Abstract
Purpose
Irradiation-avoiding strategies have been used with relative success in the treatment of infants and young children with medulloblastoma. While advances in cancer genomics have significantly improved our understanding of the tumor biology of medulloblastoma allowing for improved prognostication and risk-stratification, the molecular subgroup-specific outcomes of infants and young children with medulloblastoma treated with irradiation-avoiding strategies remains unknown.
Methods
Molecular and clinical features of children with medulloblastoma treated with irradiation-avoiding strategies at Children’s Hospital Los Angeles were analyzed. Molecular subgrouping of these patients was determined using a 31-gene TaqMan Low Density Array signature. Survival analyses were conducted based on 3 molecular subgroups (SHH, Group 3, and Group 4).
Results
Twenty-eight patients with medulloblastoma received irradiation-sparing regimens and were included in this analysis. Patients were divided into SHH (n = 16), Group 3 (n = 3) and Group 4 subgroups (n = 9). Subgroup specific 5-year progression-free and overall survival was 81.2% (95% CI 52.5–93.5) and 93.7% (95% CI 63.2–99.1) for SHH, 0% and 0% for Group 3 and 0% and 44.4% (95% CI 13.6–71.9) for Group 4.
Conclusion
The majority of young children with SHH-subgroup medulloblastoma can be treated effectively with irradiation-sparing regimens. Our results support the use of chemotherapy-only strategies for upfront treatment of young children with SHH medulloblastoma, while demonstrating the urgent need for intensification/augmentation of treatment for patients with group 3/4 medulloblastoma.
Keywords: Medulloblastoma, Radiation, Drug therapy, Pediatrics, Genomics
Introduction
Medulloblastoma is one of the most common malignant brain tumor of childhood, accounting for approximately 20–25% of all pediatric brain tumors [1, 2]. Historically, these tumors have been classified based on their histopathological features with treatment decisions made on the basis of histological subtype, age, stage of disease and extent of resection [3]. Over the past decade, molecular analyses have significantly advanced our understanding of the heterogeneity of this disease.
Recent advances have enabled us to divide medulloblastoma into subgroups based on molecular features, each with unique clinical correlates [4–7]. The current version of the World Health Organization (WHO) classification of tumors of the central nervous system (CNS) describes four distinct subgroups of medulloblastoma (WNT, SHH, Group 3 and Group 4) [8]. More recently, further expansion of this classification has revealed numerous subtypes within each subgroup [6–8]. Molecular subgroup designation has led to improved prognostication and risk stratification, in addition to the development of clinical trials investigating subgroup specific treatments such as reduced intensity chemoradiation (e.g. COG ACNS1422) and molecularly targeted therapies [9–12]. Despite advancement in molecular subgrouping, the treatment for the vast majority of patients remains largely based on clinical risk-stratification including age, extent of resection and metastatic status.
The outcome of children with medulloblastoma has improved significantly over the past few decades such that the current 5-year event-free survival (EFS) for children with average-risk medulloblastoma is approximately 80% [13, 14]. In contrast, the outcome for children with high-risk medulloblastoma remains inferior, with 5-year EFS between 60–70% [14, 15]. Despite the well-recognized long-term side effects associated with irradiation, radiotherapy remains a critical component of the multimodal approach to the treatment of many patients [16]. However, due to its deleterious neuro-cognitive effects on the developing brain, irradiation is often avoided in young children, particularly in those less than three years of age. These young patients are typically treated with irradiation-sparing regimens, utilizing a combination of induction chemotherapy followed by consolidative high-dose myeloablative chemotherapy (HDC) with autologous stem cell rescue (AuSCR) with 5-year overall survival rates of 60–70% [17–21].
The effect of molecular subgroup on the outcome of patients treated with irradiation-sparing regimens remains unclear. While several groups have previously published clinical outcomes of children with medulloblastoma based on molecular subgroup, the details regarding specific treatment regimens were not available [5, 22]. Given the heterogeneity of regimens from distinct treatment eras and the different treatment centers included in these studies, it is unclear if these prognostic factors would remain significant in the current treatment era or with specific treatment regimens. More recent publications have described the outcomes of young children with SHH medulloblastoma treated with conventional chemotherapy [23, 24]. However, patients with SHH medulloblastoma on these protocols were treated without upfront high-dose chemotherapy and autologous stem cell rescue. It is unclear if the prognostic factors identified on these studies are applicable to other commonly used irradiation-sparing regimens, especially those with high-dose chemotherapy with autologous stem cell rescue.
In this review, we summarize our experience treating infants and young children with medulloblastoma at Children’s Hospital Los Angeles (CHLA) with irradiation-avoiding strategies. We describe the clinical outcomes for these patients and correlate these findings with molecular subgroup.
Patients and methods
Patient selection
Infants and young children less than 10 years of age at the time of diagnosis of medulloblastoma between 1980 and 2016 treated at CHLA were identified via a review of an institutional database. Patients were eligible for inclusion in this study if they were treated at CHLA and their treatment included a chemotherapeutic regimen upfront with the intention of avoiding radiation therapy. Other eligibility criteria included having sufficient tumor tissue available for analysis and evaluable outcome data. Patient characteristics, treatment protocol and outcomes were assessed and analyzed. The institutional review board of CHLA approved this retrospective review.
Treatment protocols
All patients underwent initial maximal safe surgical resection as per standard of care. Following surgical resection and confirmation of histopathological diagnosis, patients were treated with chemotherapy in accordance to one of the following treatment regimens according to primary physician’s preference and treatment era. These regimens included Head Start (HS) I, HS II, HS III, CCG-99703, CCG-9008, CCG-9921, CCG-9931 and 8-in-1 chemotherapy [18, 25–31]. For the purposes of this review, these regimens were additionally subdivided into conventional chemotherapy regimens (8-in-1, CCG-9008, 9921, 9931) and HDC regimens (HS I, II, III and CCG-99703).
Molecular subgroup analysis
Molecular analysis was performed using RNA derived from fresh-frozen tissue. Molecular subgroups were identified using a previously described 31-gene TaqMan low-density array (TLDA) [32]. This method has been previously validated with other established platforms for medulloblastoma subgroup analysis [21, 32].
Statistical analysis
Progression-free survival (PFS) and overall survival (OS) were analyzed at 5 and 10 years, estimated via Kaplan-Meier method with p-value reported using the log-rank test [33]. Statistical computations were performed using the R project (https://www.r-project.org) and GraphPad Prism v7 (GraphPad Software, San Diego, CA). For all statistical tests, p-values were two-sided with p < 0.05 considered significant.
Results
Patient characteristics
A total of 74 children with medulloblastoma treated at CHLA were identified, five of whom were excluded due to a lack of evaluable outcome data. Of the 69 remaining patients, 28 patients were treated with irradiation-avoiding strategies and included in the analysis. The median age at diagnosis was 2.16 years (range 0.24–9.82), with 18/28 (64%) patients presenting with localized disease at diagnosis. Classic histology was the most common (53.6%), followed by desmoplastic/nodular (28.6%) and large cell/anaplastic (17.8%). In terms of molecular subgroup classification, tumors from majority of patients (16/28; 57.2%) were found to be SHH subgroup, followed by nine (32.1%) in Group 4 and three (10.7%) in Group 3. Majority of patients had gross-total resection (GTR) upfront (20/28; 71.4%), while five patients (17.9%) had subtotal resections (STR) with significant residual disease (> 1.5 cm2) after initial surgery. The remaining three patients had near-total resections (NTR) with ≤ 1.5 cm2 of residual disease after surgery. Four out of five of patients with significant residual disease had tumor classified as SHH subgroup, and one from Group 4. Ten patients had metastatic disease at presentation with three from SHH subgroup, two from Group 3 and five from Group 4. Six patients received radiation therapy at recurrence, three from SHH subgroup and three from Group 4. None of the patients received radiation therapy as part of upfront therapy. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics
| Irradiation-sparing cohort (n = 28) | SHH subgroup (n = 16) | Group 3 subgroup (n = 3) | Group 4 subgroup (n = 9) | |
|---|---|---|---|---|
| Age (years) | ||||
| Median | 2.16 | 1.65 | 2.6 | 5.97 |
| Range | 0.24–9.82 | 0.24–4.45 | 1.02–2.68 | 2.71–9.82 |
| n (%) | n (%) | n (%) | n (%) | |
| Race/ethnicity | ||||
| White | 7 (25) | 3 (18.7) | 1 (33.3) | 3 (33.3) |
| Hispanic/latino | 18 (64.3) | 11 (68.7) | 1 (33.3) | 6 (66.7) |
| Asian | 3 (10.7) | 2 (12.5) | 1 (33.3) | 0 |
| Disease stage | ||||
| M0 | 18 (64.3) | 13 (81.2) | 1 (33.3) | 4 (44.4) |
| M1 | 2 (7.1) | 1 (6.2) | 1 (33.3) | 0 |
| M2 | 1 (3.6) | 0 | 1 (33.3) | 0 |
| M3 | 7 (25) | 2 (12.5) | 0 | 5 (55.6) |
| Extent of resection | ||||
| GTR | 20 (71.4) | 11 (68.8) | 3 (100) | 6 (66.7) |
| NTR | 3 (10.7) | 1 (6.2) | 0 | 2 (22.2) |
| STR | 5 (17.9) | 4 (25.0) | 0 | 1 (11.1) |
| Histology | ||||
| Desmoplastic/nodular | 8 (28.6) | 8 (50) | 0 | 0 |
| Classic | 15 (53.6) | 8 (50) | 1 (33.3) | 6 (66.7) |
| Large cell/anaplastic | 5 (17.8) | 0 | 2 (66.7) | 3 (33.3) |
| Treatment regimen | ||||
| High-dose chemo | 17 (60.7) | 11 (68.8) | 1 (33.3) | 5 (55.5) |
| Conventional chemo | 11 (39.3) | 5 (31.2) | 2 (66.7) | 4 (45.5) |
| Radiation therapy | ||||
| At disease recurrence | 6 (100) | 3 | 0 | 3 |
Survival
With a median follow-up period of 7.5 years (range 1.4–19.8), the estimated 10-year PFS rate for the entire cohort was 46.4% (95% CI 27.6–63.3), with no disease relapses observed after 5 years. The estimated 5- and 10-year OS rates were 67.9% (95% CI 47.3–81.8) and 60.3% (95% CI 39.8–75.7), respectively (Fig. 1A Supplemental). Patients with disseminated disease had significantly poorer OS compared to localized disease, both at 5-years (49.6% vs 77.1%) and 10-years (40% vs 71.8%), p-value = 0.045 (Fig. 1B Supplemental).
Patients with large cell/anaplastic histology had dismal outcomes, with estimated 5-year PFS and OS of 0%. In comparison, patients with classic histology had estimated 10-year PFS of 46% (95% CI 21.3–68.7), with estimated 5- and 10-year OS of 78.7% (95% CI 49.6–92.6) and 65.4% (95% CI 35.7–83.9), respectively. Patients with desmoplastic/nodular histology had estimated 10-year PFS of 75% (95% CI 31.5–93.1) and 10-year OS of 87.5% (95% CI 38.7–98.1), respectively (Fig. 1C Supplemental). While there was a trend towards improved outcomes for patients with desmoplastic/nodular when compared to classic histology, this did not meet statistical significance [p-value = 0.26 (PFS) and 0.31 (OS)].
In terms of molecular subgrouping, patients with SHH medulloblastoma had significantly better outcomes when compared to Group 3 and Group 4, both in terms of PFS and OS (p-value < 0.0001) (Fig. 1). The estimated 5-year PFS for both Group 3 and 4 patients were 0%. The estimated 5-year OS for Group 3 was 0% while the estimated 5- and 10-year OS for Group 4 were 44.4% (95% CI 13.6–71.9) and 22.2% (95% CI 3.4–51.3), respectively. Median time-to-progression following initial therapy for these patients was 1.72 years (range 1.15–3.87). Three patients with Group 4 medulloblastoma received radiation therapy at the time of disease progression (Supp. Figure 3). All three patients died with the median time-to-death following radiation therapy of 2.84 years (range 2.24–4.21).
Fig. 1.
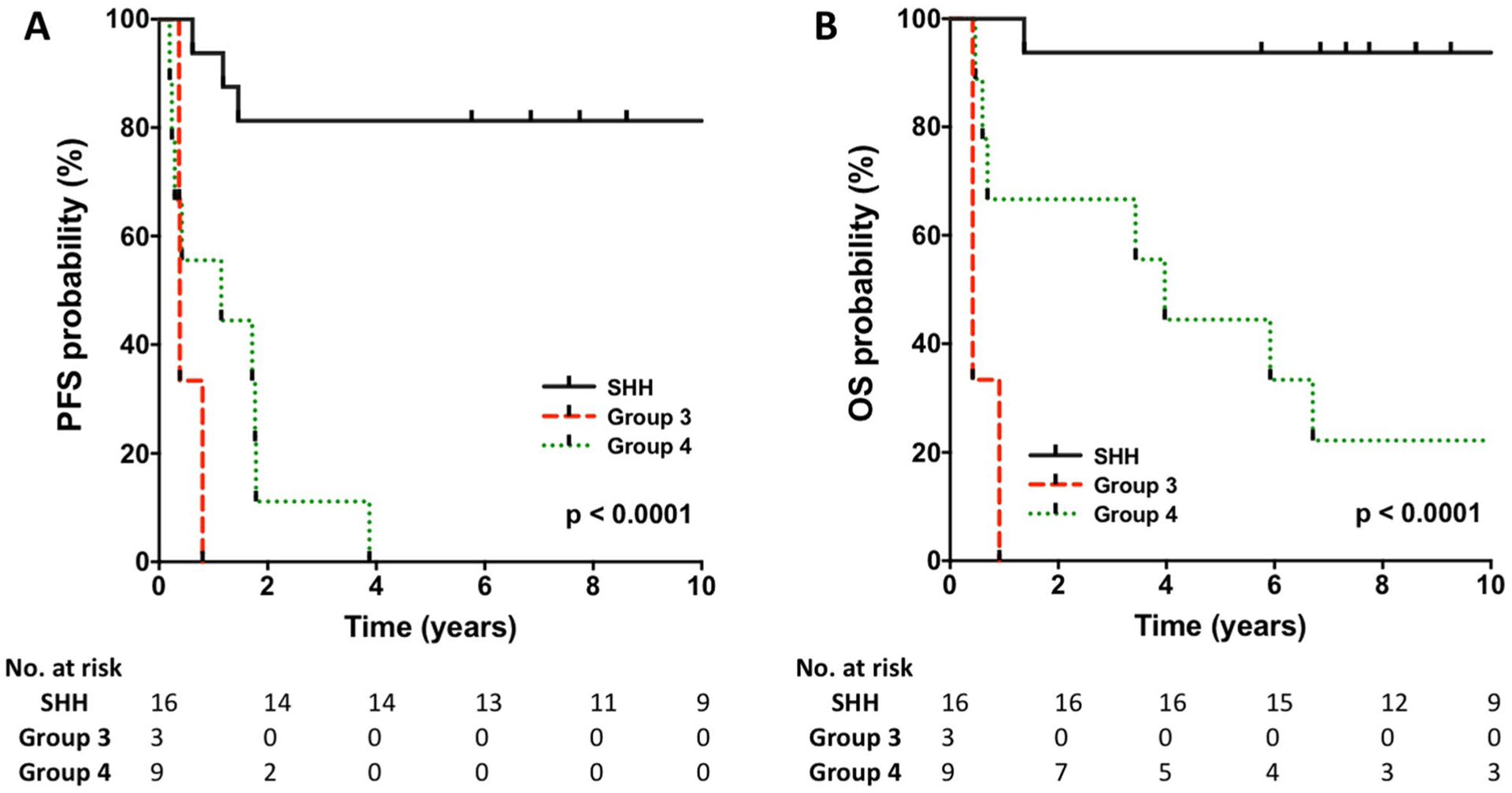
a, b Progression-free and overall survival according to molecular subgroup
With a median follow-up of 9.71 years, the estimated 10-year PFS and OS for patients with SHH medulloblastoma were 81.2% (95% CI 52.5–93.5) and 93.7% (95% CI 63.2–99.1), respectively. Among the SHH subgroup, there were no significant differences in outcome between patients with localized and disseminated disease [p-value = 0.39 (PFS) and 0.63 (OS)], as well as no difference in the outcome between patients with classic and desmoplastic/nodular histology [p-value = 0.53 (PFS) and 0.32 (OS)] (Fig. 2a, b). Regardless of extent of resection, all patients with SHH medulloblastoma had OS rates of above 90% (Supp. Figure 2). Three patients with SHH medulloblastoma relapsed and received radiation therapy. Median time-to-progression for these three patients was 1.18 years (range 0.62–1.46). Two of the three are long term survivors while the other patient progressed post-radiation and died 9 months later (Supp. Fig. 3).
Fig. 2.
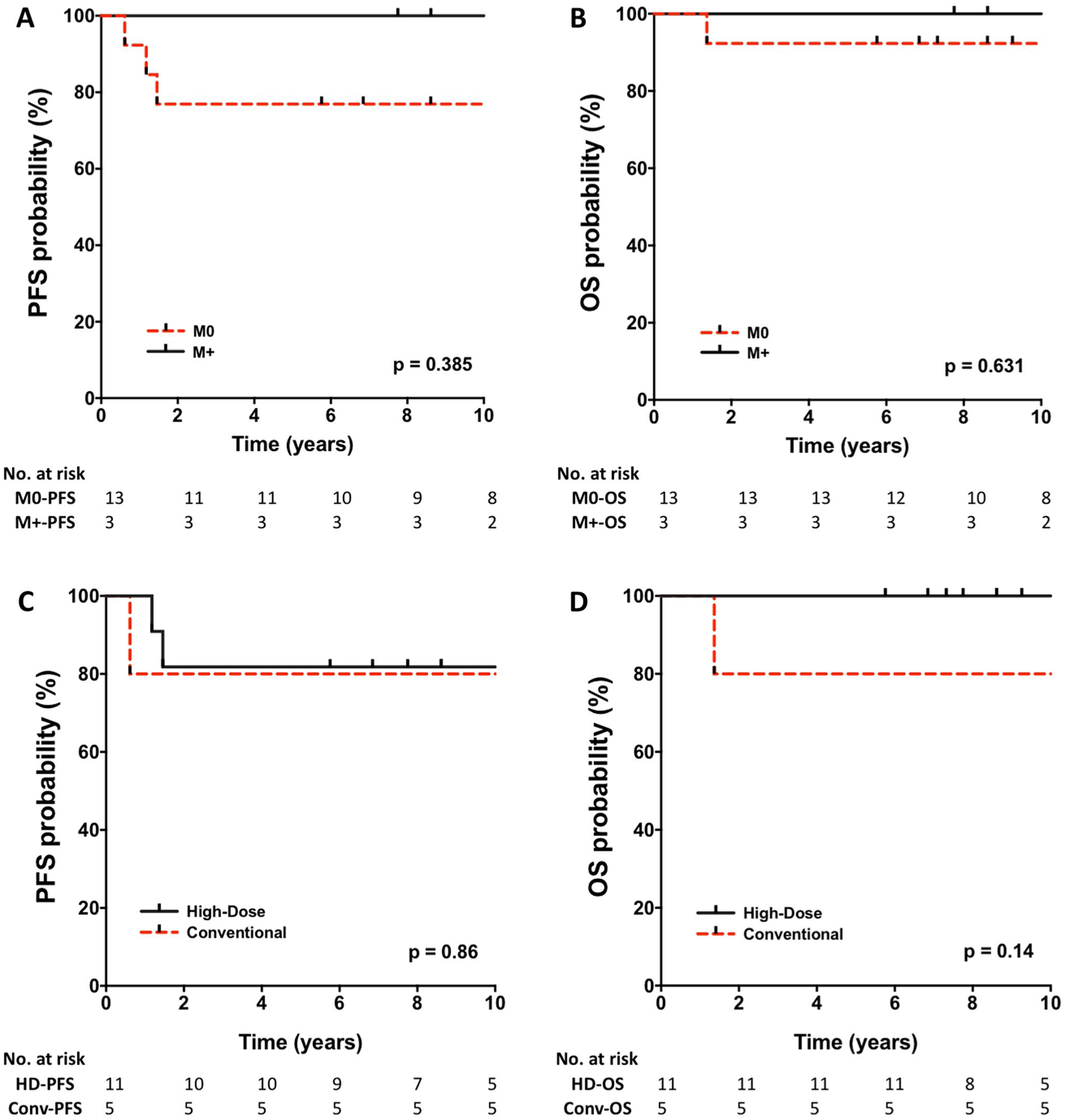
Progression-free and overall survival of patients with SHH medulloblastoma according to a, b disease stage and c, d chemotherapy regimen
Discussion
In this review, we summarize our experience treating infants and young children with medulloblastoma using irradiation-sparing regimens. We correlate the survival with histological subtype, metastatic stage, extent of resection and molecular subgroup. To our knowledge, this is one of only a few reports in the literature correlating molecular subgroup and outcomes of a large cohort of young children with medulloblastoma treated with chemotherapy-only regimens [21, 23]. Given the rarity of infant medulloblastoma and the challenges associated with irradiation-avoiding strategies, this review has broad clinical relevance as it validates previous reports supporting the continued consideration of irradiation-sparing regimens in the treatment of young children with SHH medulloblastoma and highlights the urgent need for intensification, augmentation and addition of new therapies in others.
Overall, the results for our irradiation-sparing cohort are reflective of previously published reports, with long-term OS of approximately 60–70 [17–21]. In particular, desmoplastic medulloblastoma was found in our cohort to have had excellent outcome, with a 10-year OS of 87.5% similar to previously published reports [28, 34, 35]. It is note-worthy that while we found a trend towards better survival among patients with desmoplastic medulloblastoma (vs classic histology), it did not meet significance likely due to the relatively small number of patients. More importantly, we found that patients with SHH subgroup had excellent outcome when treated with irradiation-sparing chemotherapeutic regimens (10-year OS > 90%) regardless of histology, while the vast majority of recurrences and deaths occurred in patients with Group 3/4 medulloblastoma.
All nine patients with Group 4 medulloblastoma had disease progression following/during initial therapy. Three patients received radiation therapy at time of progression; however, all ultimately died of disease. Robinson et al. also reported dismal prognosis for children < 3 years of age with Group 4 medulloblastoma treated on SJYC07, where the majority received upfront chemotherapy followed by focal irradiation [23]. In contrast, several meta-analyses have shown the overall survival of children with Group 4 medulloblastoma to be approximately 60–70% [6, 22]. While the specific treatment regimens are unknown in these analyses, given the known age distribution of Group 4 medulloblastoma (predominantly > 3 years of age), the majority of these patients would have likely received craniospinal irradiation followed by maintenance chemotherapy [6, 22]. These results suggest that chemotherapy-alone approaches are insufficient to cure young children with Group 4 medulloblastoma.
Radiation therapy was a successful salvage therapy in two of the three patients with SHH medulloblastoma who relapsed. Taken together, our data suggest the salvageability of infant SHH medulloblastoma with radiation therapy in the relapsed setting and supports the use of chemotherapy-only approach as upfront therapy for patients with infant SHH medulloblastoma as an irradiation-sparing or at least irradiation delaying strategy. These data confirms the aggressive nature of Group 3/4 infant medulloblastoma, suggests that radiation therapy is an important component of treatment and highlights the need for new innovative therapeutic options for these subgroups of patients.
Interestingly, three (2 classic, 1 desmoplastic/nodular) out of the 16 SHH patients presented with metastatic disease, with two patients having spinal disease. All three patients are long-term irradiation-free, progression-free survivors of their disease. In addition, four patients had significant residual disease (> 1.5 cm2) after initial STR, which is considered to be a significant high-risk feature in medulloblastoma [36, 37]. All four patients are long-term irradiation-free, progression-free survivors of their disease, with extent of resection not found to be a significant prognostic factor among patients with SHH medulloblastoma. Despite the small number, these examples are highly encouraging, as it highlights the chemo-sensitivity of infant SHH medulloblastoma and suggests that the criteria for risk stratification of young children with SHH medulloblastoma may be distinct from the conventional criteria used to risk stratify patients with medulloblastoma.
As previously mentioned, Robinson et al. recently reported the results of their phase II clinical trial for young children with medulloblastoma (SJYC07) treated with a risk-adapted strategy. Using methylation analysis, they described two distinct subtypes of infant SHH. These two groups (iSHH-I and iSHH-II) had significantly different 5-year PFS (iSHH-I = 27.8% vs iSHH-II = 75.4%) [23]. This observation was similarly described by Lafay-Cousin et al. on behalf of the COG at the International Symposium on Pediatric Neuro-Oncology (ISPNO) 2018, where the preliminary results of COG ACNS1221 showed a 2-year event-free survival of 27.3% and 73.3% for children with iSHH-I and iSHH-II medulloblastoma, respectively [24].
Our 31-gene panel is not able to distinguish between the two subtypes of infant SHH medulloblastoma. However, given the reported 1:1.2 ratio of iSHH-I and iSHH-II in infants with SHH medulloblastoma and our relatively sizable cohort of patients, it is reasonable to hypothesize that our cohort contained both subtypes. The exceptional outcome of infant SHH seen in our cohort is therefore of critical importance, as it supports the continued use of chemotherapy alone in these patients. Specifically, given that most of the patients in our SHH cohort were treated with high-dose chemotherapy, our result substantiates the use of induction chemotherapy followed by consolidative HDC with AuSCR in these patients. Additionally, it was recently reported that the majority of patients who relapsed from the aforementioned COG ACNS1221 study were successfully salvaged with high-dose chemotherapy. This further affirms that ability of intensive chemotherapy to successfully treat infant SHH [38].
We recognize that this study has several limitations, most of which are related to it being a single-institution retrospective review. The number of patients include in this study is limited largely by the rarity of the disease, as well as the single-institutional nature of this study. Additionally, treatment strategies in this study are heterogeneous and include both high-dose chemotherapy and non-high dose chemotherapy regimens. While this was inevitable given the varied therapeutic regimens utilized during different treatment era, we acknowledge that the difference in regimens may have had an impact on outcome. As mentioned above, given the emergence of the additional subtype data recently published on iSHH-I and iSHH-II, we recognize that our outcome data on SHH would be substantially more robust if we were able to analyze our cohort based on these findings.
Lastly, we acknowledge the lack of TP53 status on our cohort of patients with SHH-medulloblastoma. While we recognize TP53 as a critical prognostic factor in SHH medulloblastoma, patients with TP53-mutant SHH medulloblastoma are typically older children who receive radiation therapy as upfront therapy. In particular, our cohort specifically addresses the treatment of infant SHH medulloblastoma, a group known to be predominantly TP53 wildtype. This was demonstrated by Robinson et al. in the SJYC07 clinical trial, where the molecular landscape of 83 infant medulloblastoma revealed TP53 genetic alteration in 2–3% of patients [23]. Given the extremely low frequency of TP53 aberration in this population, it does not seem to be substantially relevant in the treatment of infant SHH medulloblastoma.
Despite the significant advancement in our understanding of key cellular pathways and genetic drivers associated with medulloblastoma subgroups over the past decade, the development and translation of new therapeutic agents into clinical care has been relatively slow. With the exceptions of WNT and SHH medulloblastoma, investigations into specific treatment for other molecular subgroups have not been developed. It is therefore imperative to understand and evaluate the efficacy of our previous treatment regimens based on molecular findings, with the goal of adapting and optimizing our currently available treatments based on molecular subgroups. This retrospective review provides this critical insight and validates the prognostic significance of molecular subgroups in infant medulloblastoma.
Conclusions
In this review, we describe the superior outcomes of young children with SHH medulloblastoma treated with irradiation-sparing regimens. In contrast, Group 3/4 patients have significantly poorer outcomes with these regimens, with many patients dying of disease despite attempted salvage with radiation therapy and/or additional chemotherapy. Our results support the use of chemotherapy only strategies for young children with SHH medulloblastoma. In addition, our study demonstrates the urgent need for intensification, augmentation and/or addition of new therapeutic options for young patients with Group 3/4 medulloblastoma.
Supplementary Material
Funding
This work was supported by a Grant to SA from the American Cancer Society, Alex’s Lemonade Stand Foundation, and Soccer for Hope Foundation.
Footnotes
Electronic supplementary material The online version of this article (doi:https://doi.org/10.1007/s11060-019-03307-8) contains supplementary material, which is available to authorized users.
Conflict of interest The authors report no conflicts of interest.
References
- 1.Farwell JR, Dohrmann GJ, Flannery JT (1984) Medulloblastoma in childhood: an epidemiological study. J Neurosurg 61:657–664. 10.3171/jns.1984.61.4.0657 [DOI] [PubMed] [Google Scholar]
- 2.McKean-Cowdin R, Razavi P, Barrington-Trimis J, Baldwin RT, Asgharzadeh S, Cockburn M, Tihan T, Preston-Martin S (2013) Trends in childhood brain tumor incidence, 1973–2009. J Neurooncol 115:153–160. 10.1007/s11060-013-1212-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Packer RJ, Vezina G (2008) Management of and prognosis with medulloblastoma: therapy at a crossroads. Arch Neurol 65:1419–1424. 10.1001/archneur.65.11.1419 [DOI] [PubMed] [Google Scholar]
- 4.Northcott PA, Dubuc AM, Pfister S, Taylor MD (2012) Molecular subgroups of medulloblastoma. Expert Rev Neurother 12:871–884. 10.1586/ern.12.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM (2012) Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 123:465–472. 10.1007/s00401-011-0922-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavalli FMG, Remke M, Rampasek L, Peacock J, Shih DJH, Luu B, Garzia L, Torchia J, Nor C, Morrissy AS, Agnihotri S, Thompson YY, Kuzan-Fischer CM, Farooq H, Isaev K, Daniels C, Cho BK, Kim SK, Wang KC, Lee JY, Grajkowska WA, Perek-Polnik M, Vasiljevic A, Faure-Conter C, Jouvet A, Giannini C, Nageswara Rao AA, Li KKW, Ng HK, Eberhart CG, Pollack IF, Hamilton RL, Gillespie GY, Olson JM, Leary S, Weiss WA, Lach B, Chambless LB, Thompson RC, Cooper MK, Vibhakar R, Hauser P, van Veelen MC, Kros JM, French PJ, Ra YS, Kumabe T, Lopez-Aguilar E, Zitterbart K, Sterba J, Finocchiaro G, Massimino M, Van Meir EG, Osuka S, Shofuda T, Klekner A, Zollo M, Leonard JR, Rubin JB, Jabado N, Albrecht S, Mora J, Van Meter TE, Jung S, Moore AS, Hallahan AR, Chan JA, Tirapelli DPC, Carlotti CG, Fouladi M, Pimentel J, Faria CC, Saad AG, Massimi L, Liau LM, Wheeler H, Nakamura H, Elbabaa SK, Perezpena-Diazconti M, Ponce Chico, de Leon F, Robinson S, Zapotocky M, Lassaletta A, Huang, Hawkins CE, Tabori U, Bouffet E, Bartels U, Dirks PB, Rutka JT, Bader GD, Reimand J, Goldenberg A, Ramaswamy V, Taylor MD (2017) Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell 31(737–754):e736 10.1016/j.ccell.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Northcott PA, Buchhalter I, Morrissy AS, Hovestadt V, Weischenfeldt J, Ehrenberger T, Grobner S, Segura-Wang M, Zichner T, Rudneva VA, Warnatz HJ, Sidiropoulos N, Phillips AH, Schumacher S, Kleinheinz K, Waszak SM, Erkek S, Jones DTW, Worst BC, Kool M, Zapatka M, Jager N, Chavez L, Hutter B, Bieg M, Paramasivam N, Heinold M, Gu Z, Ishaque N, Jager-Schmidt C, Imbusch CD, Jugold A, Hubschmann D, Risch T, Amstislavskiy V, Gonzalez FGR, Weber UD, Wolf S, Robinson GW, Zhou X, Wu G, Finkelstein D, Liu Y, Cavalli FMG, Luu B, Ramaswamy V, Wu X, Koster J, Ryzhova M, Cho YJ, Pomeroy SL, Herold-Mende C, Schuhmann M, Ebinger M, Liau LM, Mora J, McLendon RE, Jabado N, Kumabe T, Chuah E, Ma Y, Moore RA, Mungall AJ, Mungall KL, Thiessen N, Tse K, Wong T, Jones SJM, Witt O, Milde T, Von Deimling A, Capper D, Korshunov A, Yaspo ML, Kriwacki R, Gajjar A, Zhang J, Beroukhim R, Fraenkel E, Korbel JO, Brors B, Schlesner M, Eils R, Marra MA, Pfister SM, Taylor MD, Lichter P (2017) The whole-genome landscape of medulloblastoma subtypes. Nature 547:311–317. 10.1038/nature22973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 9.Northcott PA, Korshunov A, Pfister SM, Taylor MD (2012) The clinical implications of medulloblastoma subgroups. Nat Rev Neurol 8:340–351. 10.1038/nrneurol.2012.78 [DOI] [PubMed] [Google Scholar]
- 10.Robinson GW, Orr BA, Wu G, Gururangan S, Lin T, Qaddoumi I, Packer RJ, Goldman S, Prados MD, Desjardins A, Chintagumpala M, Takebe N, Kaste SC, Rusch M, Allen SJ, Onar-Thomas A, Stewart CF, Fouladi M, Boyett JM, Gilbertson RJ, Curran T, Ellison DW, Gajjar A (2015) Vismodegib exerts targeted efficacy against recurrent sonic hedgehog-subgroup medulloblastoma: results from phase II pediatric brain tumor consortium studies PBTC-025B and PBTC-032. J Clin Oncol 33:2646–2654. 10.1200/JCO.2014.60.1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieran MW, Chisholm J, Casanova M, Brandes AA, Aerts I, Bouffet E, Bailey S, Leary S, MacDonald TJ, Mechinaud F, Cohen KJ, Riccardi R, Mason W, Hargrave D, Kalambakas S, Deshpande P, Tai F, Hurh E, Geoerger B (2017) Phase I study of oral sonidegib (LDE225) in pediatric brain and solid tumors and a phase II study in children and adults with relapsed medulloblastoma. Neuro Oncol 19:1542–1552. 10.1093/neuonc/nox109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen K, Bandopadhayay P, Chi S, London W, Rodriguez F, Hawkins C, Yang E, Aguilera D, Castellino R, MacDonald T, Stapleton S, Ashley D (2019) MEDU-34. Pilot study of a surgery and chemotherapy-only approach in the upfront therapy of children with WNT-positive standard risk medulloblastoma. Neuro Oncol 21:ii110 10.1093/neuonc/noz036.192 [DOI] [Google Scholar]
- 13.Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, Bayer L, LaFond D, Donahue BR, Marymont MH, Muraszko K, Langston J, Sposto R (2006) Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 24:4202–4208. 10.1200/JCO.2006.06.4980 [DOI] [PubMed] [Google Scholar]
- 14.Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, Fouladi M, Broniscer A, Krance R, Hale GA, Stewart CF, Dauser R, Sanford RA, Fuller C, Lau C, Boyett JM, Wallace D, Gilbertson RJ (2006) Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 7:813–820. 10.1016/S1470-2045(06)70867-1 [DOI] [PubMed] [Google Scholar]
- 15.Tarbell NJ, Friedman H, Polkinghorn WR, Yock T, Zhou T, Chen Z, Burger P, Barnes P, Kun L (2013) High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol 31:2936–2941. 10.1200/JCO.2012.43.9984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rieken S, Mohr A, Habermehl D, Welzel T, Lindel K, Witt O, Kulozik AE, Wick W, Debus J, Combs SE (2011) Outcome and prognostic factors of radiation therapy for medulloblastoma. Int J Radiat Oncol Biol Phys 81:e7–e13. 10.1016/j.ijrobp.2010.12.042 [DOI] [PubMed] [Google Scholar]
- 17.Fangusaro J, Finlay J, Sposto R, Ji L, Saly M, Zacharoulis S, Asgharzadeh S, Abromowitch M, Olshefski R, Halpern S, Dubowy R, Comito M, Diez B, Kellie S, Hukin J, Rosenblum M, Dunkel I, Miller DC, Allen J, Gardner S (2008) Intensive chemotherapy followed by consolidative myeloablative chemotherapy with autologous hematopoietic cell rescue (AuHCR) in young children with newly diagnosed supratentorial primitive neuroectodermal tumors (sPNETs): report of the Head Start I and II experience. Pediatr Blood Cancer 50:312–318. 10.1002/pbc.21307 [DOI] [PubMed] [Google Scholar]
- 18.Geyer JR, Sposto R, Jennings M, Boyett JM, Axtell RA, Breiger D, Broxson E, Donahue B, Finlay JL, Goldwein JW, Heier LA, Johnson D, Mazewski C, Miller DC, Packer R, Puccetti D, Radcliffe J, Tao ML, Shiminski-Maher T, Children’s Cancer G (2005) Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol 23:7621–7631. 10.1200/JCO.2005.09.095 [DOI] [PubMed] [Google Scholar]
- 19.Grundy RG, Wilne SH, Robinson KJ, Ironside JW, Cox T, Chong WK, Michalski A, Campbell RH, Bailey CC, Thorp N, Pizer B, Punt J, Walker DA, Ellison DW, Machin D, Children’s C, Leukaemia Group Brain Tumour C (2010) Primary postoperative chemotherapy without radiotherapy for treatment of brain tumours other than ependymoma in children under 3 years: results of the first UKCCSG/SIOP CNS 9204 trial. Eur J Cancer 46:120–133. 10.1016/j.ejca.2009.09.013 [DOI] [PubMed] [Google Scholar]
- 20.Grill J, Sainte-Rose C, Jouvet A, Gentet JC, Lejars O, Frappaz D, Doz F, Rialland X, Pichon F, Bertozzi AI, Chastagner P, Couanet D, Habrand JL, Raquin MA, Le Deley MC, Kalifa C, French Society of Paediatric O (2005) Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol 6:573–580. 10.1016/S1470-2045(05)70252-7 [DOI] [PubMed] [Google Scholar]
- 21.Lafay-Cousin L, Smith A, Chi SN, Wells E, Madden J, Margol A, Ramaswamy V, Finlay J, Taylor MD, Dhall G, Strother D, Kieran MW, Foreman NK, Packer RJ, Bouffet E (2016) Clinical, pathological, and molecular characterization of infant medulloblastomas treated with sequential high-dose chemotherapy. Pediatr Blood Cancer 63:1527–1534. 10.1002/pbc.26042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van Meeteren A, van Vuurden D, Clifford SC, Pietsch T, von Bueren AO, Rutkowski S, McCabe M, Collins VP, Backlund ML, Haberler C, Bourdeaut F, Delattre O, Doz F, Ellison DW, Gilbertson RJ, Pomeroy SL, Taylor MD, Lichter P, Pfister SM (2012) Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol 123:473–484. 10.1007/s00401-012-0958-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson GW, Rudneva VA, Buchhalter I, Billups CA, Waszak SM, Smith KS, Bowers DC, Bendel A, Fisher PG, Partap S, Crawford JR, Hassall T, Indelicato DJ, Boop F, Klimo P, Sabin ND, Patay Z, Merchant TE, Stewart CF, Orr BA, Korbel JO, Jones DTW, Sharma T, Lichter P, Kool M, Korshunov A, Pfister SM, Gilbertson RJ, Sanders RP, Onar-Thomas A, Ellison DW, Gajjar A, Northcott PA (2018) Risk-adapted therapy for young children with medulloblastoma (SJYC07): therapeutic and molecular outcomes from a multicentre, phase 2 trial. Lancet Oncol 19:768–784. 10.1016/S1470-2045(18)30204-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lafay-Cousin L, Robinson G, Rudneva V, Northcott P, Billups C, Onar-Thomas A, Hawkins C, Eberhart C, Horbinski C, Heier L, Souweidane M, Strother D, Fouladi M, Bouffet E, Gajjar A (2018) MBCL-08. Molecular characterization of nodular desmoplastic medulloblastomas in young children treated on ACNS1221. A report from the Children Oncology Group. Neuro Oncol 20:i118–i119. 10.1093/neuonc/noy059.406 [DOI] [Google Scholar]
- 25.Dhall G, Grodman H, Ji L, Sands S, Gardner S, Dunkel IJ, McCowage GB, Diez B, Allen JC, Gopalan A, Cornelius AS, Termuhlen A, Abromowitch M, Sposto R, Finlay JL (2008) Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer 50:1169–1175. 10.1002/pbc.21525 [DOI] [PubMed] [Google Scholar]
- 26.Cohen BH, Geyer JR, Miller DC, Curran JG, Zhou T, Holmes E, Ingles SA, Dunkel IJ, Hilden J, Packer RJ, Pollack IF, Gajjar A, Finlay JL, Children’s Oncology G (2015) Pilot study of intensive chemotherapy With peripheral hematopoietic cell support for children less than 3 Years of age with malignant brain tumors, the CCG-99703 phase I/II study. A report From the Children’s Oncology Group. Pediatr Neurol 53:31–46. 10.1016/j.pediatrneurol.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finlay JL, Boyett JM, Yates AJ, Wisoff JH, Milstein JM, Geyer JR, Bertolone SJ, McGuire P, Cherlow JM, Tefft M et al. (1995) Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Childrens Cancer Group J Clin Oncol 13:112–123. 10.1200/JCO.1995.13.1.112 [DOI] [PubMed] [Google Scholar]
- 28.Dhall G, Ji L, Haley K, Gilles F, Gardner S, Sposto R, Finlay JL (2017) P11.02 long-term outcome of infants and young children with newly diagnosed non-nodular/desmoplastic medulloblastoma treated on “head start” III protocol. Neuro Oncol 19:iii91–iii92. 10.1093/neuonc/nox036.347 [DOI] [Google Scholar]
- 29.Duffner PK, Horowitz ME, Krischer JP, Friedman HS, Burger PC, Cohen ME, Sanford RA, Mulhern RK, James HE, Freeman CR et al. (1993) Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med 328:1725–1731. 10.1056/NEJM199306173282401 [DOI] [PubMed] [Google Scholar]
- 30.Allen J, Donahue B, Mehta M, Miller DC, Rorke LB, Jakacki R, Robertson P, Sposto R, Holmes E, Vezina G, Muraszko K, Puccetti D, Prados M, Chan KW (2009) A phase II study of pre-radiotherapy chemotherapy followed by hyperfractionated radiotherapy for newly diagnosed high-risk medulloblastoma/primitive neuroectodermal tumor: a report from the Children’s Oncology Group (CCG 9931). Int J Radiat Oncol Biol Phys 74:1006–1011. 10.1016/j.ijrobp.2008.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geyer RBM, Allen J, Finlay J, Jakacki R, Jennings M, Kurcznski E, Lange B, Mazewski C, Packer R, Villablanca JG, Willoughby M (1993) Intensive chemotherapy pilot for infants with malignant brain tumors. Proc Am Soc Clin Oncol 12:3 [Google Scholar]
- 32.Margol AS, Robison NJ, Gnanachandran J, Hung LT, Kennedy RJ, Vali M, Dhall G, Finlay JL, Erdreich-Epstein A, Krieger MD, Drissi R, Fouladi M, Gilles FH, Judkins AR, Sposto R, Asgharzadeh S (2015) Tumor-associated macrophages in SHH subgroup of medulloblastomas. Clin Cancer Res 21:1457–1465. 10.1158/1078-0432.CCR-14-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan EL, Meier P (1958) Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc 53:457–481. 10.2307/2281868 [DOI] [Google Scholar]
- 34.Bouffet E (2010) Medulloblastoma in infants: the critical issues of the dilemma. Curr Oncol 17:2–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leary SE, Zhou T, Holmes E, Geyer JR, Miller DC (2011) Histology predicts a favorable outcome in young children with desmoplastic medulloblastoma: a report from the children’s oncology group. Cancer 117:3262–3267. 10.1002/cncr.25856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albright AL, Wisoff JH, Zeltzer PM, Boyett JM, Rorke LB, Stanley P (1996) Effects of medulloblastoma resections on outcome in children: a report from the Children’s Cancer Group. Neurosurgery 38:265–271 [DOI] [PubMed] [Google Scholar]
- 37.Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, Allen JC, Stevens KR, Stanley P, Li H, Wisoff JH, Geyer JR, McGuire-Cullen P, Stehbens JA, Shurin SB, Packer RJ (1999) Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol 17:832–845. 10.1200/JCO.1999.17.3.832 [DOI] [PubMed] [Google Scholar]
- 38.Graham RT, Conley S, Finlay JL, AbdelBaki MS (2018) MBCL-06. Successful salvage of desmoplastic nodular medulloblastoma patients treated on ACNS1221. Neuro Oncol 20:i118–i118. 10.1093/neuonc/noy059.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


