A growing body of evidence has demonstrated higher age, male sex, and medical comorbidity as risk factors for COVID-19 mortality [1]. In particular, male sex, and older age were found to be significant determinants for severe SARS-CoV-2 phenotype supporting the hypothesis that hormonal constitution may be an etiology for both COVID-19 susceptibility and acute respiratory distress syndrome (ARDS) development. Moreover, differences between male and female immune responses is well known establishing that genetics and sex hormones are important for the immunogenic sex-bias [2]. Higher serum total testosterone (TT) levels are associated with an immunosuppressive role on different components of the immune cell-mediated response [3]. Pozzilli et al. [4] hypothesized a role for TT in the clinical course of the SARS-CoV-2 leading to multiorgan failure.
We aimed to evaluate whether serum TT levels among a cohort of 29 COVID-19 men at the time of hospital admission were associated with the need for “invasive” oxygenation strategy (i.e., Ventimask, CPAP, intubation) and may allow for patient monitoring and predict disease outcome.
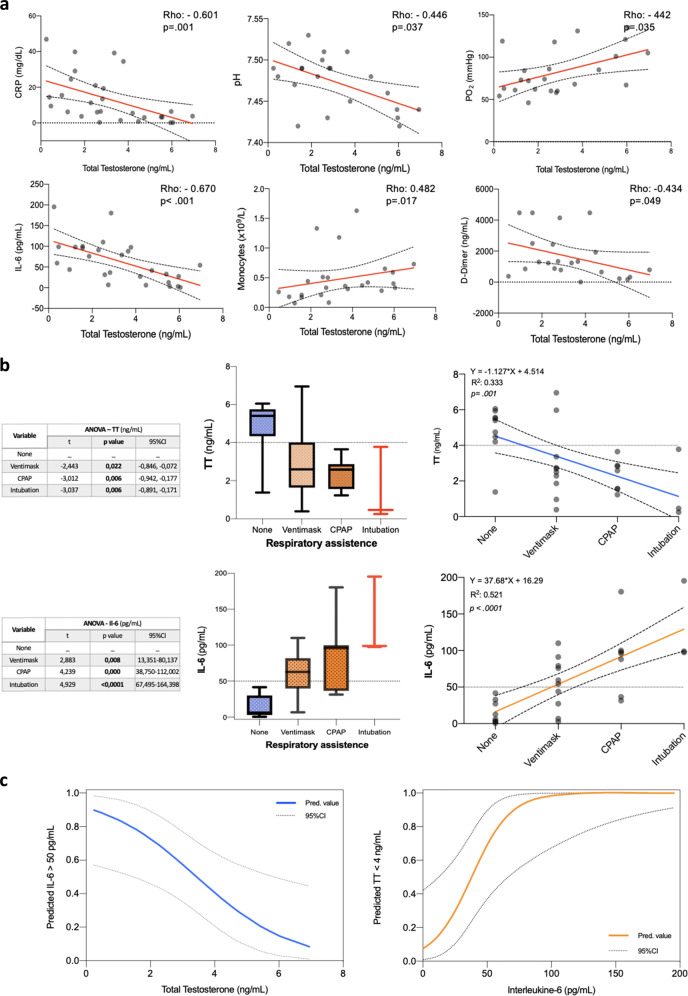
Patients’ haemato-chemical and clinical characteristics are reported in Table 1. After adjusting for Age-adjusted Charlson Comorbidity Index, history of hypertension, dyslipidemia, and smoking status, higher serum TT levels (ng/ml) were found independently associated with a lower odd of invasive oxygenation (odds ratio: 0.43, 95% CI: 0.23–0.85; p = 0.016). In addition, linear regression was used to examine the correlation between serum TT and haemato-chemical variables of interest. A significant negative correlation was found between TT and C-reactive protein, pH, Interleukine-6 (IL-6), and D-Dimer. Of note, a significant positive correlation was established among TT levels and Monocytes (×109/l) (Fig. 1a), while no significant correlation was further observed with regard of the other white blood cells lines (e.g., WBC, CD4+, CD8+ lymphocytes or NK cells). Additionally, one-way ANOVA was used to test the differences between continuous TT and IL-6 values for the different respiratory assistance strategies confirming as thresholds of interest <3.5–4 ng/ml for impaired T, while identifying >50 pg/ml for significantly elevated IL-6 (Fig. 1b). Locally weighted scatter-plot smoother function was used to graphically depict the relationship concerning these two variables and the probability of their mutual interaction for the previously defined thresholds (Fig. 1c).
Table 1.
Clinical and haemato-chemical characteristics of COVID-19 patients at hospital admission
| Variables | None O2 assistance | Invasive O2 assistance (Ventimask, CPAP, Intubation) |
p value* |
|---|---|---|---|
| Sample size, n (%) | 9 (31.0) | 20 (69.0) | – |
| Age, years | 58 (23–90) | 70 (35–86) | 0.084 |
| ACCI score, median (range) | 3 (0–5) | 3 (0–8) | 0.315 |
| Comorbidity, n (%) | |||
| Hypertension | 3 (33.3) | 12 (60.0) | 0.245 |
| Diabetes | 3 (33.3) | 6 (30.0) | 0.858 |
| Dyslipidemia | 3 (33.3) | 3 (15.0) | 0.339 |
| History of Neoplasm | 2 (22.2) | 3 (15.0) | 0.633 |
| CVD | 1 (11.1) | 3 (15.0) | 0.779 |
| CKD | 1 (11.1) | 2 (10.0) | 0.928 |
| Lung disease | 1 (11.1) | 3 (15.0) | 0.779 |
| Smoking status | 5 (55.5) | 9 (45) | 0.70 |
| Complete blood count | |||
|
HGB, g/dl Nr: 12–15.5 |
13.5 (11.7–15.3) | 11.6 (9.9–14.5) | 0.02 |
|
HCT, % Nr: 41–50 |
41.6 (36.7–43.2) | 35.7 (28.7–41.8) | 0.017 |
|
WBC, ×109/l Nr: 4.40–11.30 |
6.61 (3.90–17.26) | 7.52 (3.06–23.61) | 0.647 |
|
PLT, ×109/l Nr: 150.0–450.0 |
191.0 (167.0–633.0) | 232.0 (142.0–547.0) | 0.873 |
|
Lymphocytes, ×109/l Nr: 1.00–4.80 |
1.17 (0.67–2.39) | 0.75 (0.27–4.16) | 0.650 |
|
Lymphocytes CD4+, n°/μl Nr: 410.0–1590.0 |
384.0 (291.0–1349.0) | 524.5 (97.0–1485.0) | 0.775 |
|
Lymphocytes CD4+, % Nr: 31.0–60.0 |
45.3 (16.2–59.7) | 36.0 (20.6–74.4) | 0.461 |
|
Lymphocytes CD8+, n°/μl Nr: 190.0–1140.0 |
330.0 (224.0–1244.0) | 221.0 (78.0–1117.0) | 0.246 |
|
Lymphocytes CD8+, % Nr: 13.0–41.0 |
27.7 (16.6–69.5) | 23.3 (12.2–46.7) | 0.461 |
|
CD4+/CD8+, ratio Nr: 0.60–2.80 |
1.64 (0.23–3.61) | 1.64 (0.71–5.57) | 0.958 |
|
NK cells, n°/μl Nr: 90.0–590.0 |
160.0 (121.0–251.0) | 82.0 (13.0–604.0) | 0.360 |
|
NK cells, % Nr: 5.0–27.0 |
14.7 (6.6–18.3) | 8.5 (1.7–40.2) | 0.433 |
|
Lymphocytes B, n°/μl Nr: 90.0–660.0 |
113.0 (52.0–254.0) | 137.0 (22.0–314.0) | 0.512 |
|
Lymphocytes B, % Nr: 6.0–25.0 |
9.2 (5.4–13.3) | 10.3 (2.9–36.2) | 0.433 |
|
Monocytes, ×109/l Nr: 0.10–1.00 |
0.42 (0.28–1.63) | 0.33 (0.07–1.33) | 0.162 |
|
Monocytes, % Nr: 3.5–10.5 |
8.5 (2.2–11.2) | 5.9 (1.9–10.1) | 0.071 |
| Blood chemistry | |||
|
Creatinine, mg/dl Nr: 0.70–1.20 |
0.95 (0.80–2.00) | 0.90 (0.40–1.70) | 0.421 |
|
Testosterone, ng/ml Nr: 2.80–8.00 |
5.40 (1.38–6.05) | 2.54 (0.25–6.95) | 0.003 |
|
IL-6, pg/ml Nr: 1.50–7.00 |
9.30 (0.60–41.70) | 88.00 (6.80–195.40) | 0.001 |
|
CRP, mg/dl Nr: 0.00–0.50 |
3.14 (13.00–24.50) | 12.33 (0.31–46.91) | 0.006 |
|
LDH, U/l Nr: 135.0–225.0 |
223.5 (141.0–424.0) | 338.5 (143.0–951.0) | 0.115 |
|
Lac, mmol/l Nr: 0.3–0.7 |
0.7 (0.6–1.4) | 1.1 (0.6–3.4) | 0.175 |
|
Na+, mmol/l Nr: 136.0–145.0 |
137.0 (133.0–142.0) | 135.0 (131.0–144.0) | 0.229 |
|
K+, mmol/l Nr: 3.40–5.50 |
3.97 (3.41–4.60) | 3.80 (3.19–5.00) | 0.671 |
|
D-Dimer, ng/ml Nr: < 500 |
484.5 (170–4473) | 1146 (376–4486) | 0.124 |
| Vital signs | |||
|
pH Nr: 7.35–7.45 |
7.44 (7.42–7.48) | 7.49 (7.43–7.53) | 0.018 |
|
pO2, mmHg Nr: 83.0–108.0 |
101.0 (84.0–135.0) | 67.5 (46.0–131.0) | 0.028 |
|
PaO2/FiO2, mmHg Nr: 200–400 |
480.0 (400.0–576.0) | 286.0 (172.0–566.0) | 0.006 |
|
SO2, % Nr: 94.0–98.0 |
98.0 (91.0–99.0) | 95.5 (82.0–99.0) | 0.459 |
Results are presented as n (%) or median (range)
ACCI age-adjusted Charlson Comorbidity Index, ICU intensive care unit, CPAP continuous positive airway pressure, CVD cardiovascular disease, CKD chronic kidney disease, WBC white blood cells, PLT platelets, NK natural killer, IL-6 interleukin-6, CRP C-reactive protein, LDH lactate dehydrogenase, Lac lactate
*p values according to Fisher’s Exact test or Mann–Whitney U test when appropriate
Fig. 1.
a Scatter plots and Spearman’s rank correlation test of total testosterone (ng/ml) with haemato-chemical and vital signs among the COVID-19 cohort population. CRP C-reactive protein (mg/dl), IL-6 interleukine-6 (pg/ml). b Box plots and one-way ANOVA testing the differences between continuous total testosterone (TT) and interleukine-6 (IL-6) values for the different respiratory assistance strategies. c Locally weighted scatter-plot smoother (LOWESS) function depicting the predicted probability of reciprocal interaction between total testosterone (TT) and interleukine-6 (IL-6)
Male hypogonadism is typically of the aging male. Nevertheless, in our cohort, while age was not significantly different among the two groups (p = 0.082), TT levels were significantly lower in the ARDS group (p = 0.003) and associated with worse clinical COVID-19 phenotype. Additionally, considering the observed inverse relationship between IL-6 and TT levels, we speculate that greater TT levels could serve as hormonal shield against the COVID-19-related cytokine syndrome. Similarly, low TT levels may allow the viral infection due to a loss of immunosuppressive effect of TT. Our results are in line with the recently reported experience by Rastrelli et al. [5]. In addition, we were able to identify serum TT levels at hospital admission as a potential biomarker for the requirement for invasive respiratory assistance.
Although at first glance our thesis may appear conflicting with recent experiences suggesting possible protective role of androgen deprivation therapy (ADT) through the androgen receptor-mediated regulation of TMPRSS2 gene, relevant for SARS-CoV-2 introduction into host cells, thus outlining certain similitude to the “saturation model” proposed for the association between testosterone and prostate cancer [6], we actually believe that such theories are complementary and identify two diametrically opposite susceptibility profiles to contract the viral infection on the one hand and to develop clinically significant respiratory manifestations on the other. Evidence from unrelated studies point to a possible immunosuppressive role of TT on different components of the immune system and in different phases of the immune response [3, 7, 8]. Based on the role of the variation in androgen levels throughout life [9], testosterone could play a double-edged role in the natural history of COVID-19 infection. In the early phase, the immunosuppressive action of testosterone could explain male’s greater susceptibility to infection therefore leading to speculate a protective role of ADT. On the contrary when the infection occurred, in elderly males who frequently develop ARDS, late-onset hypogonadism could result in a lower immunosuppressive effect on the cytokine storm release as pointed out from our analysis.
Certain limitations warrant mention. First, the retrospective design and limited sample size expose the current analysis to bias and the role of chance. However, given that testicular parenchyma was recently found as a potential target of SARS-CoV-2 infection [10], we might possibly postulate Leydig cells involvement with subsequent TT levels impairment in the etiopathogenesis of the more severe ARDS cases. Moreover, our data allowed us only to make implications on the clinical severity at hospital admission but not to better define the role of TT in later history of the disease. While promising, the interplay between TT levels and COVID-19 require additional study to determine the utility of TT in clinical practice.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Stefano Salciccia, Francesco Del Giudice
These authors jointly supervised this work: Michael L. Eisenberg, Alessandro Sciarra
References
- 1.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu. Rev. Cell Dev. Biol. 2017;33:577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- 3.Trigunaite A, Dimo J, Jørgensen TN. Suppressive effects of androgens on the immune system. Cell Immunol. 2015;294(2):87–94. doi: 10.1016/j.cellimm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Pozzilli P, Lenzi A. Commentary: testosterone, a key hormone in the context of COVID-19 pandemic. Metabolism. 2020;108:154252. doi: 10.1016/j.metabol.2020.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.G. Rastrelli, V. Di Stasi, F. Inglese et al. Low testosterone levels predict clinical adverse outcomes in SARS‐CoV‐2 pneumonia patients. Andrology 1–11 (2020) [DOI] [PMC free article] [PubMed]
- 6.Montopoli M, Zumerle S, Vettor R, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann. Oncol. 2020;31(8):1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montanõ LM, Espinoza J, Flores-Soto E, et al. Androgens are bronchoactive drugs that act by relaxing airway smooth muscle and preventing bronchospasm. J. Endocrinol. 2014;222:1–13. doi: 10.1530/JOE-14-0074. [DOI] [PubMed] [Google Scholar]
- 8.Mohan SS, Knuiman MW, Divitini ML. Higher serum testosterone and dihydrotestosterone, but not oestradiol, are independently associated with favourable indices of lung function in community-dwelling mem. Clin. Endocrinol. (Oxf.) 2015;83:268–276. doi: 10.1111/cen.12738. [DOI] [PubMed] [Google Scholar]
- 9.Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J. Clin. Endocrinol. Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, A target for SARS-CoV-2 Infection in spermatogonia, leydig and sertoli cells. Cells. 2020;9(4):920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]