up in survivors was 9 years. Competing risks regression was used to assess relationship between prognostic predictors and CSM, DM, and PSA failure.
Results:
The prevalence of a positive, treatment effect and negative biopsy were 30%, 22% and 48%, respectively. Omission of androgen deprivation therapy and high-risk disease were associated with a 2.6 and 1.8-fold increase in odds of positive post-treatment biopsy, respectively. The 15-year PSA-relapse rates for negative, STE and positive post-treatment biopsy patients were 34%, 36% and 79%, respectively (p< 0.001). After controlling for known predictors, the hazard of distant metastases was 2.6-fold higher for positive biopsy patients (p<0.001) and cause-specific mortality was twice as high (HR: 2.00, p=0.022) in those with positive biopsy as compared to patients with negative and STE biopsy outcomes.
Conclusions:
A positive post-treatment biopsy after EBRT was associated with a higher hazard of distant metastases and prostate cancer related death. Patients with STE- classified biopsies have biologic characteristics more like those with a negative biopsy compared to a positive biopsy. Post-treatment biopsies were more often positive in the setting of EBRT alone without ADT or in the presence of high-risk disease.
Keywords: Radiotherapy, Prostate cancer, Biopsy, Distant Metastases
Introduction
PSA relapse-free survival outcome represents one of the most commonly used endpoints to assess the efficacy of prostate radiotherapy. While the PSA kinetics post-treatment could be considered a reflection of the local tumor control status, this may not always be the case. A rising PSA profile after therapy could possibly represent the presence of relapsing disease in regional nodes or at distant metastatic sites in the absence of local residual disease. A far more reliable assessment of the local control status could be obtained with multi-parametric MRI imaging and even more definitively with pathologic confirmation accomplished with a TRUS-guided prostate biopsy. While sampling error is possible, a negative post-treatment biopsy is nevertheless an excellent indication of intra-prostatic tumor control. A rising PSA profile after prostate radiation with a negative prostate biopsy is more likely suggestive of microscopic relapsing disease at regional or distant metastatic sites.
We have previously reported post-treatment biopsy outcomes after dose escalated radiotherapy (1–3). This report represents the first to our knowledge to correlate the post-treatment biopsy outcomes after 3D-CRT and IMRT with 15+ year survival outcomes. When correlated with 5 years outcomes, there have been conflicting conclusions regarding the prognostic implications of the biopsy classification known as “adenocarcinoma with severe treatment effect”-whether this specific classification is more consistent with a negative or a positive biopsy status (4,5). Therefore, the goals of our study were two-fold. First, we intended to confirm previous reported findings that a positive post-treatment biopsy is associated with inferior survival outcomes after radiotherapy. Second, with long-term follow up, we wanted to assess whether patients with biopsy outcomes showing adenocarcinoma with severe treatment effect have outcomes similar to patients with negative when compared to those with positive post-treatment biopsies.
Materials and Methods
Between 1990 and 2005, 382 patients underwent a post-treatment biopsy after completion of external beam radiotherapy (EBRT) for clinically localized prostate cancer. Many (n=151) of these post-treatment biopsies were performed during an ongoing dose escalation study conducted at our institution to evaluate the efficacy of EBRT dose ranging from 70.2 Gy–86.4 Gy. The median time from the completion of radiotherapy to the post-treatment biopsy was 38 months. While in general all patients receiving EBRT during this time-period were encouraged to undergo a post-treatment biopsy after radiotherapy, the majority did not undergo re-biopsy secondary to patient refusal, physician not recommending the biopsy due to the presence of multiple co-morbidities or the patient taking anti-coagulation therapy, as previously described (1,2). For this report the following eligibility criteria were required: post-treatment biopsy was performed within 5 years of completion of radiotherapy; no evidence of metastatic disease based on imaging studies at time of biopsy, and no patient receiving androgen deprivation therapy at the time of second biopsy.
The patient characteristics of the biopsy cohort compared to the patients treated during that time who did not undergo a post-treatment biopsy are shown in Table 1. Details of treatment technique, planning and delivery have been previously described (1,6).
Table 1:
Patient Characteristics of the Biopsy Cohort Compared to a Concurrent Non- biopsy Cohort
| No Biopsy | Biopsy | |
|---|---|---|
| Number of Patients | 1434 | 382 |
| T Stage: | ||
| T1c | 541 (38%) | 113 (29.6%) |
| T2 | 655 (47%) | 203 (53.1 %) |
| T3 | 238 (17%) | 66 (17.3%) |
| Gleason: | ||
| 6 or less | 738 (51%) | 151 (39.5%) |
| Greater than 6 | 696 (49%) | 231 (60.5%) |
| Pretreatment PSA (ng/mL) | ||
| 10 or less | 1009 (70%) | 182 (47.6%) |
| Greater than 10 | 425 (30%) | 200 (52.4%) |
| Age (years) | ||
| ≤ 65 | 409 (29%) | 149 (39.0%) |
| >65 | 1025 (71%) | 233 (61.0%) |
| Dose | ||
| Less than 75.6 Gy | 280 (19%) | 83 (21.7%) |
| >= 75.6 Gy | 1154 (81%) | 299 (78.3%) |
| Use of ADT | ||
| Yes | 672 (47%) | 185 (48.4) |
| No | 762 (53%) | 197 (51.6) |
Post-radiotherapy trans-rectal ultrasound guided biopsies (minimum of six cores) were performed in this cohort of patients. Among these patients, 28.3% (n=108) had manifested a PSA relapse by the time of biopsy and 71.7% (n=274) were biopsied in the absence of a PSA relapse.
The histological outcome of the prostate biopsy was classified as positive (prostatic adenocarcinoma without typical radiation–induced changes), negative (no evidence of carcinoma) or severe treatment effect. As previously described (7) this latter category was histologically characterized by residual tumor cells or poorly formed glands with abundant clear or vacuolated cytoplasm without evidence of mitotic figures. Immunohistochemical staining with antibodies to high-molecular weight cytokeratin (34BE12, Dako-Carpenteria CA) or to PSA (Biogenex-San Ramon CA) was carried out in selected patients to resolve uncertain diagnostic uncertainties when hematoxylin and eosin stains did not clearly distinguish between a treatment effect or a positive diagnosis for active cancer.
The median follow-up in survivors was 9 years (range: 0–23 years) from the time of biopsy. A PSA relapse was defined according to the nadir +2 definition. None of the patients received post-irradiation androgen deprivation or other anti-cancer therapy before documentation of a PSA relapse or the post-treatment biopsy.
Patients were classified into prognostic risk groups according to the National Comprehensive Cancer Network guidelines (www.nccn.org). As previously reported (1), after demonstrating that patients with biopsies consistent with adenocarcinoma with severe treatment effect (STE) and negative biopsies were associated with similar survival outcomes while different from those patients with positive biopsies, for subsequent analyses we merged the negative and STE biopsy cohorts together and these were compared to the patients with positive biopsies.
Statistical Methods
Prevalence of biopsy findings were described with proportions and exact 95% confidence intervals (CI). Associations between patient characteristics and biopsy finding (positive vs. negative/STE) were compared with Fisher’s Exact test and multivariable logistic regression. Overall survival (OS) was calculated from the time of biopsy until death. Patients alive at last follow up were censored. Cause Specific Mortality (CSM) was calculated from the time of biopsy until death from disease. Patients who died of other or unknown causes were treated as competing risks and patients alive at last follow up were censored. Distant metastases (DM) was calculated from time of biopsy until distant metastases diagnosis with death without DM treated as a competing risk. In patients who did not have PSA failure prior to biopsy, PSA failure was calculated from the time of biopsy until PSA failure with all deaths as competing risks. CSM, DM, and PSA failure were estimated with Kaplan Meier methods and cumulative incidence where appropriate.
Univariable and multivariable competing risks regression were used to assess the relationship between biopsy results and known prognostic factors with outcomes. Factors were included in multivariable regardless of significance. In addition, as Gleason, T-stage, and pre-RT PSA are, by definition, not independent on NCCN risk score, NCCN was not included in the main multivariable models. As we had a sufficient number of events for DM, we performed a sensitivity analysis where NCCN was included in the main effects multivariable model. Two-sided p-values less than 0.05 were considered statistically significant. All analyses were performed with SAS 9.4 (The SAS Institute, Cary, NC).
Results
Overall Outcomes for Entire Cohort
The 10 and 15-year cumulative incidence of PSA failure rates were 40% (95%CI: 34–47%) and 45% (95%CI:38–51%).The 10 and 15-year cumulative incidence of DM were 23% (95%CI: 19–28%) and 27% (95%CI: 22–32%), respectively. The 10 and 15-year cumulative incidence of cause-specific mortality were 12% (95%CI: 9–16%) and 18% (95%CI:14–23%), respectively. One hundred and ninety-one patients had died during the follow up period with a median OS of 13.2 years (95%CI: 11.6–14.2) and a 15 year OS rate of 63% (95%CI: 58–69%).
Clinical Variables Associated with a Positive Post-Treatment Biopsy Outcomes
The initial treatment details and post-treatment biopsy characteristics of this cohort are shown in Table 1. The overall incidence of a positive, STE and negative biopsy in this group were 30.1% (95%CI: 25.5–35.0%; n=115), 21.7% (95%CI: 17.7–26.2%; n=83) and 48.2% (95%CI: 43.1–53.5%; n=184). For all prognostic risk groups, a significantly higher percentage of positive biopsies was observed among patients treated with EBRT alone (53%) compared to EBRT plus ADT (38%), p=0.010. In a multivariable analysis, the omission of androgen deprivation therapy (OR: 2.56, 95%CI: 1.55–4.21, p<0.001) and the presence of high-risk disease (OR: 1.77, 95%CI: 1.09–2.86, p=0.020) were associated with an increased odds of a positive post-treatment biopsy.
It is interesting to note that 10.4% of patients overall in this cohort (16/163) had a positive biopsy in the setting of a PSA < 1 ng/ml and 23% of patients (62/272) had a positive biopsy in the absence of a PSA relapse as defined by nadir+2. As will be shown in Table 2, the biopsy outcome was independently associated with biochemical relapse in a multivariate main effects regression model. Furthermore, an enhanced model with an interaction term between PSA value and biopsy outcome showed that the effect of positive biopsy on local tumor control was statistically significant in the setting of a PSA < 1 ng/ml. All these suggest that PSA alone does not necessarily carry all relevant information on local control and pathologic confirmation with post-treatment biopsy provides valuable prognostic information.
Table 2.
Multivariable Competing Risks Regression Including Interaction Term for PSA and Biopsy Findings
| Cause- Specific Mortality | Distant Metastases | PSA Relapse | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | [95% CI] | p-value | HR | [95% CI] | p-value | HR | [95% CI] | p-value | |||
| Biopsy Result | Positive | --- | 0.027 | --- | <.001 | --- | <.001 | ||||
| Negative | --- | --- | --- | ||||||||
| #Biopsy Result*PSA | Positive | --- | 0.056 | --- | <.001 | --- | 0.049 | ||||
| <1 | 4.53 | [1.19 – 17.24] | 7.91 | [2.99 – 20.91] | 5.08 | [2.53 – 10.22] | |||||
| >1 | 1.09 | [0.61 – 1.96] | 1.23 | [0.76 – 1.99] | 2.10 | [1.23 – 3.58] | |||||
| Gleason Score | >=7 | 3.55 | [1.74 – 7.25] | <.001 | 3.34 | [1.88 – 5.94] | <.001 | 1.46 | [0.96 – 2.23] | 0.08 | |
| <=6 | --- | --- | --- | ||||||||
| PSA at Biopsy | >1 | --- | 0.001 | --- | <.001 | --- | <.001 | ||||
| <1 | --- | --- | --- | ||||||||
| Pre-RT PSA (ng/mL) | >=20 | 1.13 | [0.61 – 2.12] | 0.69 | 1.28 | [0.76 – 2.16] | 0.35 | 1.18 | [0.66 – 2.08] | 0.58 | |
| 10–20 | 0.49 | [0.24 – 0.98] | 0.042 | 0.69 | [0.42 – 1.14] | 0.15 | 0.97 | [0.62 – 1.50] | 0.88 | ||
| 0–10 | --- | --- | --- | ||||||||
The biopsy outcome with PSA demonstrates the interaction effect with biopsy in combination with the pre-biopsy PSA level
Prognostic Significance of a Severe Treatment Effect (STE) Biopsy Designation
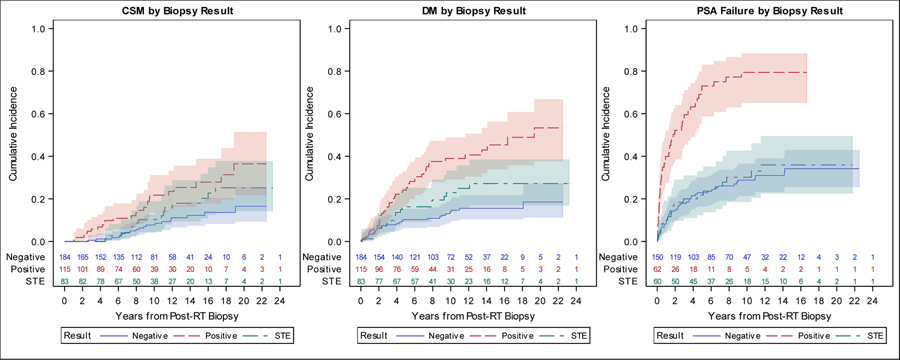
The 15-year PSA-relapse rates for negative, STE and positive post-treatment biopsy patients were 34% (95%CI: 26–43%), 36% (95%CI: 23–49%) and 79% (95%CO; 65–88%), respectively (Figure 1). In a univariable competing risks analysis, patients with positive biopsy had a significantly higher risk of PSA relapse (HR: 4.10, 95%CI: 2.43–6.93, p<0.001) compared to patients with STE, but no significant difference was found between patients with a negative biopsy (HR: 0.91, 95%CI: 0.54–1.53, p=0.72) and patients with STE. The findings were similar for DM (Figure 1). Patients with a positive biopsy had a higher risk of DM (HR: 2.00, 95%CI: 1.17–3.42, p=0.011) compared to patients with STE, but no significant differences were observed between patients with a negative biopsy (HR: 0.60, 95%CI: 0.33–1.08, p=0.09) and patients with STE. However, for CSM, no significant differences were seen between patients with positive (HR: 1.56, 95%CI: 0.82–2.96, p=0.18) or negative biopsies (HR: 0.60, 95%CI: 0.31–1.19, p=0.14) and patients with STE. However as noted below when severe treatment effect and negative biopsies are combined as one cohort (with the increase size of the cohort and number of events) there was a significant difference compared to the cohort with positive biopsies.
Figure 1.
CSM, DM and PSA Failure Cumulative Incidence Plots by Biopsy Result (Positive, Negative, and STE)
Legend: This plot displays the cumulative incidence of (a) cause specific mortality, (b) distant metastases, (c) PSA failure after biopsy stratified by biopsy findings. In this figure, STE and negative are represented by separate curves. These figures illustrate that patients with STE have similar incidence of outcomes compared to patients with negative biopsy.
Prognostic Significance of a Positive Post-Treatment Biopsy:
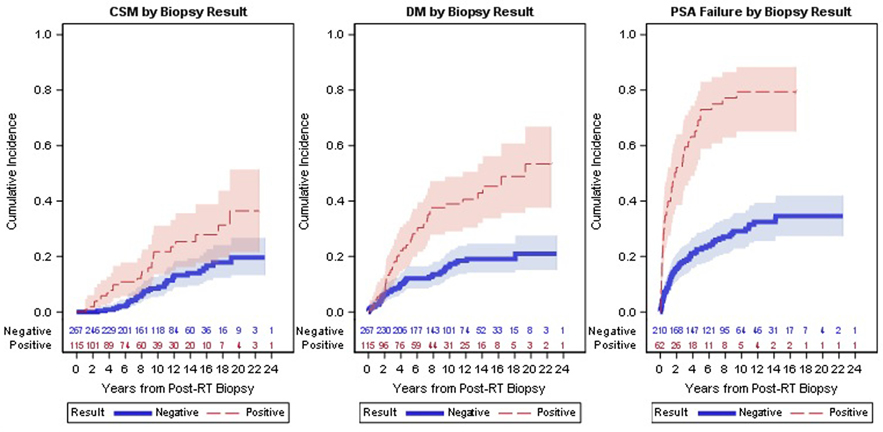
As shown in Figure 2, among patients with a positive post-treatment biopsy, the 15-year cumulative incidence of distant metastases was 45% (95%CI: 34–56%) compared to 19% (95%CI:14–25%) for those with non-positive biopsies. Similarly, among patients with a positive post-treatment biopsy 15 year CSM was 28% (95%CI: 18–39%) compared to 14% (95%CI: 10–19%) for those with non-positive biopsies. On univariable and multivariable analyses, post-RT biopsy outcome was associated with DM and CSM (Table 2). After controlling for known predictors, the risk of DM was 2.6 fold higher for those patients with a positive biopsy (HR: 2.59, 95%CI: 1.63–4.13, p<0.001) and the risk of cause specific mortality was twice as high (HR: 2.00, 95%CI: 1.11–3.62, p=0.022) in those that had a positive biopsy (Tables 3 and 4). Biopsy result was also strongly associated with PSA failure in multivariable analyses (HR: 3.87, 95%CI: 2.56–5.85, p<0.001) (Table 5). A separate analysis was performed to determine if the time between the completion of RT and obtaining the post-treatment biopsy represented a confounding factor. After controlling for time between RT and biopsy, biopsy result remained a significant predictor of outcomes in a multivariable analysis (data not shown).
Figure 2.
CSM, DM and PSA Failure Cumulative Incidence Plots by Biopsy Result (Positive and Negative)
Legend: Figure displays the cumulative incidence for the three primary outcomes: (a) cause specific mortality, (b) distant metastases, and (c) PSA failure after biopsy result. Negative biopsies include those with severe treatment effects.
Table 3.
Univariable and Multivariable Competing Risks Regression for Cause Specific Mortality (CSM)
| Univariable CSM | Multivariable CSM | ||||||
|---|---|---|---|---|---|---|---|
| HR | [95% CI] | p-value | HR | [95% CI] | p-value | ||
| Biopsy Result | Positive | 2.14 | [1.28 – 3.61] | 0.004 | 2.00 | [1.11 – 3.62] | 0.022 |
| Negative | REF | REF | |||||
| Gleason Score | >=7 | 4.07 | [2.01 – 8.23] | <.001 | 3.60 | [1.74 – 7.44] | <.001 |
| <=6 | REF | REF | |||||
| Pre-RT PSA (ng/mL) | >=20 | 1.68 | [0.93 – 3.04] | 0.021 | 1.43 | [0.78 – 2.61] | 0.25 |
| 10–20 | 0.61 | [0.31 – 1.20] | 0.57 | [0.29 – 1.13] | 0.11 | ||
| 0–10 | REF | REF | |||||
| T-Stage | T3 | 1.34 | [0.72 – 2.50] | 0.35 | 1.05 | [0.55 – 2.02] | 0.87 |
| T1-T2 | REF | REF | |||||
| Hormones | No | 0.89 | [0.53 – 1.48] | 0.65 | 0.91 | [0.51 – 1.63] | 0.75 |
| Yes | REF | REF | |||||
| NCCN Risk Score | High | 5.56 | [1.35 – 22.96] | 0.007 | --- | ||
| Medium | 2.87 | [0.68 – 12.20] | --- | ||||
| Low | REF | --- | |||||
Table 4.
Univariable and Multivariable Competing Risks Regression for Distant Metastases
| Univariable DM | Multivariable DM | Multi: Including NCCN | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | [95% CI] | p-value | HR | [95% CI] | p-value | HR | [95% CI] | p-value | ||
| Biopsy Result | Positive | 2.78 | [1.83 – 4.22] | <.001 | 2.59 | [1.63 – 4.13] | <.001 | 2.47 | [1.53 – 3.99] | <.001 |
| Negative | REF | REF | REF | |||||||
| Gleason Score | >=7 | 3.77 | [2.15 – 6.61] | <.001 | 3.20 | [1.80 – 5.70] | <.001 | 3.15 | [1.56 – 6.36] | 0.001 |
| <=6 | REF | REF | REF | |||||||
| Pre-RT PSA (ng/mL) | >=20 | 1.69 | [1.02 – 2.79] | 0.023 | 1.43 | [0.86 – 2.39] | 0.17 | 1.15 | [0.63 – 2.12] | 0.65 |
| 10–20 | 0.81 | [0.49 – 1.34] | 0.77 | [0.47 – 1.28] | 0.31 | 0.79 | [0.47 – 1.34] | 0.38 | ||
| 0–10 | REF | REF | REF | |||||||
| T-Stage | T3 | 1.28 | [0.74 – 2.21] | 0.37 | 1.06 | [0.59 – 1.89] | 0.85 | 0.87 | [0.46 – 1.65] | 0.67 |
| T1-T2 | REF | REF | REF | |||||||
| Hormones | No | 0.87 | [0.57 – 1.32] | 0.51 | 0.85 | [0.54 – 1.33] | 0.47 | 0.91 | [0.56 – 1.47] | 0.70 |
| Yes | REF | REF | REF | |||||||
| NCCN Risk Score | High | 3.56 | [1.39 – 9.09] | <.001 | --- | 1.30 | [0.36 – 4.69] | 0.69 | ||
| Medium | 1.72 | [0.66 – 4.49] | --- | 0.81 | [0.26 – 2.57] | 0.72 | ||||
| Low | REF | --- | REF | |||||||
Table 5.
Univariable and Multivariable Competing Risks Regression for PSA Failure
| Univariable PSA Failure | Multivariable PSA Failure | ||||||
|---|---|---|---|---|---|---|---|
| HR | [95% CI] | p-value | HR | [95% CI] | p-value | ||
| Biopsy Result | Positive | 4.39 | [3.00 – 6.42] | <.001 | 3.87 | [2.56 – 5.85] | <.001 |
| Negative | REF | REF | |||||
| Gleason Score | >=7 | 1.47 | [1.01 – 2.14] | 0.043 | 1.44 | [0.96 – 2.17] | 0.08 |
| <=6 | REF | REF | |||||
| Pre-RT PSA (ng/mL) | 10–20 | 1.23 | [0.81 – 1.85] | 0.60 | 1.21 | [0.79 – 1.86] | 0.37 |
| >=20 | 1.16 | [0.71 – 1.90] | 1.40 | [0.78 – 2.52] | 0.26 | ||
| 0–10 | REF | REF | |||||
| T-Stage | T3 | 1.08 | [0.69 – 1.68] | 0.75 | 0.81 | [0.48 – 1.36] | 0.43 |
| T1-T2 | REF | REF | |||||
| Hormones | No | 1.66 | [1.14 – 2.42] | 0.009 | 1.63 | [1.07 – 2.49] | 0.023 |
| Yes | REF | REF | |||||
| NCCN Risk Score | High | 1.97 | [1.07 – 3.60] | 0.07 | --- | ||
| Medium | 1.52 | [0.82 – 2.81] | --- | ||||
| Low | REF | --- | |||||
Discussion
In this report we note that the most important variable associated with distant metastases and cause-specific survival was the post-treatment biopsy outcome consistent with other reports (4, 5,8, 9). We also note that the long-term PSA-relapse free survival outcomes and distant metastases free survival outcomes at 15 years of post-treatment biopsies demonstrating STE was not significantly different from those patients with negative biopsies. As we have previously postulated (1–3), adenocarcinoma with severe treatment effect may indicate that cells are not viable or do not possess the same metastatic potential as a patient with a positive post-treatment biopsy.
Others (4, 5) have reported on the prognostic implications of the post-treatment biopsy and have noted that patients with severe treatment effect in the biopsy had clinical survival outcomes similar to those with a positive biopsy. In those studies the authors noted that among patients with STE the PSA outcomes were noted at 5 years to be similar to positive biopsy patients. Interestingly, the follow up was significantly shorter in those reports. Our study with long-term follow up was not consistent with those findings and corroborate our previous observations at earlier time points.
As emerging local therapies evaluating new radiotherapeutic interventions such a moderate and extreme hypofractionation approaches are becoming more prevalent in use for patients, it will become critical to evaluate their efficacy for achieving intra-prostatic tumor control. Escalated radiation dose levels achieved with IMRT and with further improvements observed combined brachytherapy and EBRT regimens have been associated with superior local tumor control outcomes and distant metastases-free survival outcomes. These findings are consistent with the potential impact local tumor control may have on distant metastases free survival as we have observed in the current report. While PSA serial testing has been a standard evaluation tool to assess local control, it is well recognized given the fluctuations of the values, ensuing confusion for clinicians and their patients is quite frequently encountered with rising PSA levels after treatment. Although the PSA elevation could raise suspicion for local residual disease, it could as easily reflect relapsing regional or micrometastatic disease. Quite frequently PSA elevations often trigger the need to order imaging studies to rule out metastatic disease. The post treatment biopsy is a more well-defined endpoint and may provide a more reliable assessment of the local control status and at an earlier time than biochemically relapsing disease.
Recently Kass-Illyya et al reported on results of 177 patients treated with 64–74 Gy of EBRT who underwent a 2-year biopsy with a median follow up of 7.8 years from the biopsy. PSA values at 2 years which were found between 1– 2 ng/ml were also associated with subsequent PSA failures and prostate cancer-specific survival compared with patients who had PSA lower levels <1.0 ng/ml. The authors concluded that prostate biopsies at 2 years have limited value in patients who at the time of biopsy are biochemically controlled. Yet in that study among those with PSA levels ≤ 1.0ng/ml at the time of the 2-year biopsy, approximately 20% −30% of such patients developed a subsequent PSA relapse. Indeed, in our report we noted that 23% of patients had a positive biopsy in the absence of a PSA relapse as defined by nadir+2. We agree that PSA kinetics at 2 years could possibly identify patients who may remain biochemically controlled, yet not an insignificant percentage of such patients will relapse in the follow up period which could have possibly been detected with a post-treatment biopsy at an earlier time point. In patients who have minimal co-morbidities where local salvage therapy represents a viable option, knowledge of the local disease status at earlier time points could be associated with improved salvage outcomes at time points where the PSA value was not a time point consistent with biochemical relapse.
In conclusion, post-treatment biopsies after radiotherapy provides important prognostic information and may identify patients at higher for risk for distant metastases where additional therapies could be of benefit to the patient. The classification of adenocarcinoma with severe treatment effect appears to behave in a similar fashion to those with negative post-treatment biopsies and those patients may be better served with continued surveillance. We believe that post-treatment biopsies may be a valuable tool to more effectively evaluate emerging new therapies such as hypofractionated approaches, and we have currently incorporated the 2 year biopsy into our follow-up regimen for those treated with hypofractionation to provide more clarity of the efficacy of these approaches.
Key of Definitions:
- PSA
Prostate-specific antigen
- STE
Severe treatment effect
- 3D-CRT
3-dimensional conformal radiotherapy
- IMRT
Intensity modulated radiotherapy
References
- 1.Zelefsky MJ, Leibel SA, Gaudin PB et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys 2000. 47: 1245. [DOI] [PubMed] [Google Scholar]
- 2.Levegrün S, Jackson A, Zelefsky MJ et al. Analysis of biopsy outcome after three-dimensional conformal radiation therapy of prostate cancer using dose-distribution variables and tumor control probability models. Int J Radiat Oncol Biol Phys 2002. 63:11. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky MJ, Reuter VE, Fuks Z, et al. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer.J Urol. 2008. April;179(4):1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crook J, Malone S, Perry G et al. Postradiotherapy prostate biopsies: What do they really mean? Results for 498 patients. Int J Radiat Oncol Biol Phys 2000: 48:355. [DOI] [PubMed] [Google Scholar]
- 5.Pollack A, Zagars GK, Antolak JA et al. Prostate biopsy status and PSA nadir level as early surrogates for treatment failure: analysis of a prostate cancer randomized radiation dose escalation trial. Int J Radiat Oncol Biol Phys 2002: 54:677. [DOI] [PubMed] [Google Scholar]
- 6.Zelefsky MJ, Chan H, Hunt M et al. Long term outcome of high dose intensity modulated radiotherapy for patients with clinically localized prostate cancer J Urol 2006: 176:1415. [DOI] [PubMed] [Google Scholar]
- 7.Gaudin PB, Zelefsky MJ, Leibel SA et al. Histopathologic effects of three-dimensional conformal external beam radiation therapy on benign and malignant prostate tissues. Am J Surg Pathol 1999; 23:1022. [DOI] [PubMed] [Google Scholar]
- 8.Krauss DJ, Hu C, Bahary JP et al. The importance of local control inearly stage prostate cancer : outcomes of patients with a positive post-radiotherapy biopsy treated on RTOG 94–08. Int J Radiat Oncol Biol Phys. 2015; 92:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kass- Illyya A, Jovic G, Murphy C et al. Two-years postradiotherapy biopsies: Lessons from the MRC RT01 trial Eur Urol 2018;73:968. [DOI] [PMC free article] [PubMed] [Google Scholar]