Summary:
Immune cell–derived exosomes can increase immunity against tumors. In contrast, tumor-derived exosomes can reduce the immunity and can change the tumor microenvironment to further develop and provide metastasis. These effects take place by an alteration in the innate and adaptive immune cell functions. In this experiment, we studied the natural killer (NK) cells’ effectiveness on tumor cells after expansion and thereafter incubated it with exosomes. The exosomes were derived from 2 populations of NK cells: (1) naive NK cells and, (2) NK cells previously exposed to neuroblastoma (NB) cells. Moreover, we have studied the NB-derived exosomes on NK cell function. The molecular load of the characterized exosomes (by means of nanoparticle-tracking analysis, flow cytometry, scanning electron microscopy, and western blot) from NK cells exposed to the NB cell revealed their expression of natural killer cell receptors in addition to CD56, NKG2D, and KIR2DL2 receptors. These exosomes were used to treat NK cells and thereafter administered to NB tumor cells both in vitro and in vivo. Our results showed some kind of NK cells’ education by the exosomes. This education from NK cells previously exposed to NB cell–derived exosomes caused efficient and greater cytotoxicity against NB tumors, but NB-derived exosomes act as tumor promoters by providing a tumor supporting niche. Hence, this method of preparing the exosomes has a dramatic effect on activation of anti-NK cells against NB cells.
Keywords: cancer therapy, exosome, immune-cell therapy, natural killer cell, neuroblastoma, peripheral blood
Natural killer (NK) cells as the innate immune lymphocytes are able to evade infected or transformed cells in an major histocompatibility complex independent situation.1 They are popular targets when it comes to cellular-based immunotherapies. There have been many experiences with the NK cells2–4 that have been carried out to treat hematologic malignancies (acute myeloid leukemia, acute lymphoblastic Leukemia multiple myelomas), solid tumors [neuroblastoma (NB), lung cancer, hepatocellular carcinoma], and nonmalignant disorders such as thrombocytopenic purpura and psoriasis. The number of clinical trials with the NK cell exceeds 150 trials until the March 2017 (clinicaltrials.gov).
Because of the low number of NK cells in the peripheral or cord blood, immune cell therapy with the NK cell requires an ex vivo expansion process to achieve clinically relevant numbers of reactive cells that express activation markers, natural cytotoxicity receptors (NKG2D, NKp30, NKp44, NKp46 …), and chemokine receptors.5,6 Interleukins (ILs) can activate NK cells, depending on the cell maturation stage and/or concentrations of the costimulatory factors.7 Among them, IL-21 enhances the effector functions of NK cells but unlike IL-15 limits the expansion of NK cells. In contrast, it can stimulate cytotoxicity and interferons (IFN)-γ secretion in previously activated cells8 or induce cytotoxicity in newly isolated NK cell alone.9 The combination of IL-21 and IL-15 can expand NK cells from bone marrow, cord blood, and peripheral blood.10 Many researchers have focused on successful expanding of NK cells ex vivo under Good manufacturing Practice conditions for clinical immunotherapy. Besides the expansion of NK cells, the activation methods also have a lot of importance.
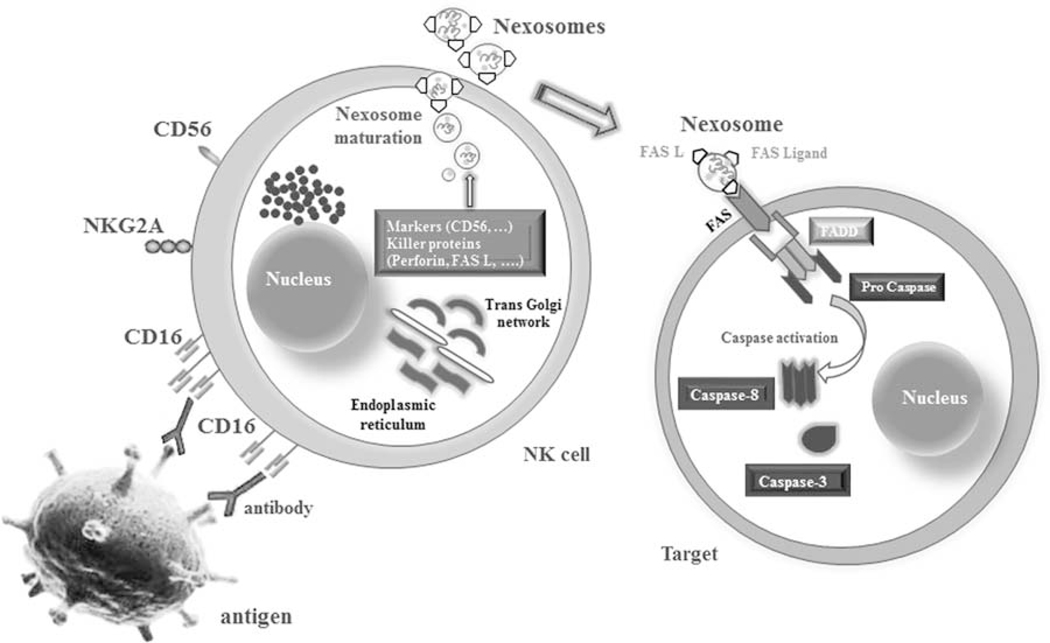
Today exosomes are attracting great attention on their role in the oncoimmunology area. These are vesicles naturally released by many of the cells as well as tumor cells to deliver several molecules to target cells.11 They act as signaling vectors between the donor cytosol and the internal compartments of a target cell.12 Tumor exosomes have crosstalk with immune cells and can attenuate them by downregulation of their antitumor activity through several ligands.13–16 However, immune cells like NKs can release exosomes that express typical NK markers (ie, CD56), killer proteins (ie, FASL and perforin) (Fig. 1), and stimulate antitumor activities.11,17 We want to name these NK cell–derived exosomes as Nexosomes. Nexosomes have a control on the cell expansion upon activation through intrinsic or extrinsic signals. Moreover, nexosome-entrapped perforin may constitute a new target to develop a direct therapy against many kinds of cancers in addition to activating other populations of NK cells against tumors. As our group works on NB and because the NK therapy has an indication for NB, we decided to use nexosomes against NB cells for better outcomes of cytotoxicity against this most prevalent extracranial solid tumor in children. We hypothesized that the NK cells exposed to NB cells can form nexosomes capable of transferring their content to the population of naive NKs to exert a strong cytotoxic effect on target cells. In fact, we thought that these NK cells can transfer the kind of experience characteristic of NK cells with a memory of target cells to naive cells to educate them for targeted therapy. This activation could help to overcome the resistance of malignant cells against immunity.
FIGURE 1.
The schematic image shows the probable process with which nexosomes (NK-derived exosomes) interact with target cells. Exosomes originate from multivesicular bodies that are produced by endoplasmic reticulum and thereafter by the Golgi apparatus. They could entrap CD markers, perforin and FAS-L, which mediate cytotoxicity by interacting with FAS on the membrane of exposed cells. The acidic pH of the tumor microenvironment may impair the antitumor immune response but favors the delivery of exosomes and promotes membrane fusion. CD16 (FcγRIII) on NK cells binds the Fc-part of antibodies coating target cells and thereby leads to an activation and lysis process. NK cell indicates natural killer cell.
MATERIALS AND METHODS
NK Isolation and Expansion
Primary rest NK cells were separated from peripheral blood mononuclear cells (50mL; from healthy donors). The mononuclear cells were purified using Ficoll Paque density gradient centrifugation with 15mL falcons (BD, CA). Cell sorting from peripheral blood mononuclear cell was performed using the antibody-conjugated magnetic beads (Miltenyi Biotech, Gladbach, Germany) by negative selection kits, according to the manufacturer’s instructions. Twenty million mononuclear cells were used as the input count of cells for all the bead sorting. Mononuclear cells underwent 2 rounds of sorting through CD3 and thereafter CD14 beads (2mL beads/1 million cells). Flow cytometry was used to assess enrichment populations (more than 95% purity). The process was followed by expansion and activation in XVIVO-20 medium (Lonza, Barcelona, Spain) supplemented with 10% fetal bovine serum (Gibco, UK), 500IU/mL IL-2 (Promokine, Germany), 10ng/mL IL-12 (Promokine), 100ng/mL Galactosyl ceramide, 50ng/mL IL-15 (Promokine), 1μM Valproic acid, and 10ng/mL IL-21 (Promokine) for about 5 days.
Phenotypic Determination of NK
Totally 20 million cells were stained in the dark room for 15 minutes on ice and thereafter washed with 5% bovine serum albumin (Promokine) in phosphate buffered saline (PBS, Gibco, UK). Propidium iodide (Sigma, CA), which intercalates the DNA of dead cells, was added before analyzing them to exclude dead cells. Cells were analyzed at the BD Accuri C6 cytometer Software (BD Bioscience). Phycoerythrin -conjugated anti-CD56, FITC-conjugated anti-CD3, anti-CD19, and anti-CD14, and allophycocyanin-conjugated anti-CD116 were prepared from BD (San Diego, CA). allophycocyanin-conjugated CD16, NKp30 (CD337), NKp44 (CD336), NKp46 (CD335), L-selectin (CD62), LFA-1, HLA-DR, CD160, granzyme A (gzm A), gzm B, KIR2DL1, and NKG2D (CD314) antibodies were obtained from Miltenyi Biotech. The expression of these markers before and after expansions and activation was analyzed by flow cytometry at the BD Accuri C6 cytometer Software (BD Bioscience).
NB Cell Culture
All methods were carried out in the experiments with human cell lines in accordance with the ethical committee of the Tehran University of Medical School. The SK-N-SH (ATCC-HTB11TM) and CHLA-255 (DSMZ-Germany) NB cell lines used in this study were cultivated in Roswell Park Memorial Institute medium supplemented with 50 μg/mL penicillin-streptomycin antibiotics, 2mM L-Glutamine, and 5% fetal bovine serum at 37°C with 5% CO2. The cell viability was measured using trypan blue (Merck, Germany) staining.
Exosome Preparation from NK and NB Cells
The cytokine-activated NKs (CANKs) were cultured with NB cell lines at the ratio 1:1. After 6 hours the NK cells were harvested from the culture by centrifugation process and purified by an MACS column (Miltenyi Biotec), according to the manufacturer’s instructions. Thereafter, the purified NKs were transferred into a serum-free medium for 24 hours. Exosomes from the supernatants were then purified by 2 centrifugation processes: 500g (5min) and 1500g (10min) to eliminate cells and debris and one ultracentrifuge step at 80,000g (Beckman Coulter, 60Ti rotor) for 100 minutes to pellet the exosomes. Before the next ultracentrifuge step, cells were passed through filters to deplete larger particles. The filtration process was sequentially continued through the 0.45, 0.22, and 0.10 μm filters. The last filtrate was collected using pipettes and individually harvested by ultracentrifugation at 100,000g for 1 hour. The exosomal pellet was washed in PBS and stored at −80°C. The exosomes from different sources were tested via flow cytometry, Nanoparticle Tracking Analysis (NTA), scanning electron microscopy (SEM), western blot (WB), and protein Assay kit (Pierce, ThermoScientific). The same process was performed to isolate the exosomes from NB cells from their 48 hours serum-free cultures in Roswell Park Memorial Institute medium-1640 for additional studies.
Characterization of Isolated Exosomes
Before incubation with the population of NKs, isolated exosomes were evaluated for their number by NTA, assuming each particle as one exosome. NTA was carried out with an NS500 nanoparticle analyzer (NanoSight, Malvern, UK) to measure the size distribution of particles. The samples were diluted in PBS between 1:500 and 1:20,000 to achieve a particle count of about 109 particles per mL. Moreover, exosomes were evaluated for the determination of their morphology by SEM and for their biological activity by flow cytometry. SEM of isolated exosomes was performed as described previously.18 Briefly, isolated exosomes were put on a copper grid coated with 0.1% Formvar in chloroform. The grids were stained with 1% (vol/vol) uranyl acetate in ddH2O, and thereafter the exosomes were examined immediately. For the flow cytometry analysis, the expression of CD56, NKG2D, NKp30, NKp44, KIR2DL2, and NKp46 was checked as described in the section of phenotypic determination of the NK cell. WB was used for the detection of Apoptosis-linked gene 2-interacting protein (Alix) and Tumor Susceptibility Gene 101 (TSG101) content of exosomes from all sources as described previously.19 Briefly, 10 μg of isolated exosomes’ protein that was measured by the Bradford protein assay after resuspending the exosomes in Radio-Immunoprecipitation Assay buffer buffer containing a 1mM phenyl-methyl-sulfonyl-fluoride and protease inhibitor mixture (Roche, Mannheim, Germany) was used for this purpose. The exosome lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membrane as described previously.19 The primary antibodies used for blotting were SAB4200476 (Sigma-Aldrich) for Alix and T5701 (Sigma-Aldrich) for TSG101. These antibodies were detected using Horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies. Protein bands were visualized and analyzed using ImageLab software (Version 3.0; Bio-Rad).
Incubation of NK Cells With Exosomes
A population of NK cells was cultured in the wells of a 24-well plate at the cell concentration of 106 cells per well in triplicate. Isolated exosomes (10 μg) from NB cells, naive NK cells, and NB-exposed NK cells were added to each well, and the plates were incubated for 12 hours at 37°C. Following extensive washing in PBS to remove exosome residues, NK cells were used to test their cytotoxicity against NB cells in vitro and in vivo. NK cells cultured in the presence of cytokines, exosomes, and a combination of exosomes and cytokines were tested for their cytotoxicity against NB cell lines and cytokine release assay.
Survey of Activating and Inhibitory Receptors
To detect the receptor changes from NK cells after treatment with exosomes and/or cytokines, the antibodies against NK surface receptors were coated to the plastic wells (96 well, Greiner) for 2 hours in PBS at 37°C at 25 μg/mL. After 3 washes, mouse anti-human CD56, NKp30, NKp44, KHI2DL2, NKp46, and NKG2D-specific monoclonal antibodies (Santa Cruise) were incubated for 45 minutes at 4°C at 20 μg/mL in PBS. After 2 steps of washing, 105 cells per well were plated and stimulated for the next 8 hours in the presence of Brefeldin-A.
Cytokine Release Assay
Enzyme-linked immunosorbent assay was utilized to detect the IFN-γ and tumor necrosis factor (TNF)-α secretion of NK cells, which were activated by NK-derived exosomes, NB exosomes, cytokines, or a combination of them. A capture antibody was incubated overnight (4°C) with coating buffer in plates (Nunc, Denmark). Thereafter, the plates were washed, blocked, and incubated with 100 μL of undiluted sample and standard buffers. After 2 hours of incubation, 3 washing steps were conducted before adding the detection antibody with peroxidase. At least 5 washing steps were performed before development reaction. Ultra TMB (Thermoscientific) served as a substrate, and after 30% of change in color, sulfuric acid was added to stop the reaction. Plates were read out in the DSX Automated Four-Plate enzyme-linked immunosorbent assay System (Dynex, UK) and evaluated with DS2 Matrix software.
In Vitro Cytotoxicity Assay
The cytotoxicity of NK cells activated with exosomes in all groups was measured by lactate dehydrogenase assay kits (Roche). The procedures of the assay were followed according to the manufacturer’s instructions.20 Briefly, the culture medium was collected after treatment and applied to the kit for the assay. The reaction took place in the dark for 30 minutes before measurement. Changes in the absorbance were measured at 492 nm by a multiplate reader (Dynex, UK). Results were expressed as a percentage of control.
Nude Mice NB Modeling and Treatment
Six week-old male Naval Medical Research Institute mice weighing 20 ± 3 g (Pasteur Institute, Amol, Iran) were housed 7 per cage in 7 ventilated cages at 25°C and 50% to 60% humidity, with free access to sterile food and water. Animal protocols were approved by the Experimental Animal Care and Ethics Committee of Tehran University of Medical Sciences and Ministry of Health and Education. The methods were carried out in accordance with the approved animal experiment protocol of Tehran University of Medical Sciences. Mice were injected with dexamethasone subcutaneously (SC) and rat anti-mouse CD122 (10 μg/g) antibody intraperitoneally 1 day before the NB cell injection and then every other week to eliminate residual murine NK cells. After this period they were injected SC with 1 million SK-N-SH cells in 200 μL PBS close to the right shoulder. Three days after NB cells inoculation their water supplemented with 300 mg/L of dichloroacetic acid (Sigma). After 4 weeks the tumors began to show obvious growth. At this time the animals distributed into 5 groups, each containing 7 animals, started to receive their regimens. The first group was only injected IL-15 and IL-21 (cytokine control), the second group received CANKs (107 cells/kg), the third group was treated with the NK preexposed NB exosome–activated NK (Nx-ANKs), the fourth group received NK cells incubated with the NB-derived exosomes (NB-Ex), the fifth group was inoculated with untreated fresh NKs (untreated control), the sixth group was injected with the NKs activated in combination with ILs and Nx-ANKs (Nx-CANKs). The last group served as the naive control group for all experiments. The volume of all of the injections was 200 μL, and they were repeated thrice with a 7-day interval between injections if the animals survived. The number of NK cells injected into the mice was 10 million per kg that was suspended in the 200 μL of PBS.
Determination of Tumor Size, Weight, and Histology
The NB tumor volume in the nude mice before and after all treatments of all groups was determined by measuring its height (X), width (Y), and depth (Z) externally with a sliding caliper. Thereafter the volume was calculated according to the volume of an ellipsoid (V = 4/3π XYZ). Mice were weighed daily and killed when they showed tumor burden as an inability to stand, and loss of skin turgor. Tumors were removed, measured, and weighed. For histologic analysis, the animals were killed, and the NB xenografts were fixed with 10% buffered formalin, embedded in paraffin, and 3mm-thick sections were stained with hematoxylin and eosin as described previously.19
Statistical Analysis
The results are expressed by mean ± SEM from 3 independent experiments. Mouse survival time was defined as the length of time from the NB cells injection date until the end of the study or time of killing because of disease progression. Statistical analysis was performed using SPSS and Sigma Plot (SD were calculated). The results were interpreted as statistically significant with P-values <0.05. The t test was used to compare the means of independent samples.
RESULTS
NK Phenotype and Cytotoxicity After Expansion
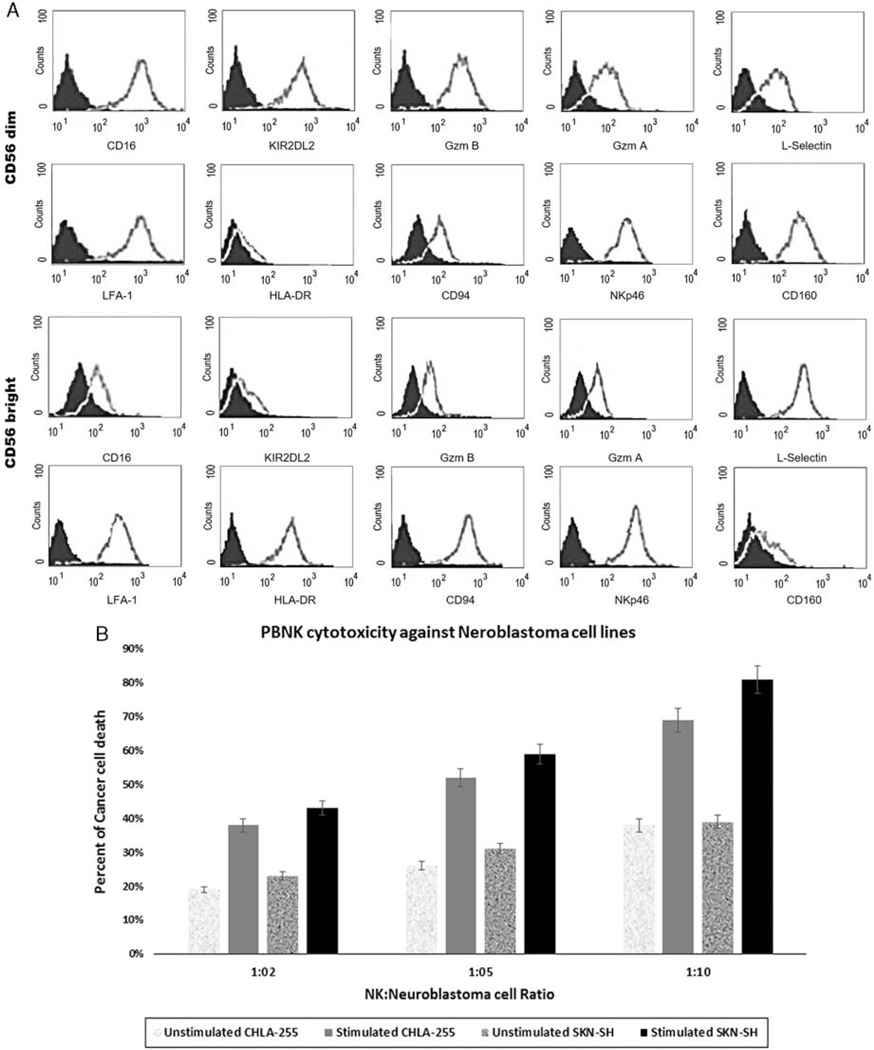
Many surface molecules such as activating, inhibitory, chemotactic, chemokine, and adhesion receptors have been described to be expressed in CD56bright and CD56dim NK cells. As shown in Figure 2A, a major subpopulation of peripheral blood natural killer cells (≥90%) belongs to the CD56dim CD16+ NK subset that expresses lytic granules such as granzymes. The remaining NK cells (≤10%) are represented by CD56bright CD16− cells, which express very low levels of granzymes and perforins (Fig. 2A). The HLA class I–specific Killer Ig–like receptors (KIR) are expressed in a considerable fraction of CD56dim CD16+ NKs (Fig. 2A), whereas the CD56bright CD16− NK subset lacks KIR (Fig. 2A). Culture with stimulating cytokines resulted in an expansion up to 100-fold after 15 days (P<0.05) and about 500-fold during a month (P<0.05) in the glass spinners. After activation with IL-21, the NK cells were tested for their cytotoxicity activity against NB cell lines (Fig. 2B). The nonactivated NK cell did not efficiently lyse NB cells even in 10:1 ratio (Fig. 2B), but the overnight treatment of IL-21 (10 ng/mL) resulted in a significant elevation in the cytotoxic activity of NKs (Fig. 2B).
FIGURE 2.
A, Phenotypes of peripheral blood natural killer cells subsets. Peripheral blood mononuclear cells were stained for CD56, CD16, granzyme A, gzm B, KIR2DL2, CD62, and CD69 and thereafter analyzed by flow cytometry. Expression of these molecules in relation to CD56 density on CD3−CD56 + NK cells is shown. B, Cytotoxicity of peripheral blood natural killer cells on the NB cell lines. Cytokine-activated NK cell did efficiently lyse NB cells, especially in the 10:1 ratio. The cytotoxicity achieved by treatment of interleukin 21 (10 ng/mL) (P < 0.05). NB indicates neuroblastoma; NK cell, natural killer cell.
Exosomes′ Characteristics
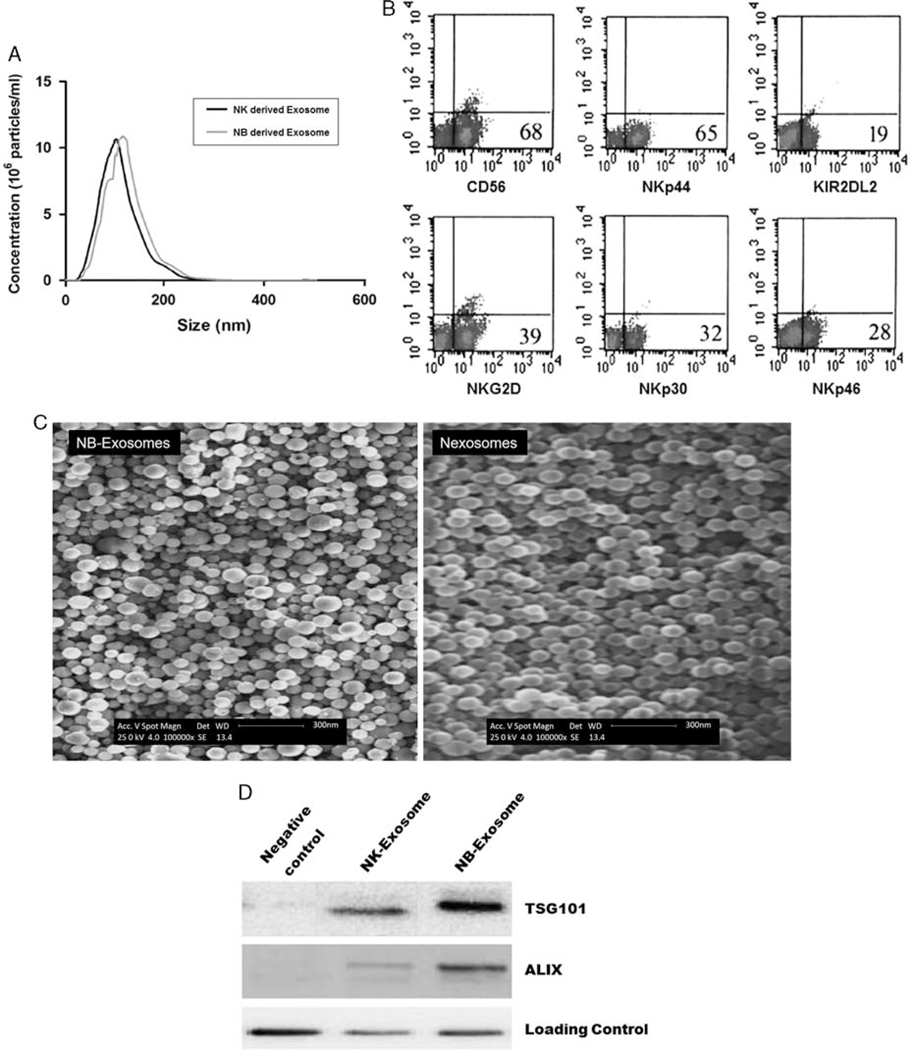
The NTA results revealed an exosome size distribution from 40 to 150nm with much frequency at 100nm that reflected the suitable filtration and centrifugation processes (Fig. 3A). These exosomes expressed NKp30, NKG2D, NKp44, and NKp46 natural killer cell receptors (NCRs). They have shown different phenotypic patterns similar to their originating cells (Fig. 3B). The freshly isolated CD56dim NK-derived exosomes showed slight expression of all NCRs especially NKp30 and NKp44 (data not shown), but, after exposure to NB cells, the NK-derived exosomes expressed higher levels of NCRs such as NKp44 (65%), NKG2D (39%), and NKp30 (32%) (Fig. 3B). This increase in NCRs’ content is directly related to the preexposure of NB cells. Moreover, exosomes isolated by differential filtration and ultracentrifugation steps were visualized by SEM. The NB exosomes showed a relative size variation between 40 and 150 nm, but NK exosomes were more uniform (Fig. 3C). The vesicular morphology and the size range of NK exosomes do not exceed 100 nm. The WB analysis of exosomes confirmed the exosome marker proteins Alix and Tsg101. These 2 specific exosome proteins were enriched in NB-Ex but expressed in lower levels in the NK cell–derived counterparts (Fig. 3D).
FIGURE 3.
A, Size of exosomes. Size distribution histogram of nexosomes and NB-derived exosomes as determined by nanoparticle-tracking analysis. B, The phenotype of exosomes. Nexosomes were stained for CD56, NKp30, NKG2D, KI2DL2, NKp44, and NKp46 NCRs. Interestingly exosomes show different phenotypic patterns similar to NK cells. C, Scanning Electron Microscopy of exosomes. Exosomes isolated by filtration and ultracentrifugation processes were placed on copper grids, stained with uranyl acetate, and examined. The left image shows the exosomes derived from NB cells and the right one shows the nexosomes. Note their vesicular morphology and the size range, which does not exceed 100nm. D, Western blot of exosomes. The western blot was conducted for the exosomal markers TSG101 and ALIX. The higher amount of these proteins is significant in NB-derived exosomes over nexosomes (each experiment was performed at least 3 times). NB indicates neuroblastoma; NK cell, natural killer cell.
Cytokine Release Assay
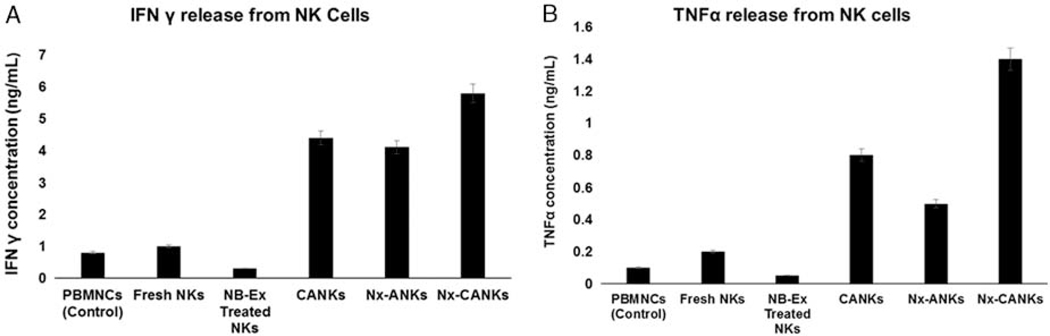
To further determine the potential antineoplastic effect of NK cells stimulated with cytokines, NB-Ex, Nx-ANKs, and Nx-CANKs, we evaluated the release of IFN-γ (Fig. 4A) and TNF-α (Fig. 4B) after coculturing treated CD56dim NK cells with CHLA-255 NB cell lines. The amount of IFN-γ in the NB-Ex–treated group decreased to 0.2ng/mL and was about one-fifth that of the control NKs (1ng/mL). In contrast, the maximum of IFN-γ production was achieved in Nx-CANKs group with 6 ng/mL (Fig. 4A). Moreover, this process was similar to the secretion levels of TNF-α (Fig. 4B). It was obvious that NB preexposed NK cells secrete exosomes that can educate the new population of NK cells to release more cytokines for better cytotoxic effects against NB tumors. NB cells alone produced a background cytokine level of <50 pg/mL in each experiment.
FIGURE 4.
(A) IFN-γ and (B) TNF-α cytokines release assay. Depicted are the results of enzyme-linked immunosorbent assay for IFN-γ and TNF-α using supernatants of NK cells after culture in different treatment or control settings. Shown are the means of triplets with SE (P < 0.05). Cytokine production of CD56dim NK after stimulation with IL-21, NB-Ex, Nx-ANKs, and Nx-CANKs. INF indicates interferons; NB, neuroblastoma; NB-Ex, NB-derived exsosomes; Nx-ANKs, NB exosome–activated NK; NK cell, natural killer cell; Nx-CANKs, NB exosome cytokine-activated NKs; TNF, tumor necrosis factor.
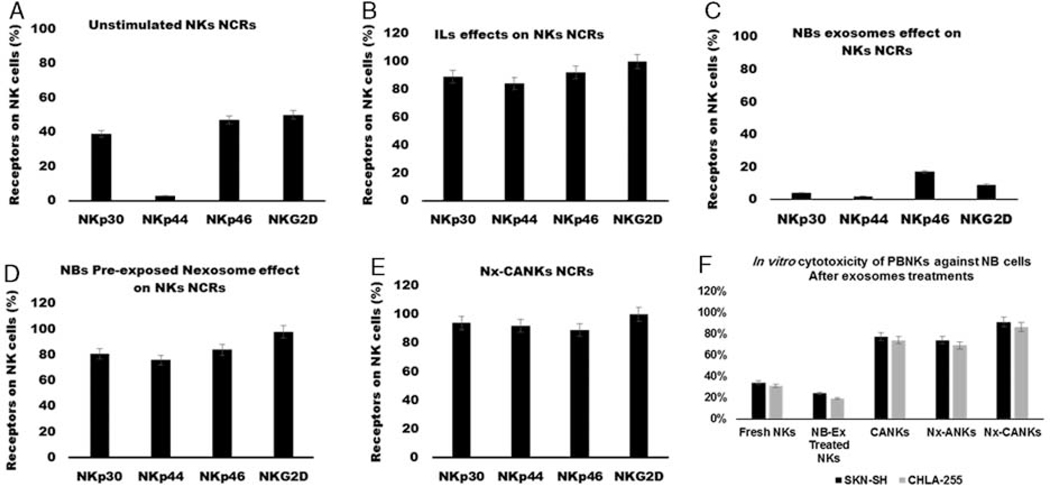
Expression of NCRs in Treated NK Cells
To kill target neoplastic cells, NK cells must express NCRs. As shown in Figures 5A–E, the exosomes could have a dramatic effect on the levels of NCRs’ expression on treated NK cells. Unstimulated NK cells can express only under 50% of their NCRs, and this level for NKp44 is just under 5% (Fig. 5A) and that means a rest situation for NKs. The cytokine cocktail activation of NKs change the rest pattern and increase the level of all tested NCRs up to 80% (Fig. 5B). The NB-Ex that carry the inhibitory signaling of tumor cells for the immune cells downregulated the NCRs to the lowest possible level. These levels for NKp30 reached under 5% and for NKp44 to 2% of the activated cells (Fig. 5C). When the NB preexposed NK exosomes were applied to the cultures, we obtained the increased levels of all tested NCRs. This pattern was similar to the effect of stimulatory cytokines (Fig. 5D). We have the most increase in all tested NCRs (between 90% and 100% of expression) in the group wherein NKs were treated with NK exosomes and cytokines (Nx-CANKs group). There was a synergistic effect between stimulatory cytokines and our obtained exosomes (Fig. 5E).
FIGURE 5.
Natural cytotoxicity receptors on NK cells. A, NK cells were stained with specific NCRs monoclonal antibodies, a resting NK cell expresses different levels of NCRs. Less than 40% of NK cells express NKp30, and the level of NKp44 is undetectable. B, Cytokine-activated NK cells express high levels of NCRs. C, NB-Ex reduces the levels of all NCRs under the resting state of NK cells. D, The Nx-ANKs induce the expression of NCRs especially NKp44 similar to CANKs. E, Nexosomes have a synergistic effect on CANKs and induce the maximum expression of all NCRs that prepare NK to have a cytotoxic function against neoplastic cells. F, In vitro cytotoxicity of peripheral blood natural killer cells against NB cells. Nexosomes strongly stimulated NK activity in the presence of IL-21. IL indicates interleukin; NB, neuroblastoma; NB-Ex, NB-derived exsosomes; NK cell, natural killer cells; Nx-ANKs, NB exosome–activated NK; Nx-CANKs, NB exosome cytokine-activated NKs.
In Vitro Cytotoxicity of Exosomes-treated NK Cells on NB Cells
As shown in Figure 5F, our NK cultures have up to 95% cytotoxicity against NB cells when incubated with NB preexposed NK-derived exosomes. These are the best cytotoxicity results from NK cells on the SK-N-SH cell line in the ratio of 10:1. The fresh isolated NKs had only been able to kill about 35% of neoplastic cells in culture condition. The condition even drops off after exposure to NB exosomes. These signal messengers decrease the cytotoxicity of NK cells under 20% of all NB cells. Standard cytokine activation of the NK cells increases the cytotoxicity up to 78% in the ratio 10:1 for SK-N-SH cell line. When the NK exosomes were added to the cytokine cocktail treatment, the NK cells killed 88% of the SK-N-SH cells. The data showed the suitable functionality of the NK exosome–treated NKs that is comparable to the standard cytokine activation method (Fig. 5F). Maybe it is an indirect evidence of the better immune synapse formation between our treated cells and targets.
In Vivo Treatment of NB Model
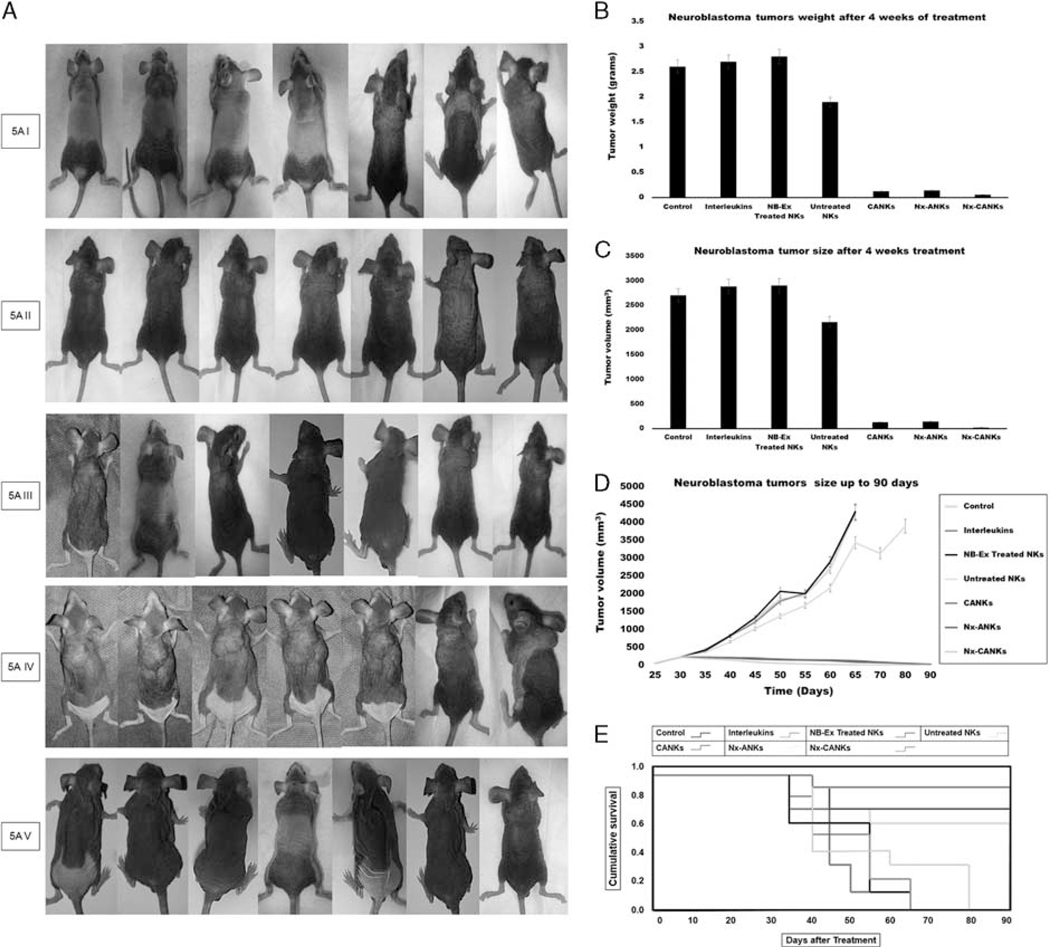
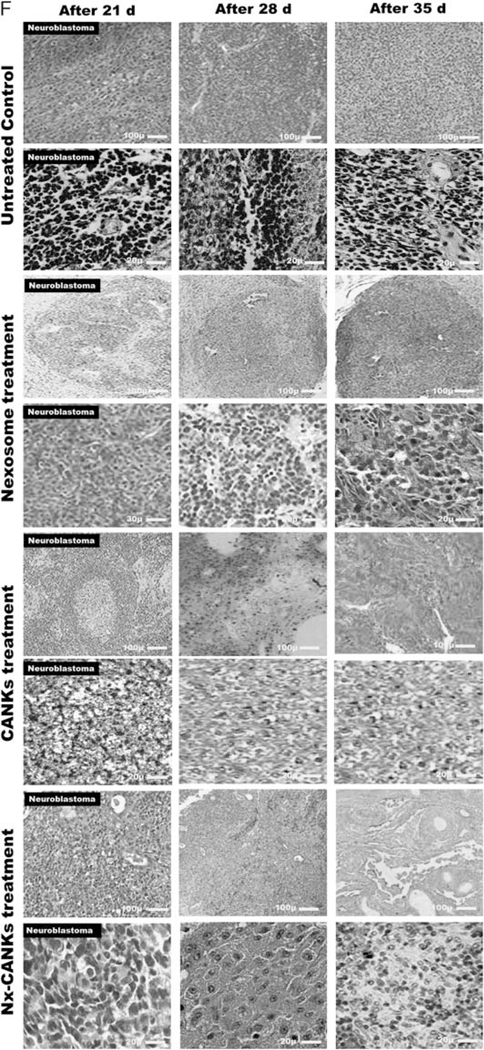
The antitumor activity of NKs in different treatment groups was tested in vivo using an SK-N-SH model of NB that was injected SC to the nude mice. The daily observation of mice detected 100% tumor formation after 4 weeks (Fig. 6A–I). In the different groups, mice were treated weekly up to four weeks by different treatment groups similar to in vitro determined groups. Beginning treatments with the fresh NKs alone was associated with reduced NB tumor growth (Fig. 6A–II), whereas tumor growth continued to increase in the control group (Fig. 6A–I). The situation gets worse after treatment with NKs exposed to NB exosomes (Fig. 6A–IV), but the tumor progression stopped after treatment with Nx-ANKs (Fig. 6A–II), and a better result was obtained after treatment with Nx-CANKs (Fig. 6A–V). In mice treated with NB exosomes, the tumor weight crossed the border of 2.81 g, whereas at the same time it was 2.59 g in the untreated control group (Fig. 6B). After 4 weeks of treatment with fresh NK the tumor weight got close to 1.94 g, but when the NK were treated with cytokine cocktails it did not even reach to 0.2 g. There was a similar result from the case of Nx-ANKs and improved results after treatment with Nx-CANKs (≤ 0.1 g; P<0.05) (Fig. 6B). The results of tumor size were similar to that of the tumor weight. In mice treated with Nx-CANKs in the fourth week, there was no detectable tumor (Fig. 6C). However, the NB exosomes resulted in tumors that had a volume of up to 3000mm3 (Fig. 6C). The mice in the untreated control group were killed on day 65 because of ethical reasons, and some of them did not reach day 65 because of tumor progression. This process was continued up to 80 days for the group that was treated with the freshly isolated NK cells (Fig. 6D). There was an increasing survival time for CANKs, Nx-ANKs, and Nx-CANKs groups for up to 90 days after tumor formation (Fig. 6D). Tumor progression in untreated mice was significantly greater than that of all nexosome-treated groups (P<0.05), but the NB-Ex treatment prepares the best environment for tumor growth and metastasis. This significant difference in mice survivals can be seen at the Kaplan-Meier plot of 7 different groups of NB tumor–bearing mice (*P<0.05) (Fig. 6E). The 90-day survival in mice models that were treated with only cytokines or untreated controls was zero. In contrast, the survival rate for Nx-ANKs, CANKs, and Nx-CANKs groups were 56%, 71%, and 86%, respectively (*P<0.05) (Fig. 6E). Survival was much greater after treatment with Nx-CANKs than that in other groups. These results emphasize in vivo antineoplastic function of NK cells treated with NK-derived exosomes that express NCRs and can uptake by resting CD56dim NK. hematoxylin-eosin staining of resected NB tumors after 3, 4, and 5 weeks of treatment in all groups have shown a minimum residue or even complete destruction of neoplastic cells after 5 weeks of treatment with Nx-CANKs (Fig. 6F).
FIGURE 6.
A, Tumor formation in different groups of mice. Neuroblastoma tumor growth in nude mice is obvious after 4 weeks. The row I shows tumor formation in mice that did not receive any other treatment and served as tumor control group. Row II shows the fresh NK effect on the mice bearing NB cells after 28 days of injection. Row III shows the nexosome-treated NK effect on mice bearing NB cells after 28 days of injection. Row IV shows NB-Ex-treated NK effect on mice bearing NB cells, and row V shows the Nx-CANKs-treated mice after 28 days of injection. B, NB tumor weights after 4 weeks of treatment. The diagram shows a −3 g NB tumor in mice treated with NB-Exosomes. The fresh NK can reduce the tumor weight, but nexosome-treated NKs have a similar effect to that of cytokine-activated NKs to reduce tumor weight under 0.2 g (P < 0.05). C, NB tumor volumes after treatments. The best effect of treatment on tumor volumes after 4 weeks was achieved by Nx-CANKs that destroyed 86% of tumors completely. D, NB tumor sizes after 90 days of treatment. The nude mice were checked every 3 days and killed on day 95. The tumor volumes in CANKs, Nx-ANKs, and Nx-CANKs were drastically smaller than that of other groups in order (*P < 0.05). E, Kaplan-Meier Graph. There is a significant difference in survival of 7 different groups of NB tumor–bearing mice of up to 90 days. An overall 86% survival for Nx-CANKs and no survival for NB-Ex-treated groups (*P < 0.05). F, Histology. Hematoxylin-eosin staining of resected NB tumors after 3, 4, and 5, weeks of treatment shows that we had a minimum of neoplastic cells after 5 weeks’ treatment with Nx-CANKs as our best complementary treatment group. NB indicates neuroblastoma; NB-Ex, NB-derived exsosomes; NK cell, natural killer cells; Nx-ANKs, NB exosome–activated NK; Nx-CANKs, NB exosome cytokine-activated NKs.
DISCUSSION
One major barrier for translation of NK cells for therapeutic use in many cancers is the lack of an efficient method to activate them against tumors of interest. In this study, we showed that naive NK cells exposed to the exosomes derived from NK cells, which were previously cocultured with NB cells, had greater cytotoxicity against NB cells. However, in this work, we performed the experiments with NB cells that may differ from the real human tumor, as it would be a mixture of microvesicles originating from different cell types in various stages of the disease and causing inconsistency in results; however, the in vivo model of the work confirmed the in vitro results and showed the effectiveness of using nexosomes to strengthen the NK cells’ cytotoxicity against NB. To the best of our knowledge, this was the first study exploring the in vivo effects of NK-derived exosomes (nexosomes) from previously exposed NB cells, on the expanded peripheral blood NKs to kill the NB cells. Repeated administration of highly activated NK cells provides an effective strategy to treat minimal residual malignancies after chemotherapy or even serious refractory chemoresistant cancers. Although naive NK cell therapy might be effective to treat malignancies,21 NK cell activation has been found to be necessary because of the shortcomings in naive NK functionality, including low-level cytokine secretion, inadequate NCRs expression, low functional cytotoxicity, and anergy in malignancies, that have been demonstrated in several studies.21 Although genetic manipulation of NK is an expensive process and also challenging because of the low efficiency and inefficient yield of effective cells for clinical purposes, we decided to choose an alternative complementary method to boost the NK functionality against malignancies. It has been known that CD56bright NK has a higher capacity to proliferate after in vitro culture with IL-2 and IL-15. In this experiment, we observed that CD56dim NK also has increased proliferation capacity in the presence of these cytokines, as well (data not shown). The IL-2 and IL-15 belong to the common gamma chain family that enhances the expression of NKG2D on NK cells.22 They can activate STAT523 and are associated with an increased surface protein expression of activation receptors.24 There is more evidence that confirms their role in the effective expansion of NK. A 2009 study by Fujisaki et al18 has confirmed that IL-2, IL-15, and 4–1BB ligand can stimulate a mean of 280-fold increase in NK from peripheral blood of normal donors. Because of this, we decided to use these cytokines in addition to IL-21 for the expansion and activation of NKs as the standard and simple method for NK culture.
For NK therapy, the activation levels are considered as the key factors of success.25 Hence, NKs should express detectable amounts of NKG2D receptor and other NCRs such as NKp44, NKp30, and NKp46 to get ready to respond against neoplastic cells. These NCRs that are less expressed in resting NKs undertake for cytotoxicity. Among these NCRs, NKp44 is more important to strike against the target cells.26 Therefore, we can overcome tumor growth and even tumor metastasis, if we are able to regulate NCRs’ expression. It has been reported previously that activated NKs can secrete specific exosomes.17 The NK-released exosomes (nexosomes) express both NKcell markers and cytotoxic molecules. Exosomes are strong intercellular communicators that transfer the active mRNA, proteins and also miRNA to allow striking target cell modulation.27 The feasibility of using nexosomes therapeutically has not been shown yet. Our study confirms the production of these exosomes by NKs (Fig. 3A) and demonstrated the NK markers and NCRs as an important part of their protein contents (Fig. 3B). Their shape was different from exosomes derived from neoplastic cells in shape, manner, and uniformity (Fig. 3C). The nexosomes can affect NK cells and have interactions with their targets by various mechanisms. Previously Gesierich et al28 and Nazarenko et al29 have reported that exosomal tetraspanin complexes account for target selection. In our experiments the isolated exosomes from different sources directed their effect on NK cells (Figs. 4A, B) in a short incubation time; hence, we can conclude that the tetraspanin complexes are capable of targeting NKs, too. It has been known that phagocytic cells rapidly take up microvesicles,30 and our data confirmed the rapid uptake of nexosomes by NK as phagocytes. This uptake has increased the levels of NCRs even without cytokine induction (Fig. 5D). Theoretically, it has been known that the exosomes can activate resting NK cells. In fact, activation of NK by immune cell exosomes has been previously ascertained in 2006. At that time, Chaput et al31 showed that dendritic cell–derived exosomes are the potent activators of NK cells. In addition, the use of dendritic-derived exosomes in a phase I clinical trial was successful in the activation of NKs in patients with non–small cell lung cancer.32 Nonetheless, our study was the first of its kind to use the nexosomes for activation of the NK cells. We observed that nexosomes were able to express some NCRs, typically. In addition, NCRs’ expression was increased after coculture of NKs with NB cells (Fig. 3B). This preexposure to NB cells made this phenomenon robust. The flow cytometry data confirmed that this preexposure led to the production of NKs that can produce a greater amount of nexosomes with higher expression of NKp44 and NKp30 (Figs. 5D, E). Hence, the process was important both in quantitative and qualitative conditions of nexosomes. By increasing the cytotoxicity of NKs (Fig. 5F), it seems that nexosomes can control innate immunity even by a paracrine effect and/or systemically. It is obvious that as well as the cellular origin of an exosome, its interaction with the target cell is a critical step to transfer information between cells. Depending on the type of the target cell, exosomes may be internalized or fused to the cell membrane.30 In 2008, it has been reported that exosomes derived from cultured neoplastic cells can modify the mRNA expression profile of the exposed fibroblasts.33 Maybe this modification happened to our NK cells as they were forced to express a greater amount of cytokines that are important to their cytolysis activity. Our data confirmed the altered releasing patterns of the IFN-γ (Fig. 4A) and also TNF-α (Fig. 4B) cytokines from NK treated in different groups. Moreover, IL-21 exerted a synergistic effect on the Nx-ANKs for induction of these 2 important cytokines. It has been previously identified that IL-21 enhances proliferation and survival of the NK cells,26 which has been found to be due to the conservation of telomere length.34 Therefore, the cultured cells did not show senescence after a period of 4 weeks’ expansion in the culture.35–37 In addition to these characteristics, we found that IL-21 intensifies the cytotoxicity of NKs (Fig. 5F) and their activity on the NB cells because of an increase in IFN-γ (Fig. 4A) and TNF-α (Fig. 4B) production. There are no data until now to compare the mechanism of exosome action on NK with IL-21, but it is obvious that we face a very complex agent that may harbor molecules similar to the cytokines too. Furthermore, as reported previously the IL-21 induces terminal differentiation of NKs and results in enhanced cytotoxicity against neoplastic cells.38 This is so close to our findings of enhanced cytotoxicity against NB cells (Figs. 5F, 6E). The nexosomes solely have similar effects and can play an immune regulatory inscription. They have induced a number of striking phenotypic changes, including alterations in NCRs expression (Figs. 5A–E), which might be due to the exosomes’ origins. Moreover, these extracellular vesicles are very important for the transfer of genetic materials. There are some confined reports in this context that show the role of exosomes in hepatitis C and hepatitis B transmission. The transfer of genetic elements subsequently caused NK dysfunction.39,40 Hence, as mentioned previously here, on the basis of the exosomes’ source they could be a double-edged swordplay. This study demonstrated that NB-Ex (Fig. 3C) reduce the levels of IFN-γ and TNF-α secretion by NKs and also decrease the levels of NCRs’ expression, especially NKp44 (Fig. 5C). These changes finally lead to the dramatic fall in the cytotoxicity of NKs against NB cells both in vitro (Fig. 5F) and in vivo (Fig. 6E). As we know the NK cytotoxicity is regulated by a combination of activating and inhibitory signals.41,42 Our data showed that nexosomes can carry the activator signals and induce the expression of NKG2D and KIR2DL2 (Fig. 3B) on resting NKs without addition of any cytokines; nonetheless, the cocktail of cytokines and nexosome (Nx-CANKs) has a more potent synergistic effect on the in vitro (Fig. 5F) and in vivo cytotoxicity of NKs (Figs. 6A–D). Just 4 weeks after nexosome treatments of the mice model, the weight and volume of tumors decreased dramatically (Fig. 6D). In vivo suppression of NB tumors were sufficient to suppress tumor metastases (Fig. 6F). When the animals were killed for histology experiments, microscopic studies confirmed the effectiveness of nexosomes in NK therapy at the tissue level, as there were minimal residues of neoplastic cells (Fig. 6F).
It is worth saying that this was a complicated process because the NB tumors of our models also can secrete exosomes in vivo and exert their inhibitory effect on NKs in the tumor microenvironment. Hence, this change in tumor niche promotes the secretion of inflammatory cytokines by monocytes through Toll-like receptors.43 As a long known fact, inflammation is an important factor in tumor initiation. Although inflammation is a beneficial response to restore tissue injury and to eradicate pathogens, if it continues and becomes chronic, it can induce malignant cell transformation in the surrounding tissue. In addition, inflammatory cytokines help cancer progression by enhancing metastasis as reported previously by Nicolini et al.44 They reported cytokines such as IL-1, IL-6, IL-11 and transforming growth factor-b regulate the inflammatory tumor microenvironment to stimulate cancer cell proliferation and invasion. There are many similar reports that are an emphasis on the complicated environment of tumors, such as a study by Baj-Krzyworzeka et al,45 that demonstrated that tumor-derived exosomes harbor several mRNA of tumor cells and surface determinants that can transfer them into the monocytes. These data can be generalized to our study and, of course, are in accordance with our findings of the NB-exosomes’ behavior on NK immunity. Adding another point is necessary to know that; however, it is true that NKs are able to respond against tumors in an antigen-independent manner, but as described by Cooper et al46 the NK memory formation is beneficial for their better function for therapeutic use in cancers. Here we can propose that nexosomes are beneficial tools to form this memory even in naive cells.
CONCLUSIONS
The nexosomes derived from NB preexposed NK cells that express NCRs and activating signals can activate human-resting NK and educate them to enhance NK-mediated anti-NB tumor reaction. These results encourage further evaluation of nexosomes as the potential novel oncology complementary therapeutics in immune cell therapy.
Acknowledgments
FINANCIAL DISCLOSURES
This work was supported by a grant NO. 94-02-87-28675 from Tehran University of Medical Sciences.
Footnotes
CONFLICTS OF INTEREST
All authors have declared that there are no financial conflicts of interest with regard to this work.
REFERENCES
- 1.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxicreactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–229. [DOI] [PubMed] [Google Scholar]
- 2.Curti A, Ruggeri L, Addio A, et al. Successful transfer ofalloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–3279. [DOI] [PubMed] [Google Scholar]
- 3.Geller MA, Cooley S, Judson PL, et al. A phase II study ofallogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011; 13:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iliopoulou EG, Kountourakis P, Karamouzis MV, et al. Aphase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother. 2010;59:1781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg TK, Szmania SM, Khan JA, et al. Highly activated andexpanded natural killer cells for multiple myeloma immunotherapy. Haematologica. 2012;97:1348–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Cu Y, Voong N, et al. Activating signals dominate inhibitory signals in CD137L/IL-15 activated natural killer cells. J Immunother. 2011;34:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldmann TA. The biology of interleukin-2 and interleukin15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. [DOI] [PubMed] [Google Scholar]
- 8.Kasaian MT, Whitters MJ, Carter LL, et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559–569. [DOI] [PubMed] [Google Scholar]
- 9.Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. [DOI] [PubMed] [Google Scholar]
- 10.Cooper MA, Fehniger TA, Caliguiri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;11:633–640. [DOI] [PubMed] [Google Scholar]
- 11.Fais S NK cell-released exosomes. Oncoimmunology. 2013; 2:e22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parolini I, Federici C, Raggi C, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashiru O, Boutet P, Fernández-Messina L, et al. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zech D, Rana S, Büchler MW, et al. Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal. 2012;10:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiners KS, Topolar D, Henke A, et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood. 2013;121:3658–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller L, Mitsuhashi M, Simms P, et al. Tumor-derived exosomes regulate expression of immune function related genes in human T cell subsets. Sci Rep. 2016;6:20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lugini L, Cecchetti S, Huber V, et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 2012;189:2833–2842. [DOI] [PubMed] [Google Scholar]
- 18.Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoae-Hassani A, Keyhanvar P, Seifalian AM, et al. lambda Phage nanobioparticle expressing apoptin efficiently suppress human breast carcinoma tumor growth in vivo. PLoS One. 2013;8:e79907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho YS, Yu MS, Lai CSW, et al. Characterizing the neuroprotective effects of alkaline extract of Lycium barbarum on beta-amyloid peptide neurotoxicity. Brain Res. 2007;1158:123–134. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez CJ, Le Treut T, Boehrer A, et al. Natural killer cells and malignant haemopathies: a model for the interaction of cancer with innate immunity. Cancer Immunol Immunother. 2011;60:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Decot V, Voillard L, Latger-Cannard V. Natural-killer cell amplification for adoptive leukemia relapse immunotherapy: comparison of three cytokines, IL-2, IL-15, or IL-7 and impact on NKG2D, KIR2DL1, and KIR2DL2 expression. Exp Hematol. 2010;38:351–362. [DOI] [PubMed] [Google Scholar]
- 23.Pillet AH, Bucault F, Theze J, et al. A programmed switch from IL-15- to IL-2-dependent activation in human NK cells. J Immunol. 2009;182:6267–6277. [DOI] [PubMed] [Google Scholar]
- 24.Park YP, Choi SC, Kiesler P. Complex regulation of human NKG2D-DAP10 cell surface expression: opposing roles of the gc cytokines and TGF-b1. Blood. 2011;118:3019–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng M, Chen Y, Xiao W, et al. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10: 230–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch J, Steinle A, Watzl C, et al. Activating natural cytotoxicity receptors of natural killer cells in cancer and infection. Trends Immunol. 2013;34:182–191. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed KA, Xiang J. Mechanisms of cellular communication through intercellular protein transfer. J Cell Mol Med. 2011;15:1458–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gesierich S, Berezovskiy I, Ryschich E, et al. Systemic induction of the angiogenesis switch by the tetraspanin D6.1A/CO-029. Cancer Res. 2006;66:7083–7094. [DOI] [PubMed] [Google Scholar]
- 29.Nazarenko I, Rana S, Baumann A, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosomeinduced endothelial cell activation. Cancer Res. 2010;70:1668–1678. [DOI] [PubMed] [Google Scholar]
- 30.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaput N, Flament C, Viaud S, et al. Dendritic cell derived-exosomes: biology and clinical implementations. J Leukoc Biol. 2006;80:471–478. [DOI] [PubMed] [Google Scholar]
- 32.Morse MA, Garst J, Osada T, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skog J Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu S, Phatarpekar PV, Denman CJ, et al. Transcription of the activating receptor NKG2D in natural killer cells is regulated by STAT3 tyrosine phosphorylation. Blood. 2014;124:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somanchi SS, Senyukov VV, Denman CJ, et al. Expansion, purification, and functional assessment of human peripheral blood NK cells. J Vis Exp. 2011;48:2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7:e30264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Wu HW, Sheard MA, et al. Growth and activation of natural killer cells ex vivo from children with neuroblastoma for adoptive cell therapy. Clin Cancer Res. 2013;19:2132–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins SH, Nguyen KB, Takahashi N, et al. Cutting edge: inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J Immunol. 2002;168:2585–2589. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Han Q, Hou Z, et al. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol. 2017;14:465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Liu K, Liu Y, et al. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat Immunol. 2013;14:793–803. [DOI] [PubMed] [Google Scholar]
- 41.Raulet DH, Vance RE, McMahon CW. Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol. 2001;19:291–330. [DOI] [PubMed] [Google Scholar]
- 42.Colucci F, Di Santo JP, Leibson PJ. Natural killer cell activation in mice and men: different triggers for similar weapons? Nat Immunol. 2002;3:807–813. [DOI] [PubMed] [Google Scholar]
- 43.Bretz NP, Ridinger J, Rupp AK, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013; 288:36691–36702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17:325–337. [DOI] [PubMed] [Google Scholar]
- 45.Baj-Krzyworzeka M Tumor-derived microvesicles carry several surface determinants and MRNA of tumor cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006;55:808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper MA, Elliott JM, Keyel PA, et al. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106:1915–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]