Abstract
As the coronavirus pandemic evolves, the focus of radiology departments has begun to change. The acute phase of imaging a new disease entity whilst rationalising radiology services in the face of lockdown has passed. Radiologists are now becoming familiar with the complications of COVID-19, particularly the lung parenchymal and pulmonary vascular sequelae and are considering the impact follow-up imaging may have on departments already struggling with a backlog of suspended imaging in the face of reduced capacity. This review from the British Society of Thoracic Imaging explores both the thoracic and extra-thoracic complications of COVID-19, recognising the importance of a holistic approach to patient follow-up. The British Thoracic Society guidelines for respiratory follow-up of COVID-19 will be discussed, together with newly developed reporting templates, which aim to provide consistency for clinicians as well as an opportunity for longer-term data collection.
Introduction
The arrival of a global pandemic is a rare event in medicine. Yet the rate at which knowledge of coronavirus disease 2019 (COVID-19) has disseminated across today's globally connected world and the level of universal collaboration witnessed between medical specialties has been astonishing. As radiologists, we have rapidly rationalised services, designed new imaging pathways, and reorganised working patterns, whilst maintaining the “bread and butter” of urgent diagnostic work for patients, including those with COVID-19. Common imaging appearances of a new disease entity have been described, disseminated, and reported in a rapid and structured way, aiding the diagnostic and follow-up patient pathway.
In the UK, the peak of infection in early April has passed, with the number of new cases falling each day. As the country emerges from lockdown, radiologists now need to look forward and be ready for new challenges. Many departments benefitted from the brief interlude in scanning as breathing space to clear the existing backlog of reporting generated by a chronic shortage and underinvestment in the radiology workforce. Yet following this brief period of altered workflow, new challenges are emerging. Firstly, the imbalance between a new backlog of demand for previously suspended imaging and further reduced capacity due to increased scan time; secondly, the recognition of a new patient cohort with post-COVID-19 complications who require a holistic approach to follow-up. It is this particular challenge associated with post-COVID-19 follow-up that will be the main focus of this article.
Following on from initial statements in the acute phase of the pandemic,1 , 2 this update from the British Society of Thoracic Imaging group aims to provide an overview of the multisystem complications of COVID-19 with a focus on the thoracic manifestations, in particular the lung parenchymal and pulmonary vascular sequelae. The authors will also explore the British Thoracic Society (BTS) guidance on the respiratory follow-up of patients with COVID-19, including the resource implications for departments, discuss the potential use of structured reporting of follow-up imaging and outline future opportunities for longitudinal data collection and research.
Complications of COVID-19
The precise prevalence of post-COVID-19 complications in the UK is unknown and will be difficult to ascertain due to the lack of testing in asymptomatic and mildly symptomatic patients not admitted to hospital. Until there is widespread access to immunoglobulin testing with confirmation of the duration of maintenance of seropositivity, it will be impossible to know the denominator for this calculation. The only similarly investigated comparator groups are those with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). Data from these pandemics will provide a contextual background, particularly for the long-term pulmonary sequelae of COVID-19.3 , 4
Thoracic complications
Acute/subacute
Acute thromboembolic disease
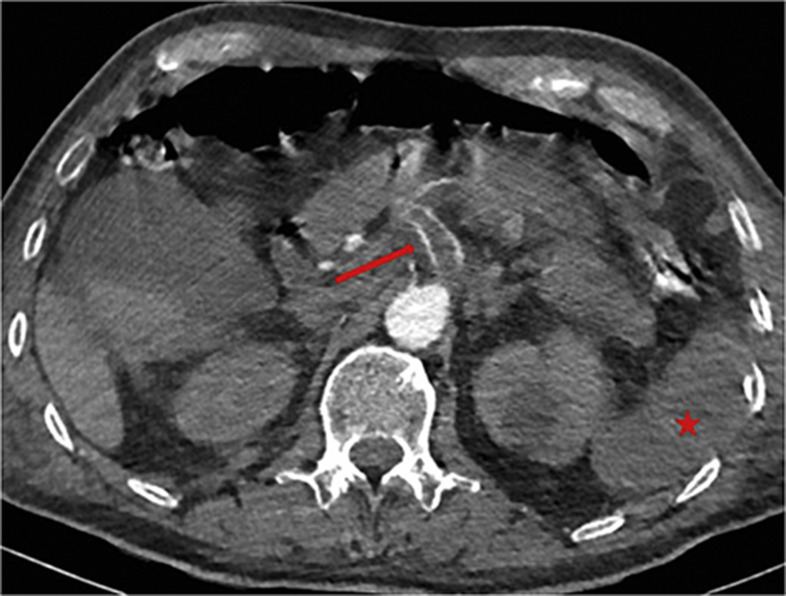
There is emerging evidence that patients with COVID-19 experience a higher rate of thromboembolic disease, both venous and arterial, with pulmonary embolism (PE) tending to be the most frequent thrombotic complication.5 , 6 The inflammatory nature of this infection, together with hypoxia, immobilisation, and disseminated intravascular coagulation are all likely to contribute to this phenomenon.5 Rare cases of arterial thrombus and cerebral vein thrombus have also been reported in association with COVID-195 (Fig 1 ).
Figure 1.
A 68-year-old man presented with shortness of breath and tested positive for COVID-19. CTPA undertaken to investigate persistent oxygen requirement showed an incidental finding of splenic infarction (star) due to an acute coeliac axis thrombus (arrow).
Acute respiratory distress syndrome
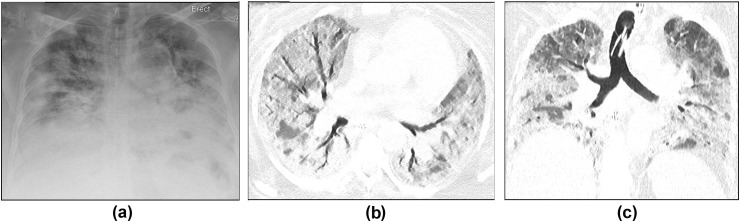
Respiratory failure in COVID-19 is poorly understood, with disparity between the severity of hypoxaemia and the degree of impairment in respiratory mechanics. Although it would seem that acute respiratory distress syndrome (ARDS) is not responsible for all cases of respiratory compromise associated with COVID-19, it is one of the commonest reasons for transferring patients to critical care and is a major cause of death.7 Its reported prevalence in COVID-19 is up to 31%,8 , 9 significantly higher than rates of injury to other organ systems (Fig 2 ).
Figure 2.
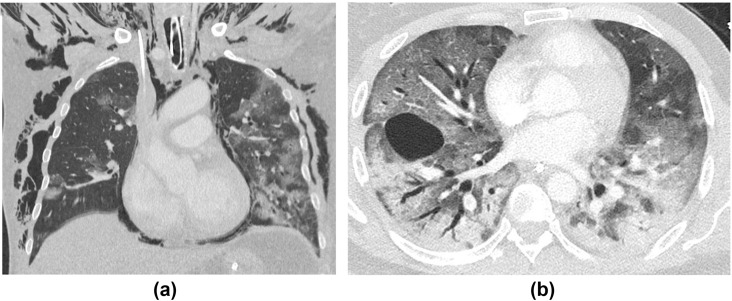
A 60-year-old woman with hypertension, type 2 diabetes mellitus, and obesity was admitted to the emergency department (ED) with severe shortness of breath and hypoxia. (a) CXR on admission showed bilateral peripheral and basal predominant consolidation in keeping with COVID-19. She was intubated in the ED and transferred to the ICU. She tested positive for COVID-19. Several days later, she was diagnosed clinically with ARDS. (b,c) CT showed progression of lung parenchymal changes with much more diffuse GGO and consolidation, areas of bronchial dilatation, and interlobular septal thickening.
Secondary infection
Respiratory infection in COVID-19 can be complicated by viral co-infection and secondary bacterial pneumonia, including ventilator-associated pneumonia. Secondary bacterial pneumonia has been well studied in pandemic and seasonal influenza, when it contributes significantly to mortality.10 In COVID-19, it can occur in the acute phase of the disease and also in the recovery period.11 It is also more common in older patients, and in a retrospective study in China, it was reported in 15% of all patients and 50% of non-survivors.12 Radiologists should be familiar with the classical and genuinely indeterminate features of COVID-19 on chest radiography (CXR) and computed tomography (CT) already described2 and be alert to “non-COVID-19” findings such as lobar consolidation with air-bronchograms, more typical for bacterial pneumonia (Fig 3 ), and bronchial wall thickening, mucous plugging, and tree-in-bud changes, more typical of airway-centred infection (Fig 4 ).
Figure 3.
An 80-year-old man with chronic obstructive pulmonary disease, type 2 diabetes mellitus, and atrial fibrillation presented to the ED with 4 days of a productive cough with green sputum, shortness of breath, lethargy, and fever. He had elevated C-reactive protein, lymphopenia, and tested positive for COVID-19. CXR showed left lower lobe consolidation and he was treated with supportive care and antibiotics for COVID-19 with superadded bacterial infection.
Figure 4.
A 19-year-old woman with cystic fibrosis and Pseudomonas spp. colonisation was admitted with small bowel obstruction requiring laparotomy. During admission she developed fever, hypoxia, and tested positive for COVID-19. She was treated in the ICU with continuous positive airway pressure (CPAP) and intravenous antibiotics in view of her Pseudomonas lung infections. (a) Admission CXR (left) compared to baseline (right) showing new bilateral lower lobe consolidation and features of cystic fibrosis. (b) CTPA to investigate acute desaturation shows bronchiectasis with bronchial wall thickening, mucous plugging, and tree-in-bud changes in keeping with cystic fibrosis together with bilateral peripheral, basal predominant consolidation, and GGO with peri-lobular opacities in keeping with COVID-19.
Pneumothorax and pneumomediastinum
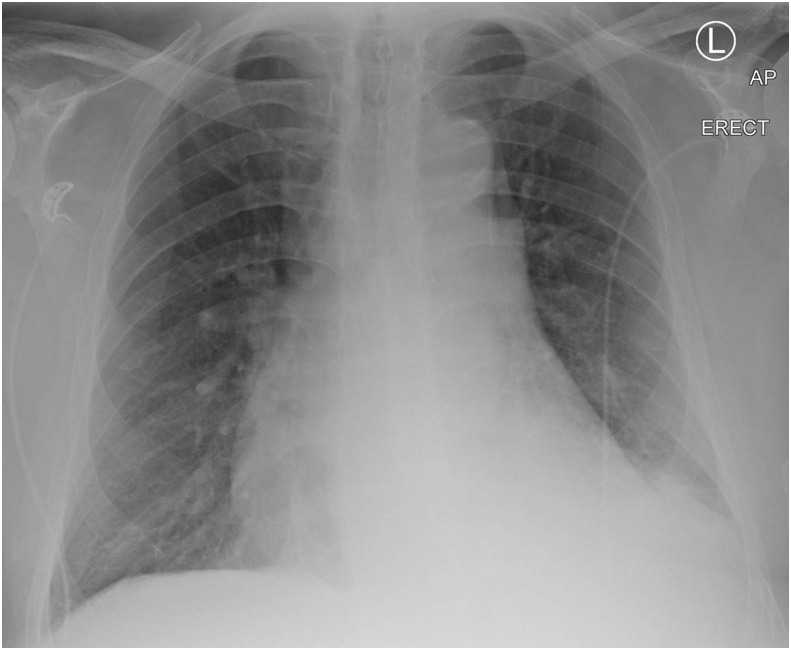
Pneumothorax and spontaneous pneumomediastinum in COVID-19 are predominantly seen as case reports in the literature and are uncommon complications.13 Pneumothorax was reported as a rare presenting sign (1%) in a case series of 99 patients in China14 and has been recognised as a rare occurrence in progressive disease7 (Fig 5 ). It is already established that pneumothorax and tension pneumothorax are recognised complications of mechanical ventilation, with a predisposition in patients with underlying lung disease and this would seem to hold true in COVID-19 pneumonia also.13 , 15 Spontaneous pneumomediastinum has been described in the literature and observed by the authors, and although it is rare in viral pneumonia, it was also reported in cases with SARS16 , 17 (Fig 6 ).
Figure 5.
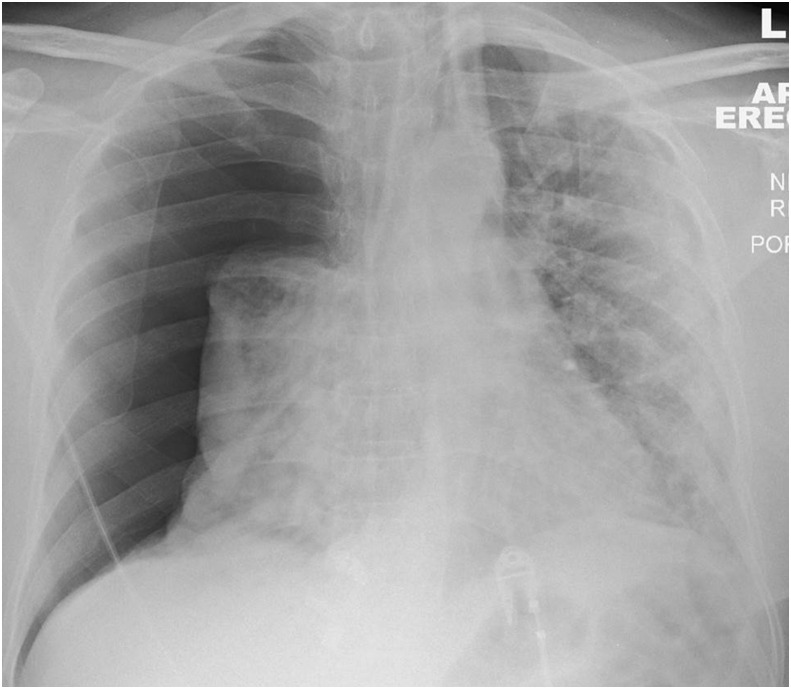
A 64-year-old man presented to the ED having acutely deteriorated following 14 days of intermittent fever and progressive shortness of breath. CXR on admission showed a large right pneumothorax and left-sided consolidation in a predominantly peripheral distribution. He tested positive for COVID-19.
Figure 6.
A 66-year-old man was admitted with COVID-19. He was intubated and ventilated on day 7 of his admission. (a) CT performed on the same day due to an acute deterioration showed extensive pneumomediastinum and surgical emphysema. A 40-year-old man with COVID-19 was intubated and ventilated on day 5 of his admission. (b) CT showed a loculated pneumothorax with air in the right major fissure. Lung parenchymal changes were consistent with COVID-19 (bilateral basal predominant GGO and consolidation with bronchial dilatation).
Chronic
Thrombosis, chronic thromboembolic disease, and pulmonary hypertension
Reports in the literature highlight both a high incidence of macrovascular injury (e.g., acute PE), but also microvascular injury in COVID-19. In a study by Klok et al.,5 the incidence of thrombotic complications in 184 intensive care unit (ICU) patients with COVID-19 was 31%. Anecdotal reports from large UK critical care units suggest potentially higher figures. Autopsy findings from the first 12 consecutive COVID-19 deaths at a single German academic medical centre18 revealed deep venous thrombosis in 58% in whom venous thromboembolism was not suspected, with PE a direct cause of death in four patients; however, COVID-19 has also been associated with microvascular coagulopathy, with findings similar to those seen in disseminated intravascular coagulopathy.19 It would seem reasonable, therefore, to suggest that although patients may develop acute PE secondary to a severe illness and hospitalisation, the pronounced inflammatory response seen in a cohort of COVID-19 patients results in a substantial pro-thrombotic tendency.
There is insufficient literature on the use of dual-energy CT (DECT) in COVID-19 to know whether this technique provides additional assessment of microvascular disease in the form of “perfusion abnormalities” on iodine maps; however, Lang et al. highlighted the presence of perfusion defects (in the absence of visible PE) on DECT in 12 patients.20 This has also been the experience of some of the authors, who also note that when present, acute PE has often developed in segmental/subsegmental vessels, rather than in main or lobar pulmonary arteries.
It is possible (but still unknown) that those recovering from COVID-19 may develop chronic thromboembolic disease (CTED), chronic thromboembolic pulmonary hypertension (CTEPH) and/or pulmonary hypertension (PH) secondary to lung disease (e.g., fibrotic organising pneumonia).
It is important that both clinicians and radiologists be alert to the diagnosis of PH. CTEPH particularly is under-diagnosed; in 2015 it was estimated that, on average, only 16% of patients in the USA, Europe and Japan who had CTEPH were diagnosed.21
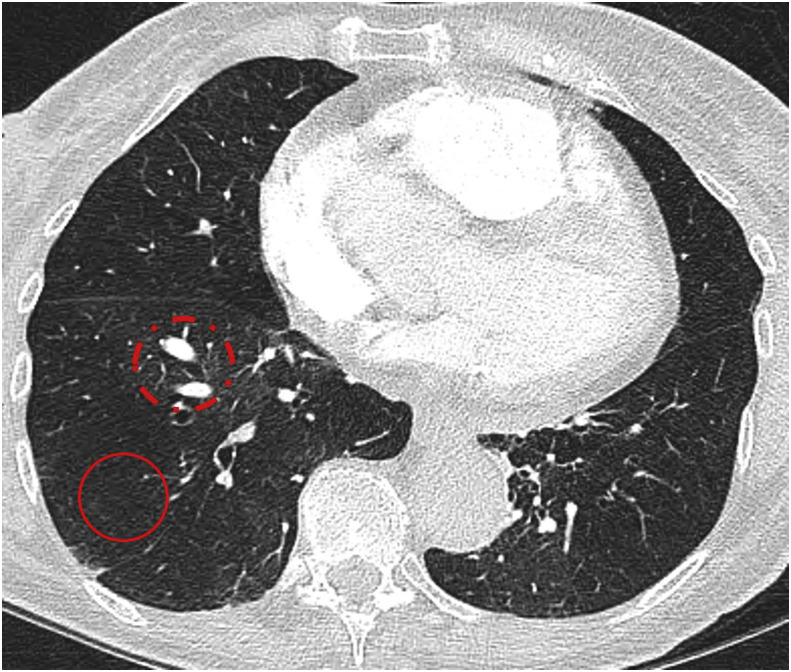
Diameter of the main pulmonary artery (mPA) exceeding 30–31mm should raise the suspicion of PH; however, in the setting of established pulmonary fibrosis, a ratio of the mPA to the adjacent ascending aorta >1.1 may be a more reliable predictor.22, 23, 24 The presence of increased pulmonary artery to bronchus ratio in at least three vessels has also been shown to have a high specificity for the diagnosis of PH.25 A right ventricle to left ventricle ratio (RV:LV) >1.0 has consistently been shown to be a useful risk stratification biomarker and predictor of poor outcome in acute PE, CTEPH, and interstitial lung disease25, 26, 27, 28; however, the RV:LV ratio is substantially under-reported, as highlighted in the 2019 National Confidential Enquiry into Patient Outcome and Death (NCEPOD).29 These findings, together with reflux of contrast medium into the hepatic veins, may be present in all forms of PH, irrespective of aetiology. In CTED/CTEPH, the radiologist should review the pulmonary arteries for vascular webs and occlusions (Fig 7 ). DECT may also show multiple peripheral perfusion defects (Fig 8 ). The lung parenchyma can often show mosaic attenuation with geographic areas of low attenuation associated with peripheral scarring, the residuum of previous infarction (Fig 9 ). Subtle pulmonary vascular abnormalities, particularly those observed in more distal chronic thromboembolic disease can be difficult to detect with CT and, as in the work-up of suspected PH, ventilation/perfusion scintigraphy (VQ) should be considered a more sensitive imaging tool to exclude chronic thromboembolic disease, if this is suspected.30
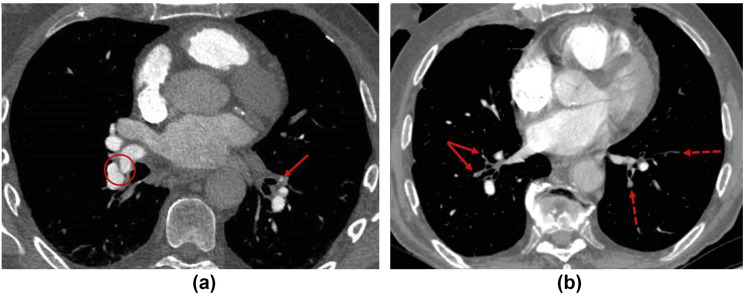
Figure 7.
CTPA in CTEPH. (a) Web across the right lower lobe pulmonary artery trifurcation (red circle) and occlusion of the anterior segment of the left lower lobe (red arrow). (b) Occlusive disease with only attenuated “ghost vessels” of the anterior and lateral segments of the right lower lobe (red arrows) with patent medial and posterior segments. In the left lower lobe, there is occlusive disease of the anterior and posterior segments (dotted arrows) with a patent lateral segment.
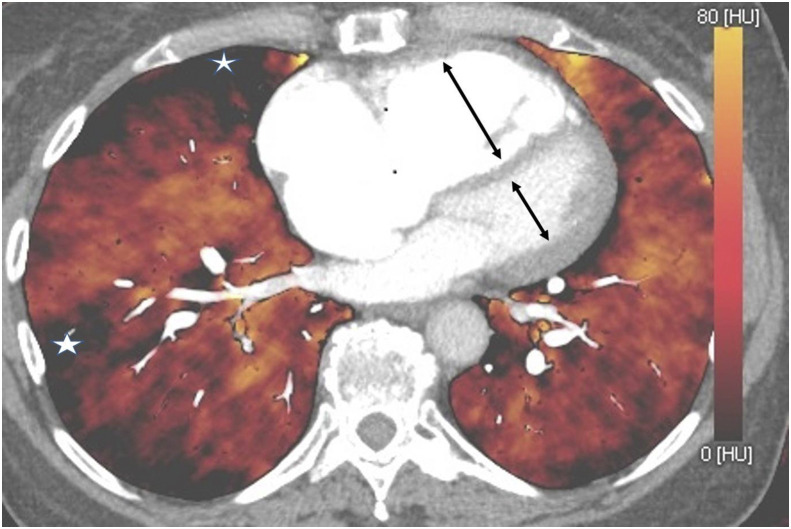
Figure 8.
DECT. Multiple iodine map “perfusion defects” in a case of CTEPH (white stars). Note also an increased RV:LV ratio with mild paradoxical bowing of the interventricular septum (black arrows).
Figure 9.
CTPA, lung windows. Mosaic perfusion pattern in CTEPH. Hypoattenuating lung (red circle) with paucity and attenuated vasculature, compared with relative increase in attenuation (dotted red circle) with greater vascular markings and dilatation of segmental pulmonary arteries.
Lung parenchymal complications
CXR follow-up will suffice for the majority of COVID-19 patients, and should show improvement or even total resolution of abnormalities detected in the acute phase. In the absence of robust analyses to date, it is reasonable to expect that COVID-19 follows a similar pattern of recovery and debilitation to SARS, where follow-up (after a mean of 6.5 weeks) suggested approximately 20% of patients could have residual CXR and HRCT abnormalities, usually accompanying a physiological deficit.
It is worth remembering that, if guidance is dutifully followed, patients undergoing CT follow-up should be those who had a protracted or critical course of illness and have insufficiently improved, suggesting that a high pre-test probability of abnormality on CT could be expected. The key aims of parenchymal CT evaluation post COVID-19 are firstly, to identify any realistic chance of stabilising or even reversing active inflammation in the lung and secondly, to detect residual fibrosis, both of which should prompt consultation with interstitial lung disease (ILD) teams, to consider whether immunomodulatory or even anti-fibrotic therapy could be appropriate.
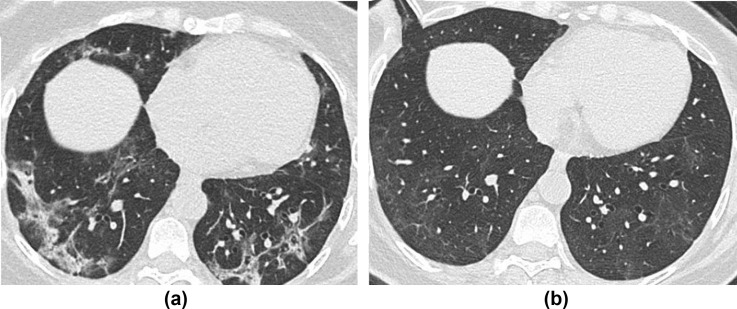
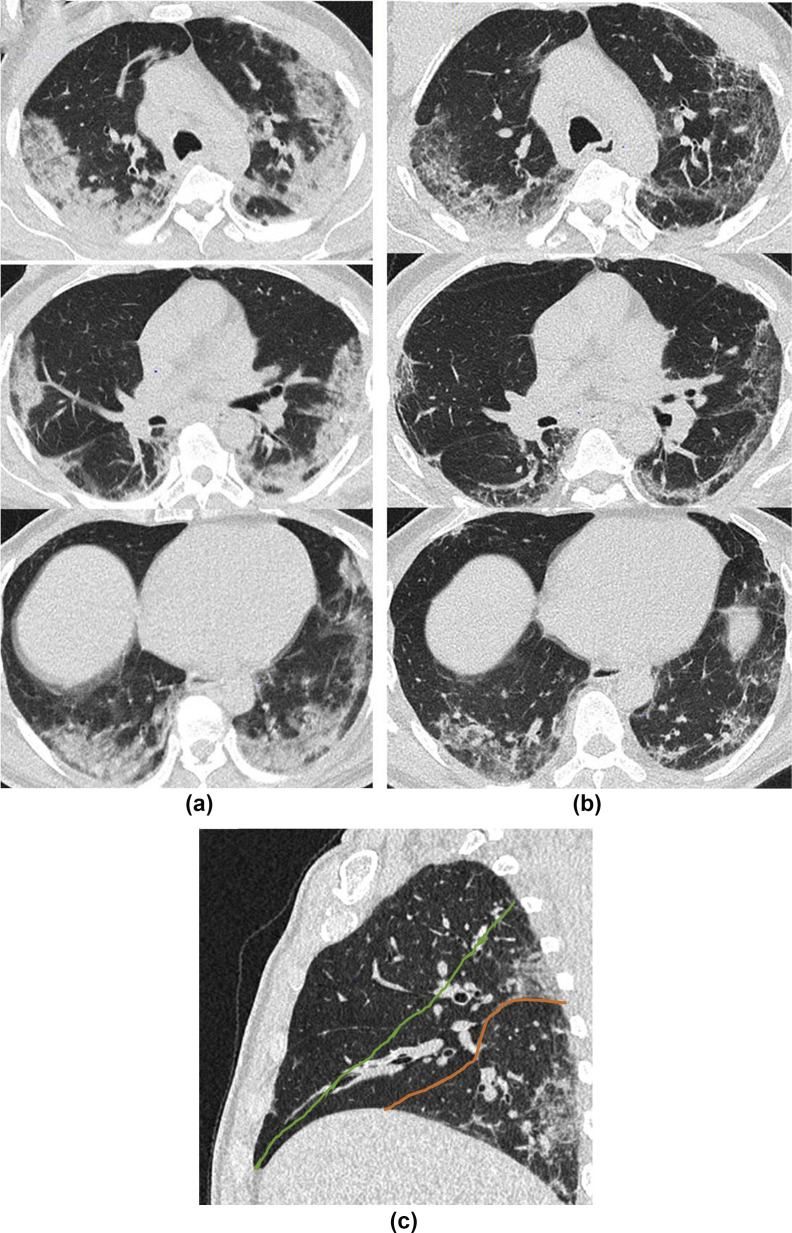
CT follow-up experience thus far mirrors that of preliminary reports.31 Unsurprisingly, patterns of fibrosis or diminishing abnormality with no fibrosis representing the organising/fibrotic and resolving phases of lung injury, respectively, are seen to different extents and often co-exist in the same patient. The cardinal signs of fibrosis (traction bronchial dilatation with architectural distortion and volume loss) can be noted, together with reticulation, and may be either bronchocentric or peripheral, usually reflecting the distribution of the acute abnormality. A perilobular pattern of organising pneumonia,32 recognised in the acute phase of the disease, may also be present on follow-up but may dissipate on later imaging, especially if unaccompanied by signs of fibrosis (Fig 10 ). It is worth noting that if CT is performed too early following the acute phase of the infection, transient architectural distortion and even apparent traction dilatation within areas of radiological organising pneumonia may be spuriously present, leading one to overcall fibrosis, but this can subsequently return to normal. Conversely, ground-glass opacity (GGO) with no signs of fibrosis (so-called “free-standing GGO”) may be seen and reflect a lingering inflammatory infiltrate, or perhaps even a superimposed viral infection, as it may do in ILD.33 , 34 Radiological distinction of these two underlying causes of GGO is virtually impossible and relies on correlation with the patient's clinical course and other laboratory parameters; however, the presence of such freestanding GGO could prompt physicians to consider additional investigations, including bronchoalveolar lavage to aid therapeutic decision-making, particularly as consideration of immunomodulatory therapy requires careful negotiation of a precarious risk–benefit balance after recent infection or in the presence of an opportunistic infection. As such, it seems sensible to provide a pragmatic estimate of whether the pattern on CT is predominantly fibrotic or inflammatory, and to use all available tools, for example sagittal reformats (Fig 11 ), to assess for early signs of volume loss.
Figure 10.
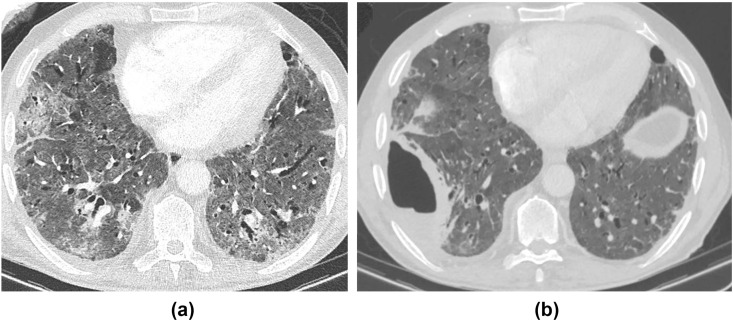
A 58-year-old woman underwent CT thorax for completion staging for breast cancer, 4-weeks post-COVID-19 infection requiring 2 weeks of hospital admission but no mechanical ventilation. (a) Axial unenhanced CT shows peripheral consolidation and admixed GGO, with a perilobular pattern. (b) Follow-up CT at 11 weeks shows complete resolution of consolidation and the perilobular pattern, but a residual “free-standing” GGO in the same regions with no fibrotic features. The patient was already completely asymptomatic at 4 weeks and remained so at 11 weeks, suggesting radiological improvement may lag behind short-term clinical improvement; however, the longer-term impact on respiratory function, if any, is currently unclear.
Figure 11.
A 61-year-old man presented with 1 week history of feeling generally unwell with symptoms of fever, fatigue, and myalgia. Swab for COVID-19 was positive. (a) CT performed 7 days after the onset of symptoms showed bilateral basal predominant pulmonary infiltrates consistent with COVID-19. He was discharged after 8 days but represented at 32 days post-initial symptom onset with progressive shortness of breath. (b) CT showed improvement in the previous GGO but with some architectural distortion and volume loss best appreciated on sagittal reformats (c) showing inferior displacement of the right major fissure (red line) from its normal position (green line) due to loss of volume in the right lower lobe.
Extra-thoracic complications
COVID-19 is predominantly a respiratory disease mostly affecting adults. In most people who experience symptoms, the illness is mild but it may progress to pneumonia, ARDS, multi-organ failure, and death35; however, over the course of 3 months, we have begun to disentangle the wider multisystem effects of this disease. These have included coagulopathy (as described above),18 and damage to the cardiovascular,36 , 37 neurological,38 and renal systems, making a multidisciplinary evaluation, including mental health and rehabilitation, key aspects of patient management. The extent to which radiology input will be required for the diagnosis and follow-up of extra-thoracic COVID-19 complications is uncertain.
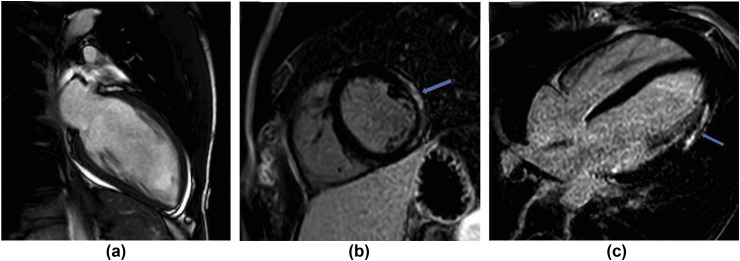
Cardiac complications including left ventricular dysfunction, arrhythmias, and myocardial injury are common in patients with pneumonia and have also been seen with COVID-19.39 Studies report acute myocardial injury in 12–17%, inferred from an elevation of cardiac biomarkers12 , 40 , 41 and there are also case reports and case series of myocarditis42 , 43(Fig 12 ). Myocardial damage originating from COVID-19 can relate to the cytokine storm initiated by the body's immune response to the virus, or from virus-mediated damage to the heart itself.44 Cardiac involvement has recently been described in recovered COVID-19 patients, with cardiac MRI showing myocardial oedema, fibrosis, and impaired right ventricular function.45 Furthermore, COVID-19 is now known to affect children46 and rare case reports of Kawasaki's disease in patients who also tested positive for SARS CoV-2 have been published,47 although there is no evidence of a definite causal association.48
Figure 12.
An 18-year-old man presented to the ED with non-sustained ventricular tachycardia (NSVT). He reported a history of anosmia for 2 weeks and tested positive for COVID-19. Echo showed a small pericardial effusion and mildly impaired left ventricular systolic function. Troponin-I was elevated and myocarditis was suspected. (a) Cardiac MRI confirmed the small pericardial effusion and (b,c) phase-sensitive inversion recovery (PSIR) sequences showed late gadolinium enhancement in a sub-epicardial (non-ischaemic) distribution in the mid and apical lateral and inferolateral wall, which can be seen in myocarditis.
Acute kidney injury is frequently observed in COVID-19 and is an independent predictor of mortality.49
Gastrointestinal (GI) symptoms are also recognised, sometimes in the absence of respiratory manifestations,50 but whether there are any long-term sequelae of GI involvement is unknown. Liver injury was common in SARS and MERS51 and is also recognised with COVID-19; several studies have reported abnormal elevated liver enzymes in up to 53% of cases, and this correlated with more severe disease.50
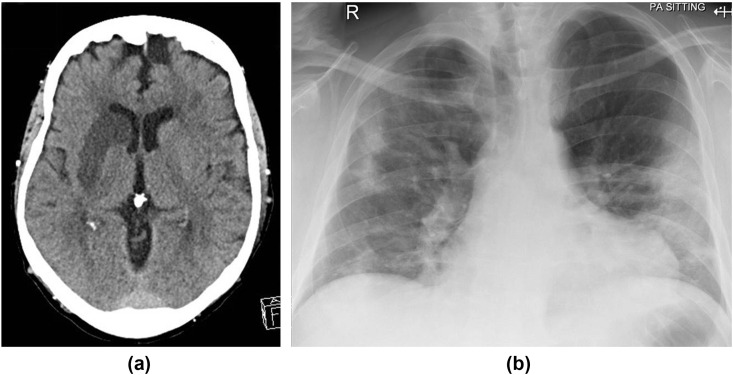
Neurological sequelae appear in the literature as case reports and small case series and, as well as the recognised symptoms of headache, dizziness, encephalopathy and delirium, complications include acute stoke (Fig 13 ), Guillain–Barré syndrome, acute transverse myelitis, acute encephalitis, and posterior reversible encephalopathy syndrome (PRES; Fig 14 ).52, 53, 54
Figure 13.
A 64-year-old man presented to ED with left hemiparesis. (a) CT showed right middle cerebral artery territory infarct. He was also noted to be hypoxic; blood tests revealed lymphopenia with elevated C-reactive protein and (b) CXR showed features of COVID-19.
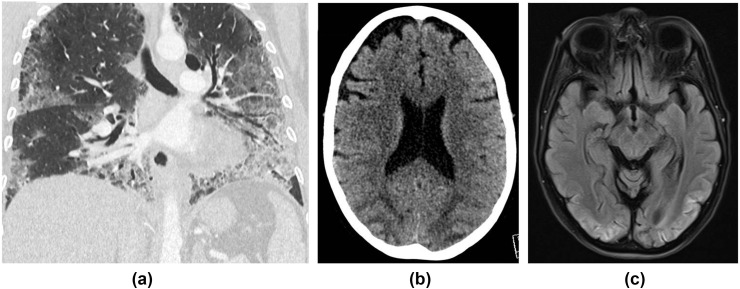
Figure 14.
A 53-year-old woman was admitted with a 2-week history of fever, cough, diarrhoea, and shortness of breath. She tested positive for COVID-19 and was ventilated for 2 weeks. (a) CT chest showed typical features of COVID-19 pneumonitis (peripheral basal ground glass with septal lines and bronchial dilatation). During her stay in critical care, she had a generalised tonic–clonic seizure. (b) CT head showed bilateral symmetrical occipital and posterior parietal low attenuation with sulcal effacement in keeping with oedema, which was confirmed on MRI (c), which showed corresponding high signal posteriorly on fluid-attenuated inversion recovery (FLAIR) sequences. A diagnosis of PRES was made. She remained delirious for a few days post-extubation, and was found to have cortical blindness on examination. She has made a good recovery since and is currently undergoing neuro-rehabilitation.
The pathophysiology of all these complications is not fully understood but is likely to be multifactorial. Whether they reflect the consequences of any severe viral illness, with associated inflammatory insult, together with the expected complications of any critical care admission, or are more specifically related to COVID-19 is yet to be clearly established. Several studies explore the relevance of angiotensin-converting enzyme 2 as the main host receptor of human coronaviruses and note its expression by epithelial cells of the lung, intestine, kidneys, cardiovascular system, and blood vessels.55 , 56 Regardless of the exact mechanisms at work in COVID-19, a holistic approach to ongoing follow-up will be necessary for some patients and, as radiologists, it is important to be mindful of this, particularly when involved in clinico-radiological discussions in the follow-up of this patient cohort.
Overview and implications of the British Thoracic Society guidance
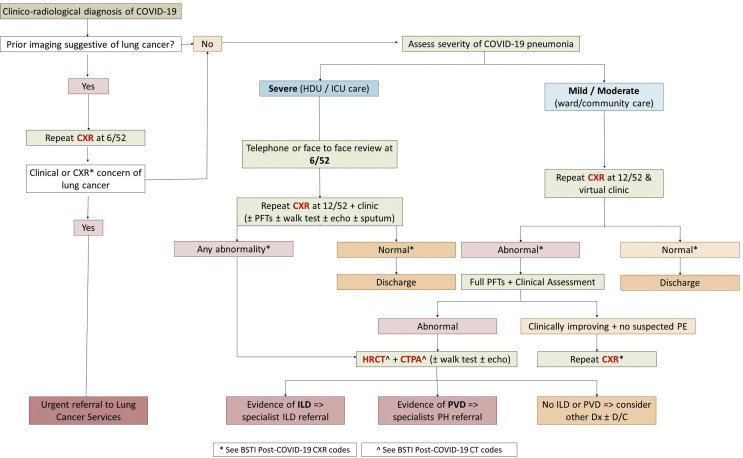
The British Thoracic Society (BTS) has published guidance57 on the respiratory follow-up and management of patients with a clinicoradiological diagnosis of COVID-19 (Electronic Supplementary Material Appendix S1). The BTS guidance∗ has defined two follow-up algorithms for COVID-19 patients according to disease severity and functional capacity of patients on discharge, and encompasses suggestions for holistic rehabilitation: severe COVID-19 pneumonia (patients cared for in critical care and a subset of ward-based patients) and mild–moderate pneumonia (patients typically cared for on a non-critical care hospital ward or in the community).
Those who had a severe pneumonia will undergo a telephone clinical consultation 4–6 weeks post-discharge with CXR and face-to-face consultation at 12 weeks. For those with mild–moderate pneumonia, the telephone consultation can occur slightly later at 12 weeks post-discharge, together with a CXR.
Irrespective of the above advice, if lung malignancy is suspected, either an early repeat CXR 6 weeks after discharge or referral to local cancer services is advised; however, individual hospitals may adapt their own recall and urgent cancer pathways to suit, tailoring the timing of further investigation on an individualised basis according to the level of suspicion and physiological impairment. Importantly, follow-up CXR is not required in patients without radiological evidence of pneumonia, or in those whose radiological changes have resolved by the time of discharge.
Progression to CT in either of group will be determined by clinical review, physiological impairment and CXR findings. If there are persistent CXR changes and/or evidence of physiological impairment, unenhanced CT and CT pulmonary angiography (PA) should be considered. The rationale for performing both unenhanced and contrast-enhanced imaging relates to the potential prevalence of thromboembolic disease in COVID-19 and the superior diagnostic quality of unenhanced inspiratory CT in ILD. Contrast enhancement of the lung parenchyma, together with an expiratory phase of imaging (often seen with non-breath hold rapid CTPA), can make the differentiation between true and artefactual GGO challenging, particularly posteriorly at the bases, areas commonly involved in COVID-192 (Fig 15 ).
Figure 15.
CT Technique. (a) CT Thorax obtained in inspiration with the patient supine showing fully inflated normal lungs. (b) CTPA on the same patient acquired at the same time also in the supine position. The CTPA was performed without a breath-hold and is partly in expiration as observed from the bowing of the posterior tracheal membrane (arrow). This, together with IV contrast, increases lung density giving the spurious impression of ground glass opacity.
Reporting templates
The reliance on imaging at 12 weeks post-discharge would suggest that a standardised template reporting system for CXR and CT could add significant benefit in post-COVID-19 follow-up and provide consistency for both community and hospital clinicians in terms of managing complications, as well as providing an opportunity for efficient long-term data collection.
Post-COVID-19 CXR guidance and reporting template (Electronic Supplementary Material Appendix S2)
In early March 2020, BSTI developed a simple, internationally recognised CXR reporting template for index COVID-19 presentation to help facilitate standardised reporting. This had embedded CXR reporting codes allowing retrospective radiology information system (RIS) keyword searches for audit purposes.1
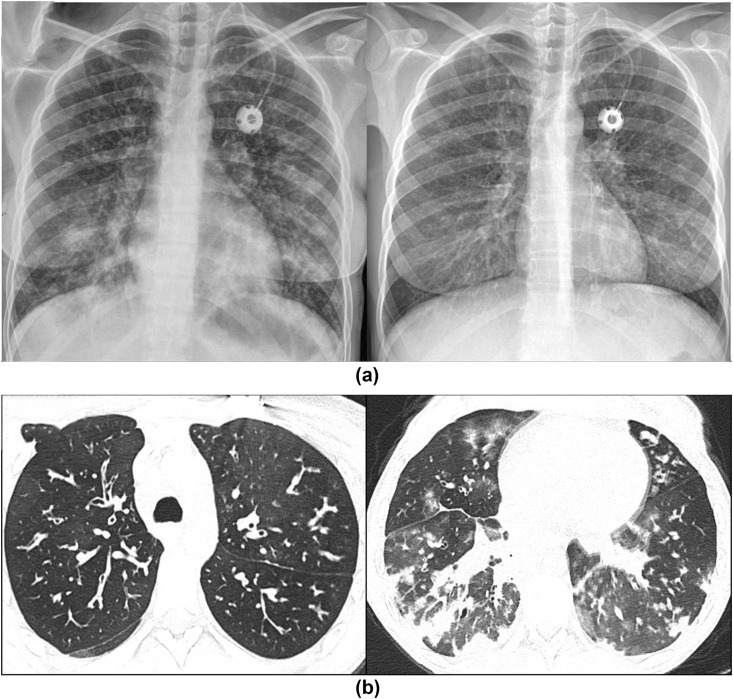
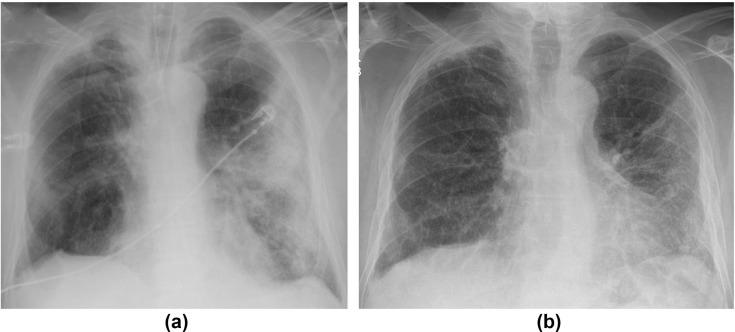
This new template is tailored for a post-COVID-19 CXR follow-up at 12 weeks and stratifies patients into one of five groups based on CXR findings: resolved (PCVCX0); significantly improved (PCVCX1); stable (PCVCX2); worsening, for example development of fibrosis even if alveolar opacity has improved (Fig 16 ; PCVCX3), and other findings, for example, lung malignancy, pneumothorax, or new infection (PCVCX4).
Figure 16.
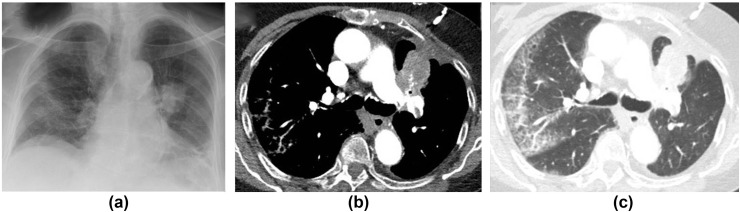
A 68-year-old man with COVID-19. (a) CXR on admission showed bilateral asymmetrical peripheral consolidation. (b) Follow-up CXR at 12 weeks shows improvement in the consolidation, but some volume loss and suggestion of architectural distortion concerning for fibrosis.
Post-COVID-19 CT reporting guidance and template (Electronic Supplementary Material Appendix S3)
The BSTI suggests similarly themed reporting codes for CT follow-up: normal (PCVCT0); improving (PCVCT1); stable (PCVCT2); fibrosis (PCVCT3), and other findings, similar to above (PCVCT4; Fig 17 ). An additional PE code can also be incorporated: no PE (PCVPE0); new acute PE (PCVPE1); chronic PE (PVCPE2); for example, PCVCT1 + PCVPE1 would indicate resolving COVID-19 changes with new acute PE. Fig. 18 illustrates another example of the application of several of these RIS codes.
Figure 17.
A 71-year-old man had a 6-week stay in hospital with COVID-19. (a) CT prior to discharge showed diffuse GGO with areas of consolidation and bronchial dilatation. Six-weeks later, he presented again unwell with raised inflammatory markers. (b) CT showed a focal thick-walled pleural collection of fluid and gas, confirmed to be an empyema.
Figure 18.
A 73-year-old man was admitted with COVID-19. (a) CXR at presentation showed a left hilar mass concerning for lung cancer and bilateral peripheral consolidation typical for COVID-19. (b) Early follow-up staging CT confirmed a left upper lobe tumour and pulmonary emboli and (c) peripheral GGO with inter- and intralobular septal thickening in keeping with COVID-19. RIS codes to be applied in this case would be PCVCT2 + PCVCT4 + PCVPE1.
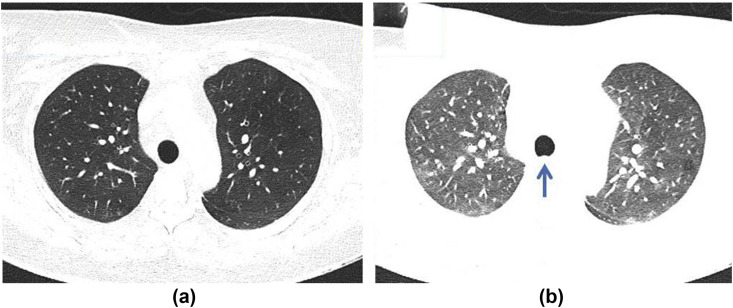
Follow-up of pulmonary nodules
We are aware that many departments have rationalised imaging across all subspecialties within radiology during the COVID-19 pandemic. Routine imaging services were temporarily suspended and subspecialties have adjusted guidelines to manage the demand and pressures for imaging locally; however, given the relatively rapid median growth rates of lung cancer, nodule surveillance should be appropriately prioritised. Expert consensus broadly recommends that during the pandemic, the initial intended point of surveillance could be deferred by 3–6 months where the risk of malignancy is low (e.g., for a solid nodule sub-8 mm in diameter).58 It would thus seem pragmatic to perform surveillance CT as close as possible to intended time points, while deferring nodules where stability has already been demonstrated. For example, a nodule requiring 3-month surveillance CT, which has now been delayed by another 6 months (i.e., 9 months from baseline) should be performed immediately. If stable, current recommendations59 would suggest performing the next follow-up at 12 months from baseline; however, this would then be only 3 months from the most recent CT. A multidisciplinary team may consider it appropriate and pragmatic to perform such a follow-up at a later time interval to allow for a longer duration over which to assess stability. We must stress that such approaches are currently not evidenced based, but may help to judiciously reduce a small percentage of potentially unnecessary examinations. In the recovery phase post-COVID-19, even such a modest reduction is vital.
Practical challenges for radiology departments
As follow-up imaging will mostly be performed at 12 weeks, it would seem unlikely there will be active shedding of the virus at this time. Imaging should therefore ideally be performed at designated clean sites/scanners. Nevertheless, it should be noted that, as yet, there is no formal evidence stating that re-infection is unlikely, and it remains unclear how long any acquired immunity to SARS-CoV-2 will last.
Post-COVID-19 follow-up could generate a significant volume of new radiology workload in departments that are already stretched with capacity issues and significant backlogs. Radiology departments will also need to retain the flexibility to juggle workflow at short notice to cope with any future surges in infection including a potential second peak of the pandemic.
Looking forward
Given the sheer number of patients that have experienced severe lung damage from COVID-19, chronic pulmonary morbidity is likely to be one of the major lasting impacts on our healthcare system. Therapeutic trials of anti-fibrotic medication in COVID-19 are being constructed. It is hoped that automated image analysis tools combined with data science applied to health records will be able to identify patients at risk of pulmonary complications at an early stage and enable recruitment into clinical trials.
Given the interdependence of cardiac disease on lung health and lung disease on cardiac health, and the effects of both on neurological disease, the long-term health consequences for COVID-19 patients will necessitate close surveillance and reporting to national registries. To this end, imaging repositories such as the National COVID-19 chest imaging database60 could provide a valuable resource that identifies healthcare needs and enables prioritisation of resources as the pandemic begins to stabilise.61 In the UK, the PHOSP-COVID study62 is also standardising outpatient CT acquisition protocols in patients that had been admitted acutely for COVID-19. In particular, the PHOSP-COVID study62 should allow a comprehensive evaluation of the prevalence and impact of COVID-19 on lung parenchymal, airway and vascular damage, as well as determining the effects of COVID-19 across various organ systems.
Conclusion
As the coronavirus pandemic begins to mature, the focus of imaging departments in the UK is changing direction to encompass the re-initiation of elective work whilst beginning to engage in the follow-up of patients previously diagnosed with COVID-19. Over the last 3 months, the multisystem effects of this disease have been recognised and, although comprehensive knowledge of their pathophysiology is still being sought, the need for a holistic approach to ongoing patient management and rehabilitation is acknowledged.
Thromboembolic complications and lung parenchymal damage are likely to be the commonest clinically significant sequelae in survivors of COVID-19. Although the epidemiology of pulmonary fibrosis has been inferred from previous SARS and MERS epidemics, the actual impact and prevalence of such complications will only be truly defined in the coming months.
Radiologists should be aware of the guidelines and imaging techniques for follow-up of patients with ongoing respiratory morbidity and such follow-up is likely to have an impact on service demand, stretching capacity further.
Standardised reporting templates and RIS codes can provide an element of consistency for clinicians involved in ongoing patient management and provide an opportunity for robust data collection.
Although there are specific guidelines for the follow-up of thoracic complications, patients with pulmonary fibrosis and pulmonary vascular disease (with or without pulmonary hypertension) will likely ultimately feed into existing clinicoradiological services, particularly with respect to the hub-and-spoke model for UK tertiary interstitial lung disease and pulmonary hypertension services.
Our knowledge of COVID-19 is changing at such a rate that speculation on its long-term impacts and relevant clinical questions may well be outdated by the time a manuscript goes to press, and there is also potential for the trajectory of this coronavirus pandemic to have changed within that timeframe.
Conflict of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Joseph Jacob reports fees from Boehringer Ingelheim and Roche unrelated to the current submission and is supported by a Clinical Research Career Development Fellowship 209553/Z/17/Z from the Wellcome Trust. Arjun Nair reports non-financial support from AIDENCE BV. No other declarations.
Acknowledgements
The authors thank Dr Gerard Meachery, Dr Michelle Muller, Dr Andrew Stanton, Dr Hilary Tedd, and Dr Sophie West (Newcastle upon Tyne Hospitals NHS Foundation Trust), Dr Neil Archibald (South Tees Hospitals NHS Foundation Trust), Dr Azer Majeed (South Tyneside and Sunderland NHS Foundation Trust), Dr Seung Choi, Dr Shishir Karthik, and Dr Anshuman Sengupta (Leeds Teaching Hospitals NHS Foundation Trust) for their invaluable contribution of images. J.J. is supported by a Clinical Research Career Development. Fellowship 209553/Z/17/Z from the Wellcome Trust and the Biomedical Research Centre at University College London. J.J. reports fees from Boehringer Ingelheim and Roche unrelated to the current submission. A.N. reports non-financial support from AIDENCE BV.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crad.2020.09.025.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Hare S.S., Rodrigues J.C.L., Nair A. The continuing evolution of COVID-19 imaging pathways in the UK: a British society of thoracic imaging expert reference group update. Clin Radiol. 2020;75(6):399–404. doi: 10.1016/j.crad.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues J.C.L., Hare S.S., Edey A. An update on COVID-19 for the radiologist — a British society of thoracic imaging statement. Clin Radiol. 2020;75(5):323–325. doi: 10.1016/j.crad.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui D.S., Joynt G.M., Wong K.T. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das K.M., Lee E.Y., Singh R. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imag. 2017;27(3):342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danzi G.B., Loffi M., Galeazzi G. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salehi S., Abedi A., Balakrishnan S. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;14:1–7. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 8.Li X., Ma X. Acute respiratory failure in COVID-19: is it "typical" ARDS? Crit Care. 2020;24(1):198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Morales A., Cardona-Ospina J.A., Gutierrez-Ocampo E. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metersky M.L., Masterton R.G., Lode H. Epidemiology, microbiology and treatment considerations for bacterial pneumonia complicating influenza. Int J Infect Dis. 2012;16(5):e321–e331. doi: 10.1016/j.ijid.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Wu C.P., Adhi F., Highland K. Recognition and management of respiratory coinfection and secondary bacterial pneumonia in patients with COVID-19. Cleve Clin J Med. 2020;11:ccc015. doi: 10.3949/ccjm.87a.ccc015. [DOI] [PubMed] [Google Scholar]
- 12.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao W., Wang T., Jiang B. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020;10:S0007–0912(20):30203–30208. doi: 10.1016/j.bja.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu C.W., Sun S.F. Iatrogenic pneumothorax related to mechanical ventilation. World J Crit Care Med. 2014;3(1):8–14. doi: 10.5492/wjccm.v3.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J., Su X., Zhang T. Spontaneous pneumomediastinum: a probable unusual complication of coronavirus disease 2019 (COVID-19) pneumonia. Korean J Radiol. 2020;21(5):627–628. doi: 10.3348/kjr.2020.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsiao C.H., Wu M.Z., Hsieh S.W. Clinicopathology of severe acute respiratory syndrome: an autopsy case report. J Formos Med Assoc. 2004;103:787–792. [PubMed] [Google Scholar]
- 18.Wichmann D., Sperhake J.P., Lutgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19. A prospective study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connors J.M., Levy J. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang M., Som A., Mendoza D. Hypoxaemia related to COVID-19:vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020;Apr;S1473–3099(20):30367–30374. doi: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gall H., Hoeper M.M., Richter M.J. An epidemiological analysis of the burden of chronic thromboembolic pulmonary hypertension in the USA, Europe and Japan. Eur Respir Rev. 2017;26(143):160121. doi: 10.1183/16000617.0121-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terpenning S., Deng M., Hong-Zohlman S.N. CT measurement of central pulmonary arteries to diagnose pulmonary hypertension (PHTN): more reliable than valid? Clin Imaging. 2016;40(4):821–827. doi: 10.1016/j.clinimag.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Grosse A., Grosse C., Lang I. Evaluation of the CT imaging findings in patients newly diagnosed with chronic thromboembolic pulmonary hypertension. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0201468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandey A.K., Wilcox P., Mayo J.R. Predictors of pulmonary hypertension on high-resolution conputed tomography of the chest in systemic sclerosis: a retrospective analysis. Can Assoc Radiol J. 2010;61(5):291–296. doi: 10.1016/j.carj.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Deveraj A., Wells A.U., Meister M.G. Detection of pulmonary hypertension with multidetector CT and echocardiography alone and in combination. Radiology. 2010;254(2):609–616. doi: 10.1148/radiol.09090548. [DOI] [PubMed] [Google Scholar]
- 26.Bax S., Jacob J., Ahmed R. Right ventricular to left ventricular ratio at CT pulmonary angiogram predicts mortality in interstitial lung disease. Chest. 2020;157(1):89–98. doi: 10.1016/j.chest.2019.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meinel F., Nance J.W., Schoepf U.J. Predictive value of computed tomography in acute pulmonary embolism: systematic review and meta-analysis. Am J Med. 2015;128(7):747–759. doi: 10.1016/j.amjmed.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Ema R., Sugiura T., Kawata N. The dilatation of main pulmonary artery and right ventricle observed by enhanced chest computed tomography predict poor outcome in inoperable chronic thromboembolic disease. Eur J Radiol. 2017;94:70–77. doi: 10.1016/j.ejrad.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava V., McPherson S.J., Smith N.C.E. National Confidential Enquiry into Patient Outcome and Death; 2019. Know the score. A review of the quality of care provided to patients aged over 16 years with a new diagnosis of pulmonary embolism. [Google Scholar]
- 30.Gopalan D., Delcroix M., Held M. Diagnosis of chronic thromboembolic pulmonary hypertension. Eur Resp Rev. 2017;26(143):160108. doi: 10.1183/16000617.0108-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei J., Lei P., Yang H. Analysis of thin-section CT in patients with coronavirus disease (COVID-19) after hospital discharge. J Xray Sci Technol. 2020;28(3):383–389. doi: 10.3233/XST-200685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ujita M., Renzoni E.A., Veeraraghavan S. Organising pneumonia: perilobular pattern at thin-section CT. Radiology. 2004;232:757–761. doi: 10.1148/radiol.2323031059. [DOI] [PubMed] [Google Scholar]
- 33.Akira M., Kozuka T., Yamamoto S. Computed tomography findings in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:372–378. doi: 10.1164/rccm.200709-1365OC. [DOI] [PubMed] [Google Scholar]
- 34.Nair A., Walsh S.L., Desai S.R. Imaging of pulmonary involvement in rheumatic disease. Rheum Dis Clin North Am. 2015;41:167–196. doi: 10.1016/j.rdc.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Jiang F., Deng L., Zhang L. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35(5):1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalised patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao L., Wang L., Chen S. Neurological manifestations of hospitalised patients with COVID-19 in Wuhan, China: a retrospective case series study. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babapoor-Farrokhran S., Gill D., Walker J. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalised patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fried J.A., Ramasubbu K., Bhatt R. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141(23):1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paul J.F., Charles P., Richaud C. Myocarditis revealing COVID-19 infection in a young patient. Eur Heart J Cardiovasc Imaging. 2020;21(7):776. doi: 10.1093/ehjci/jeaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clerkin K.J., Fried J.A., Raikhelkar J. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 45.Huang L., Zhao P., Tang D. Cardiac involvement in recovered COVID-19 patients identified by magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;S1936–878X(20):30403–30404. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Licciardi F., Pruccoli G., Denina M. SARS-CoV-2-induced Kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Paediatrics. 2020;146(2) doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- 47.Jones V.G., Mills M., Suarez D. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Paediatr. 2020;10(6):537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 48.Schroeder A., Wilson K., Ralston S. COVID-19 and Kawasaki disease: finding the signal in the noise. Hosp Pediatr. 2020 May 13 doi: 10.1542/hpeds.2020-000356. hpeds.2020-000356. [DOI] [PubMed] [Google Scholar]
- 49.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee I.C., Huo T.I., Huang Y.H. Gastrointestinal and liver manifestations in patients with COVID-19. J Chin Med Assoc. 2020 Jun;83(6):521–523. doi: 10.1097/JCMA.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu L., Liu J., Lu M. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kishfy L., Casasola M., Banankhah P. Posterior reversible encephalopathy syndrome (PRES) as a neurological association in severe COVID-19. J Neurol Sci Jul. 2020;414:116943. doi: 10.1016/j.jns.2020.116943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaya L., Kara S., Akinci C. Transient cortical blindness in COVID-19 pneumonia; a PRES-like syndrome: case report. J Neurol Sci Jun. 2020;413:116858. doi: 10.1016/j.jns.2020.116858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciaglia E., Vecchione C., Puca A.A. COVID-19 infection and circulation ACE2 levels: protective role in women and children. Front Pediatr Apr. 2020;8:206. doi: 10.3389/fped.2020.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng H., Wang Y., Wang G.Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92(7):726–730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.George P.M., Barratt S.L., Condliffe R. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020 Aug 24 doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 58.Mazzone P.J., Gould M.K., Arenberg D.A. Management of lung nodules and lung cancer screening during the COVID-19 pandemic: CHEST Expert Panel Report. J Am Coll Radiol. 2020;17(7):845–854. doi: 10.1016/j.jacr.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Callister M.E., Baldwin D.R. Akram AR on behalf of the British Thoracic Society Standards of Care Committee et al. British Thoracic Society Guidelines for the investigation and management of pulmonary nodules: accredited by NICE. Thorax. 2015;70:ii1–ii54. doi: 10.1136/thoraxjnl-2015-207168. [DOI] [PubMed] [Google Scholar]
- 60.NHSX . 2020. National COVID-19 chest image database.https://nhsx.github.io/COVID-chest-imaging-database/ [Google Scholar]
- 61.Jacob J., Alexander D., Baillie J.K. Using imaging to combat a pandemic: rationale for developing the UK National COVID-19 Chest Imaging Database. Eur Resp J. 2020;56(2):2001809. doi: 10.1183/13993003.01809-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.NIHR . 5 July 2020. Major study into long-term health effects of COVID-19 launched in the UK.https://www.nihr.ac.uk/news/major-study-into-long-term-health-effects-of-COVID-19-launched-in-the-uk/25200 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.