Highlights
-
•
SARS-CoV-2 expresses viral S-protein that exploits ACE2 to gain host cell entry.
-
•
The renin-angiotensin-aldosterone system influences SARS-CoV-2 pathogenicity.
-
•
ACE2-mediated endothelial infection partially explains vascular pathologies in COVID-19.
-
•
NSAIDs enhance viral replication and shedding in several known viruses.
-
•
Corticosteroids mediate pathways that may aggravate early infection.
Keywords: NSAIDs, SARS-CoV-2, Corticosteroids, Viral infection, Renin-angiotensin system, Paracetamol
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the etiologic agent of coronavirus disease 19 (COVID-19), and is genetically related to the 2003 SARS and Middle East respiratory syndrome (MERS-CoV) coronaviruses. Recent studies have reported that similar to SARS-CoV, this strain expresses a spike protein (S) with a receptor binding domain (RBD) that binds to angiotensin-converting enzyme 2 (ACE2) – an enzyme expressed mostly in the endothelium, kidneys, heart, gastrointestinal tract and lungs – to facilitate viral entry and intracellular replication. Incidentally, the renin-angiotensin-aldosterone system (RAAS) is integral to physiologic control of both ACE and ACE2 expression, and is an essential system utilized by SARS-CoV-2, albeit with varying schools of thought on how it can affect viral entry. In this paper, we will review current knowledge on the RAAS and how it can be affected by non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroid use at the organ and cellular levels. We will then discuss the relevance of these interactions on organ-specific ACE2 expression, and provide scientific insights on how this mechanism can potentially affect SARS-CoV-2 infection in the early phases of disease. From the standpoint of other known viruses, we will then aim to discuss the potential uses or restrictions of these drugs in viral infection, and provide an update on relevant studies about COVID-19.
1. Introduction
Coronavirus disease 19 (COVID-19) is a highly contagious and potentially fatal respiratory disease caused by SARS-CoV-2, a strain of coronavirus that originated in the city of Wuhan of the Hubei province in China, which was first discovered to circulate in the Huanan Food Market early of December 2019 (Fan et al., 2020; Rothan and Byrareddy, 2020). In a span of ten months, the virus has already infected more than 39, 261, 533 people worldwide, and has been attributed to more than 1, 104, 202 deaths (WorldOMeter, 2020a), making the current crude fatality rate (CFR) of COVID-19 to be 2.81 %. The crude CFR currently varies from 0.048 % (Singapore) to 10.21 % (Mexico) among countries with more than 50,000 cases (WorldOMeter, 2020a), and although the USA has become the greatest contributor to the case tally with a total of 8,219,831 cases to date, they have a lower CFR of about 2.71 % (WorldOMeter, 2020b). An article published at The Lancet this March indicated risk factors for mortality among patients with COVID-19 in Wuhan, which included an older age (>69 yrs; median age of 58 yrs), a higher Sequential Organ Failure Assessment (SOFA) score, and a d-dimer level greater than 1 μg/mL (Zhou et al., 2020). Data from the Centers for Disease Control and Prevention (CDC) reported that 20 % of hospitalized patients were from the 20–44 years age group, with more than half of hospitalized (55 %) coming from the workforce (20–64 years) (Centers for Disease Control and Prevention, 2020a). These data indicate that the risk for severity should be interpreted on the basis of age, the presence of comorbidities such as sepsis, shock or organ dysfunction, as well as any coagulopathies or diseases that may predispose to blood clot formation, such as pneumonia or disseminated intravascular coagulation (DIC). Likewise, it is prudent to assume that CFR is better interpreted after age-standardization is done, or when it is adjusted for the presence of comorbidity, which has been done in some reports including that of the Centers for Disease Control and Prevention (CDC) (Centers for Disease Control and Prevention, 2020b)
A possible contributor to the risk of severity or to variations in the CFR, however, may also come from the patient’s medications. There have been studies proposing that COVID-19 morbidity and mortality are a result of excessive inflammation, such as airway inflammation and cytokine storms (Hu et al., 2020), and infected patients in the early phases of disease may experience malaise or fever and may seek symptomatic relief through analgesics like paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen. Similarly, patients with chronic inflammatory diseases may take NSAIDs or corticosteroids as management for their disease, which may increase their susceptibility to microbial infection. Since COVID-19 is a viral illness, there have been concerns that anti-inflammatory drug use can aggravate SARS coronavirus infections (Fang et al., 2020; Auyeung et al., 2005), but evidence on this speculation has not been unified and is even contradicting. Interestingly, the membrane receptor of SARS-CoV-2, which is ACE2, is an essential component of the regulatory mechanism of the renin-angiotensin-aldosterone system (RAAS), and this hormone pathway may interact with the pathophysiology of COVID-19. To this end, this paper will review the mechanisms of the RAAS and its coincidence with the anti-inflammatory pathways of NSAIDs and corticosteroids both at the cellular and organ levels. We will discuss current evidence on the effects of these anti-inflammatory drugs on SARS-CoV-2 infection, as well as the potential interactions of pathways upstream or downstream of ACE2 on the RAAS, in an effort to bridge these pathways in the setting of COVID-19. Based on the evidence collated, we will then compare the anti-inflammatory drug classes included in this review to determine the potential uses or restrictions of these drugs in early SARS-CoV-2 infection.
2. Materials and methods
For this review, we did a literature search at NCBI PubMed and MEDLINE databases to gather current studies on the COVID-19-RAAS-anti-Inflammatory drug interactions using the terms 'SARS-CoV-2′ or 'COVID-19′, matched with 'renin-angiotensin system', 'renin-angiotensin-aldosterone-system', 'RAAS' or 'RAS', which yielded 374 unique results. We further narrowed the search by including only the studies which investigated the effects of anti-inflammatory drugs or corticosteroids on COVID-19, using additional terms for the string such as ‘corticosteroids’, ‘NSAID’, ‘steroids’, ‘anti-inflammatory’ or ‘inflammation’, and by narrowing the publication type to ‘Journal article’, ‘Clinical trial’, ‘review’, ‘systematic review’, ‘meta-analysis’, or ‘randomized controlled trial’. We then analyzed each article to include only those that proposed either the mechanisms of action of SARS-CoV-2 on the RAAS or of the drugs that interact with SARS-CoV-2 or the RAAS, to a final count of 63. From the following studies, all journal articles, clinical trials, systematic reviews and meta-analyses, excluding literature reviews, were included only if they provided experimental or clinical evidence, which narrowed the inclusion to five articles (two retrospective cohort studies, one case series, one in silico analysis, and one in vitro study). A second, less-stringent search was done which included studies on anti-inflammatory or corticosteroid drug use for COVID-19 with or without associations to the RAAS, using the similar terms as mentioned above. This search returned seven studies which were either systematic reviews or meta-analyses or both, and were included in this review (Supplementary File 1). For the said searches, we considered articles that are not only in the English language, but also those written in Chinese, Dutch, French and Russian. The two literature searches were accomplished on August 6.
On the other hand, we included studies on the epidemiology of COVID-19 and the molecular biology of SARS-CoV-2 based on chronology to reflect the turn of events during the pandemic. For most of the review, basic science researches that explain or complete mechanistic pathways on the physiology and biology of the RAAS or of the mechanisms of anti-inflammatory drugs and corticosteroids were referenced from PubMed and Google Scholar at our discretion. In addition, pertinent references suggested by the reviewers were included as needed, especially those that were not covered at the time of the literature search.
It is important to note that since the approach of this paper is to provide current knowledge on the anatomic, physiologic and molecular bases of anti-inflammatory drug and corticosteroid action on the RAAS, this paper will not demonstrate a systematic review or meta-analysis of current clinical evidence, but will only provide insight on the probable influences of the discussed pathways on early SARS-CoV-2 infection. Further, since there is a present lack of definitive studies investigating the association between NSAID or corticosteroid drug use and COVID-19 outcome severity, such clinical analyses are only warranted as more evidence is obtained.
3. SARS-CoV-2 and its human receptor
In late of December 2019, Dr. Wang and researchers from the Institute of Pathogen Biology in China obtained the complete sequence of SARS-CoV-2 from bronchoalveolar lavage specimens of patients in Wuhan presenting with acute respiratory distress syndrome (ARDS) (Ren et al., 2020). A subsequent study then extracted SARS-CoV-2 DNA from nine patients with COVID-19, which found a 79 % and 50 % identity with SARS-CoV and MERS-CoV, respectively (Lu et al., 2020). Similarly, another study reported an 86 % similarity between the SARS-CoV and SARS-CoV-2 genomic sequences, with high degrees of homology to the SARS-like coronaviruses isolated from bats (Chan et al., 2020). Since SARS-CoV was known to bind to human angiotensin-converting enzyme 2 (ACE2) for viral entry, these data suggested that SARS-CoV-2 may also utilize its high affinity binding for human ACE2 to enter host cells, by virtue of its conserved receptor binding domain (RBD), which was later confirmed to be true (Chan et al., 2020; Wilder-Smith et al., 2020; Baig et al., 2020). In 2017, Khan et al. developed a pilot clinical trial using human recombinant soluble ACE2, also known as GSK2586881, as a potential treatment for acute lung injury. The two-part clinical trial, although showed a successful interaction of hrsACE2 and its targets by evaluating the modulation of RAS, did not elaborate any clinical changes or improvements upon administration of GSK2586881 (Khan et al., 2017). It did, however show an increase in Ang(1–7) synthesis from Ang II, offering protection against heart failure with reduced and preserved ejection fraction largely due to ACE2 deficiency, as well as preventing Ang II-induced myocardial hypertrophy, diastolic dysfunction, and myocardial fibrosis (Patel et al., 2016; Guo et al., 2020). Earlier this year, Monteil et al. did a similar study where they engineered human blood vessel organoids and human kidney organoids, both of which expressed a sufficient amount of ACE2, to emulate SARS-CoV-2 infection in human tissues and organs. This idea was built on the knowledge that SARS-CoV-2 mainly uses ACE2 as the entry point into cellular invasion. By introducing human recombinant soluble ACE2 (hrsACE2), the S-protein region of SARS-CoV-2 was competitively bound by the enzyme, which effectively limited the binding and entry of SARS-CoV-2 thereby preventing infection in a dose-dependent manner (Monteil et al., 2020 Apr). Several more clinical trials emerged which suggested the importance of hACE2 to SARS-CoV-2 infection, and which led hrsACE2 to become one of most promising therapeutics for the early prevention of COVID-19 to date. There were six amino acids identified in the RBD that is crucial for ACE2 binding, and while five of these differed between SARS-CoV and SARS-CoV-2, the binding affinity of SARS-CoV-2 RBD to human ACE2 receptors was found to be 10 times higher than its counterpart spike protein in SARS-CoV (Andersen et al., 2020; Wrapp et al., 2020)). This ability of SARS-CoV-2 to bind the ACE2 enzyme with a stronger affinity than SARS-CoV and HCoV-NL63 under a non-ideal configuration strongly implies that prior mutations have been occurring as a form of natural selection. That is, SARS-CoV-2 may have been circulating the wet animal markets for several years before the outbreak and infecting individuals without triggering any symptoms (Andersen et al., 2020; Fehr and Perlman, 2015).
The downstream effect of the ACE2 ligand-receptor complex is the degradation of angiotensin II (Ang II) to produce angiotensin (1–7), initiating a negative feedback mechanism that inhibits further production of Ang II. Decreased Ang II results in decreased salt and water reabsorption, which lowers blood pressure and vascular tone, and effectively prevents the excessive effects of Ang II (Tikellis and Thomas, 2012; Fountain and Lapin, 2019). When the SARS-CoV-2 viral spike S protein binds the human ACE2 enzyme, it fuses with the cell membrane to initiate viral entry by activating signal transduction pathways, resulting to cell uptake of the virus-receptor complex via a process called clathrin-mediated endocytosis. Moreover, the S protein of SARS-CoV-2 possesses an S1/S2 site, which is cleaved by furin, to encourage entry of SARS-CoV-2 into the cell while evading the host immune response (Hoffmann et al., 2018; Howley and Knipe, 2013; Burkard et al., 2014; Hoffmann et al., 2020). In the process, ACE2 is inhibited and internalized, and reduced membrane ACE2 affects the homeostasis of RAAS due to decreased Ang II breakdown. Ang II is a direct vasoconstrictor known for its predominantly vasoactive properties, and its excessive production is associated with acute hemodynamic changes, such as hypertension and a drop in glomerular filtration rate (GFR). This triggers chronic inflammation if left unaddressed, and eventually leads to end-organ failure due to fibroid formation (Vukelic and Griendling, 2014; Elmarakby et al., 2016; Muñoz-Durango et al., 2016).
4. Tissue distribution of ACE2 and its relevance to SARS-CoV-2 infection
ACE2 is an active homologue of ACE seen in a diverse group of tissues including the oral and nasal mucosa, the lungs, the gastrointestinal tract, blood forming organs, liver, kidney, brain, and endothelial cells, with the majority seen localizing in the tongue and esophageal epithelial cells, enterocytes, male reproductive cells, type II lung alveolar epithelial cells, renal tubules, ductal cells, bladder urothelial cells, endothelial cells, and cardiomyocytes (Hamming et al., 2004; Clarke and Turner, 2012; Hikmet et al., 2020; Xu et al., 2020). Interestingly, the number of organs that express ACE2 continues to increase as more data is published, which can be monitored using the COVID-19 Cell Atlas database (https://www.covid19cellatlas.org/). The ramifications on the ability of the virion to provoke cellular or tissue damage remain intriguing, but for one, it is dependent not only on the presence or absence of ACE2 but also on the amount of ACE2 on a certain organ or tissue.
One of the most commonly studied regions of SARS-CoV-2 entry is the respiratory system, for which it is thought to be the main route of infection, transmission, and pathophysiology. The specialized type II alveolar cells express an abundant amount of ACE2, and ACE2 inactivity due to SARS-CoV-2-induced depletion early in the infection may disturb the RAS homeostasis, leading to impaired tissue repair mechanisms, increased vascular permeability, fluid accumulation in extra-alveolar spaces, and oxidant/antioxidant imbalance (Perrotta et al., 2020). In a study by Ackerman et al., which examined postmortem lungs of patients who died from COVID-19 and compared them to 7 lungs obtained from patients who died from ARDS secondary to AH1N1 and to 10 age-matched healthy lungs, COVID-19 patients were found to suffer more frequently from severe endothelial injury due to cell membrane damage by presence of SARS-CoV-2. Widespread thrombosis with microangiopathy was seen, and patients were 9 times more likely to experience alveolar capillary microthrombi, and 2.7 times more likely to experience intussusceptive angiogenesis than with flu (Ackermann et al., 2020). This phenomenon of thrombosis and other vascular events in COVID-19 is likely the result of an abundance of ACE2 in endothelial cells, which permits SARS-CoV-2 infection along the endothelium. This will be further discussed in later sections.
SARS-CoV-2 has also been studied to disseminate into the CNS, by initially invading the peripheral nerve terminals via the synapse-connected route, through the mechanoreceptors and chemoreceptors in the lungs, and into the medullary-cardiorespiratory center (Abate et al., 2020; Li et al., 2020a). However, ACE2 is not particularly abundant in neurons, and other pathways of infection are being investigated. The oral and nasal mucosa also holds a significant distribution of ACE2, especially on the tongue epithelial cells, which may explain its route of infection (Xu et al., 2020). If and how the virus affects the activity of the tongue epithelial cells were investigated by a cross-sectional study in L. Sacco Hospital in Milan, Italy, where the prevalence of olfactory and taste disorders in hospitalized COVID-19 patients were obtained. Of the 88 hospitalized COVID-19 patients, all 59 patients who were coherent and responsive reported the persistence of olfactory and taste disorders throughout the disease (Giacomelli et al., 2020), indirectly supporting the hypothesis of oral and mucosal routes of invasion to the nervous system. Interestingly, ACE2 population is also pervasively seen in pancreatic cells, which in fact shows a higher expression profile than pulmonary cells. This possibly makes the pancreas, by virtue of ACE2-dependent viral entry, one of the major targets of SARS-CoV-2. Complications may include acute pancreatitis resulting from direct injury to pancreatic acinar cells or uncontrollable systemic inflammatory response from cytokine storm syndrome, both of which have been documented in the disease (Thaweerat, 2020). Acute pancreatitis due to SARS-CoV-2 infection could pose an additional threat to people afflicted with diabetes mellitus (DM) types 1 and 2 by promoting metabolic complications as the infection continues to alter pancreatic β-cell function, which may in fact explain the feared risks of severe COVID-19 among patients with DM (Apicella et al., 2020). In the kidneys, the ACE2 expression in the proximal tubules remains the highest in concentration, with markedly lower concentrations in the glomeruli (Mizuiri and Ohashi, 2015). STZ-treated rats in previous experiments were found to have higher ACE mRNA/ACE2 mRNA ratio, a marker of kidney damage that resulted in renal injury similar to human diabetic nephropathy (Tesch and Allen, 2007; Ma et al., 2014). Due to a higher ACE/ACE2 ratio, there was less Ang-(1–7) synthesized in the kidney, which can effectively eliminate its renoprotective effects (Mizuiri and Ohashi, 2015). In an experimental study using C57BL/L mice models, investigators compared the physiology of normal 20–22 week-old C57BL/6 mice against ACE2−/y mice which lacked ACE2 expression. The eNOS expression in both protein and mRNA levels and NO concentrations in the aortas of ACE2−/y mice were decreased, along with urine and plasma nitrite concentration. On the other hand, lipid peroxidation was markedly increased, in contrast to a decreased superoxide dismutase level in the aorta homogenates of ACE2−/y mice subjects – an indication of impaired antioxidant activity leading to the formation of reactive oxygen species (ROS) (Sena et al., 2018) and observed vascular dysfunction in ACE2-negative C57BL/6 mice (Rabelo et al., 2016). Disturbances in the oxidative activity of the vascular system could pose a risk for acquiring inflammation, vascular remodelling and vascular injury, eventually leading to activation of the coagulation cascade that could cause microthrombi formation (Sena et al., 2018; Schwarzmaier et al., 2016). Taken together, these correlate to the negative consequences impacting the vascular homeostasis of SARS-CoV-2-induced ACE2 deficiency, with partial relations to the renin-angiotensin-aldosterone system (RAAS), which will be discussed.
5. ACEi and ARB drugs and their associations with COVID-19 symptom severity
A correspondence by Fang et al. published at The Lancet this March discussed that hypertensives and diabetics taking ACE2 inhibitor (ACEi) and angiotensin receptor blocking (ARB) drugs may be at increased risk of infection by SARS-CoV-2 and severity of COVID-19, citing three studies wherein diabetes and hypertension were major comorbidities of patients with severe COVID-19 and of non-survivors (Fang et al., 2020). Several criticisms were received by this article, mainly due to the lack of clinical evidence for many of the author’s claims at the time. A descriptive study on the pharmacologic characteristics of 96 COVID-19 patients in the Toulouse University Hospital Intensive Care Unit (ICU) in France showed that many of the patients who were intubated had either taken paracetamol (33.8 %), ARBs (28.2), calcium channel blockers (15.5 %), corticosteroids (15.5 %), or metformin (14.1 %). However, these patients also had pre-existing co-morbid conditions that warranted medication intake, such as obesity, arterial hypertension, and diabetes mellitus, which may regard the evidence as coincidental at best. Since the study was also descriptive (case series), the results and their implications can only be suggestive (Montastruc et al., 2020). However, a drug interaction cannot be ruled out. In terms of the RAAS, interaction between ARB/ACEi drugs and viral entry is plausible, since these drugs either inhibit ACE or the receptor of its enzymatic product angiotensin II, and a known physiologic response of cells to inhibition or internalization is feedback upregulation (Bittner and Martyn, 2019). Since the known human receptor of SARS-CoV-2 is ACE2, then it is possible for hypertensives chronically taking ACEis to be susceptible to SARS-CoV-2 infection, if feedback upregulation of ACE2 expression does occur, or due to the patients’ impaired ability to decrease Ang II secondary to renin production, all of which may increase membrane ACE2 levels (Ferrario et al., 2005; Huang et al., 2010) (Fig. 1 ). A similar pathway is expected for ARBs, since inhibition of angiotensin receptors can trigger a feedback mechanism to increase Ang II synthesis resulting to an upregulation of ACE2 expression (Fig. 1), and this has been shown in previous studies (Ferrario et al., 2005; Igase et al., 2008; Gallagher et al., 2008; Furuhashi et al., 2015). Several other studies have since followed this hypothesis closely, and have in the process unraveled the role of the renin-angiotensin-aldosterone system (RAAS) on the observed clinical outcomes of some COVID-19 patients, albeit with a surprisingly different mechanism. Two experimental studies, one in silico and another in vitro, have clarified and suggested the role of the RAAS, as follows. An in silico approach, which utilized the COXPRESdb v7, Kyoto Encyclopedia of Genes and Genomes (KEGG) PATHWAY and THE HUMAN PROTEIN ATLAS databases to determine the co-expression, molecular interactions and organ-specific expression of ACE2, neprilysin and carbonic anhydrase (CA), have shown that these three enzymes were highly co-expressed in the local RAS, with important overlapping functions on the production of Ang-(1–9) and Ang-(1–7) for the regulation of systemic vascular resistance and the conversion of CO2 in times of hypoxia. Further, the authors found that these enzymes were most likely co-expressed at organs of interest such as digestive, renal, respiratory, and reproductive systems, with the exception of CA which is ubiquitous (Emameh et al., 2020). Likewise, an in vitro study showed that lung epithelial cells infected with SARS-CoV expressed 11 unique RAS genes within a 48-hr period, which correlated well with the time of viral infection. These genes were divided into those that were significantly upregulated during first 24 h (ACE, ACE2, insulin-like growth factor 2 receptor (IGF2R), angiotensinogen (AGT), epidermal growth factor receptor (EGFR), matrix metalloendopeptidase (MME), alanyl aminopeptidase (ANPEP)) and those that were significantly upregulated by the 48th hr (arginyl aminopeptidase (ARPEP), EGFR, ANPEP, neurolysin, ACE2, IGFR, leucyl and cysteinyl aminopeptidase (LNPEP) and cathepsin D), all of which has clearly shown the early response of the RAAS on SARS infection (Turk et al., 2020). Studies have also recently reported that cleavage of ACE2 due to the interactions with the viral S protein leads to inhibition of ACE2 function through the RAS ACE/Ang II/AT1a arm (Vrigkou et al., 2017; Alfano et al., 2020). Another study has suggested that excessive activation of RAAS by SARS-CoV-2 plays a role in the onset of acute respiratory distress syndrome (ARDS), a severe form of acute lung injury (Zhang et al., 2020). In the presence of ARDS, fluid builds up in the alveoli, damaging the surfactant and promoting inflammation, eventually leaving fibrotic scars that prevents complete oxygen uptake. It is important to note that the binding of the SARS-CoV-2 spike RBD to ACE2 effectively inhibits the enzyme receptor, leading to endocytosis and early ACE2 depletion. This mechanism, which can interact with many other pathways, can also lead to the onset of ARDS, by virtue of feedback activation of the RAAS or by other mechanisms dependent solely on ACE2.
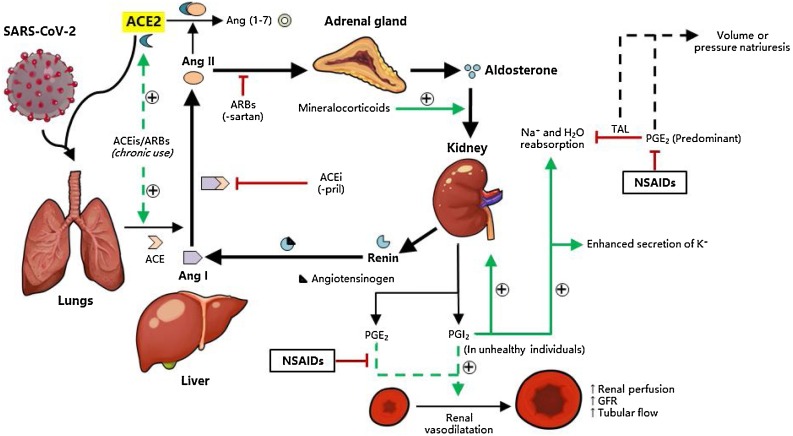
Fig. 1.
(ARB-ACEi mismatch was now addressed) Interaction of the renin-angiotensin-aldosterone system (RAAS) with SARS-CoV-2. The RAAS is an important homeostatic mechanism of the body that involves the kidney, liver, lungs and adrenal glands, which responds mainly to electrolyte and volume changes as well as to renal blood flow. In times of reduced plasma volume, reduced perfusion to the kidneys and to a concomitant loss of Na+, the juxtaglomerular cells of the kidney produce renin, which catalyzes liver angiotensinogen to produce angiotensin I (Ang I). The lungs then upregulate angiotensin-converting enzyme (ACE) that converts Ang I to Ang II, which influences the adrenal glands to synthesize more aldosterone. In addition, Ang II has a direct vasoconstrictive effect. Aldosterone (mimicked by other mineralocorticoids), which is released from the adrenal glands then influences the kidneys to increase Na+ and H2O reabsorption in the distal convoluted tubules and collecting ducts. Under normal conditions, prostaglandins E2 and I2 are formed which, in the kidneys, generally vasodilate the afferent renal arterioles to increase the glomerular filtration rate (GFR) and renal perfusion. In addition, PGE2 predominantly inhibits Na+ and H2O reabsorption in the thick ascending loop of Henle (TAL) to induce volume or pressure natriuresis, which harmonizes with the RAAS to maintain homeostasis. In hypertension and other conditions of reduced renal blood flow, the effects of PGI2 are marked and there is increased reabsorption of Na+ and H2O. Chronic use of ACE inhibitors (ACEis) and angiotensin receptor type 1 blockers (ARBs) can lead to a compensatory upregulation of ACE and ACE2 expression, which is the probable mechanism for the aggravation of SARS-CoV-2 infection among chronic hypertensives taking these drugs.
To date, there seems to be no sufficient clinical evidence that can confirm the biological relevance of these experimental results. In a retrospective multicenter cohort study of 154 COVID-19-positive geriatric patients in Belgian nursing homes, a non-significant association was observed between ARB/ACEi use and severe or poor outcomes (OR 0.48; CI 0.10–1.97) (Spiegeleer et al., 2020). In another retrospective cohort study of 117 geriatric COVID-19 patients with diabetes mellitus (DM) in Daegun, South Korea, use of ACEi/ARB drugs did not significantly affect the severity of outcome of COVID-19, apart from an incidental protective effect against acute cardiac injury (OR 0.048), which was expected from the mechanism of action of the drugs (Chung et al., 2020). Another review article discussing the interaction between RAAS inhibitors and ACE2 in the context of COVID-19 claimed that although angiotensin-receptor blockers and mineralocorticoid blockers increased ACE2 expression in a number of experimental and clinical models, ACE2 inhibitors, while increasing cardiac ACE2 mRNA levels, had no effect on ACE2 activity and expression. A direct inhibitor of renin, aliskiren, in contrast was associated with a decrease in ACE2 expression but the mechanism of action remains to be studied further (Mourad and Levy, 2020). In contrast, Wysocki and authors investigated the effects of ACEis and ARBs on membrane and total ACE2 expression in lung and kidney cells and lysates, respectively, and found that use of these drugs do not significantly affect both the membrane and total ACE2 protein levels in mouse lungs. Likewise, ACEi (captopril) and ARB (telmisartan) administration did not increase membrane ACE2 levels in mouse idneys, but rather decreased them. Although preliminary, this study provided direct evidence supporting continued use of ACEis and ARBs in COVID-19, which also implied that feedback upregulation of ACE2 does not occur even if the RAS pathway is inhibited, at least for a span of 2 weeks (Wysocki et al., 2020). It will also be interesting to determine similar responses to ACEis and ARBs in mouse models of hypertension, either genetic or non-genetic, to determine the consistency of these results in the setting of compensatory mechanisms.
Besides these studies, others that directly assessed the clinical effects of ACEi/ARB drugs on COVID-19 outcome severity were not found during the literature search. Hence, the direct negative effects of ACEi/ARB drugs on COVID-19 outcome, as earlier hypothesized by clinicians and researchers, are at best experimental and, to the best of our knowledge, not yet supported by clinical evidence. A more in-depth discussion on the interactions between SARS-CoV-2 entry and ACEi/ARB drugs can be found elsewhere, and readers are referred to those materials for more information.
6. The renin-angiotensin-Aldosterone system
The renin-angiotensin-aldosterone system (RAAS), or simply the renin-angiotensin system, is a strong physiologic pathway that controls various processes relating to salt and water homeostasis (Fig. 1). Classically, the chronological order of the RAAS begins with the cleavage of angiotensinogen into Ang I by renin, followed by the conversion of Ang I into Ang II by ACE which occurs extensively in the lungs. ACE2 catalyzes both Ang I and Ang II to produce Ang (1–9) and Ang (1–7), which provides negative feedback to Ang II thereby modulating its effects. Prior to ACE2 interaction, Ang II modulates the RAAS promotes vasopressin release from the posterior pituitary gland, and directs the adrenal gland to produce aldosterone, which then promotes Na+ and H2O retention in normal physiology. In patients with cardiovascular disease, available Ang II reacts with angiotensin 1 receptor (AT1R) to stimulate pathologic behavior such as vasoconstriction, oxidative stress and profibrotic events. Ang II is then converted into Ang (1–7) by ACE2, followed by the activation of AT2R and MasR. Elevation of Ang II may increase plasma aldosterone levels through the regulatory action of adrenocorticotropic hormone (ACTH) mediated by AT1R, elevating blood pressure by increasing Na+ reabsorption and K+ excretion. In contrast, Ang-(1–7) increases urinary Na+ excretion, endothelial oxide activity, insulin sensitivity (Yatabe et al., 2011). This further shows that the ACE 2/MasR/AT2R axis performs an opposite reaction with the ACE/Ang II/AT1R axis, offering protection against hypertension, fibrosis, and oxidative stress (Gaspari et al., 2012). As mentioned above, SARS-CoV-2 exploits and depletes these ACE2 receptors to facilitate viral host cell entry and increase its pathogenicity. The RAAS is dysregulated following ACE2 depletion, promoting effects downstream of Ang II and therefore increasing aldosterone secretion. Aldosterone acts by inhibiting sodium excretion in the distal convoluted tubule and collecting ducts via the mineralocorticoid receptors. While Na+ is reabsorbed and H2O is retained, potassium is consequently excreted, and an excess can lead to wastage and hypokalemia. This is consistent with the findings of Chen et al. from a cohort study of 175 COVID-19-positive patients, wherein they classified patients as either being severely hypokalemic (17.71 %), hypokalemic (36.57 %), or normokalemic (45.71 %). They found that patients with higher hypokalemia also had increased creatinine kinase (CK), lactate dehydrogenase (LD) and C-reactive protein (CRP) levels, along with a higher body temperature, which were consistent with inflammatory and catabolic pathways (Chen et al., 2020). Some suggest that the observed hypokalemia among COVID-19 patients is not isolated but rather is part of a general electrolyte imbalance. On the other hand, aldosterone in the setting of a dysregulated RAAS may or may not be the cause of hypokalemia in some COVID-19 cases, but rather tubular dysfunction, such as renal tubular acidosis. However, studies on this aspect remain elusive. Nonetheless, there is evidence that potassium levels, which are mainly regulated by aldosterone, are commonly deranged in COVID-19 patients, and this could be due to interactions with the RAAS.
7. Non-steroidal anti-inflammatory drugs (NSAIDs) and peripheral prostaglandin synthesis
In the same correspondence published at The Lancet recommending the exercise of caution for COVID-19 patients under ACEi and ARB drugs, ibuprofen and thiazolidinediones were mentioned to potentially increase ACE2 expression (Fang et al., 2020). Ibuprofen is under the class known as non-steroidal anti-inflammatory drugs (NSAIDs), and acts by non-selectively inhibiting cyclooxygenase enzymes (COX) 1 and 2, which confers its anti-inflammatory and antipyretic effects through the inhibition of prostaglandin synthesis from arachidonic acid (Bushra and Aslam, 2010; Ricciotti, 2011). Of the four prostaglandins synthesized by this pathway, PGE2 and PGI2, along with the prostanoid thromboxane TXA2, are produced specifically in the kidneys, while PGD2 is produced in the airways, and they act by interacting with rhodopsin-like G-protein coupled receptors or GPCRs (Ricciotti, 2011). Interestingly, prostaglandins, particularly PGE2 and PGI2, have been well-studied and have been shown to affect kidneys by improving blood flow through vasodilation. Under conditions of reduced renal flow, this mechanism leads to increased tubular flow, increased renal perfusion, and increased glomerular filtration rate, with a concomitant increase in the secretion of potassium (Fig. 1, Fig. 2 ). This is supported by the fact that PGE2 regulates sodium and water reabsorption, while PGI2 increases the production of renin -- with the latter increasing salt and water retention and hence blood volume, while PGE2 counteracts this process via volume/pressure natriuresis (Kim, 2008).
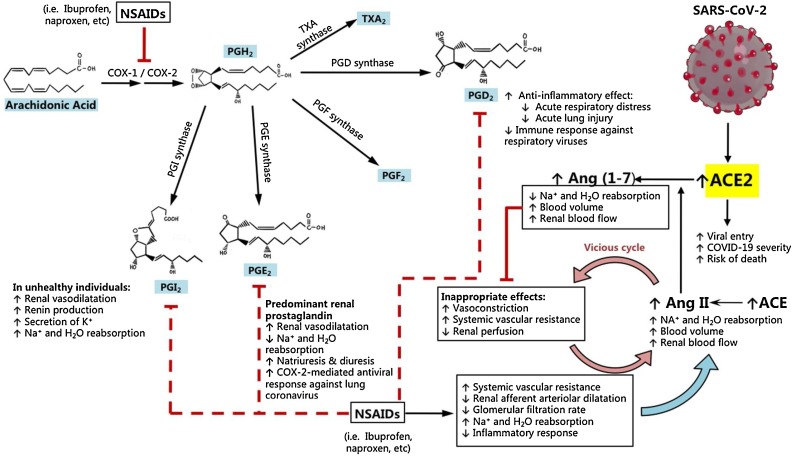
Fig. 2.
NSAIDs inhibit the synthesis of prostaglandins that protect against COVID-19. Arachidonic acid is converted to the prostaglandins and thromboxanes by the action of cyclooxygenases COX-1 and COX-2. Of the four prostaglandins, PGE2 and PGI2 promote optimal renal function by dilating afferent renal arterioles and promoting natriuresis in normal conditions, with PGI2-mediated Na+ and H2O reabsorption seen more evidently in unhealthy individuals with reduced renal perfusion. When NSAIDs like ibuprofen or naproxen are taken continuously, the net effects of COX inhibition are (1) increased Na+ and H2O reabsorption, (2) reduced renal afferent arteriolar dilatation, (3) reduced glomerular filtration rate (GFR), and (4) an increase in systemic vascular resistance. Due to reduced GFR and renal perfusion, the body produces greater amounts of angiotensin II (Ang II) to increase blood flow via increased plasma volume. However, increased Ang II levels leads to inappropriate vasoconstriction and increased systemic vascular resistance, which may ultimately result in a vicious cycle due to feedback compensation from Ang II in susceptible patients. Subsequently, an upregulation of ACE2 expression is expected to counteract Ang II providing its catabolic pathway to produce vasodilator and natriuretic angiotensins (1-7). This compensatory increase in ACE2 expression from NSAID intake, which is aggravated in unhealthy individuals such as those with hypertension, is the proposed mechanism by which SARS-CoV-2 can more aggressively infect the cell, by binding to greater amounts of ACE2 for viral entry. The concomitant immunosuppression by NSAIDs also promotes viral replication and shedding of SARS-CoV2, which can complicate the early phases of COVID-19.
Since PGE2 is the main prostaglandin in the kidney, COX inhibition leads to an overall increase in sodium and water reabsorption. Because ibuprofen and other NSAIDs decrease renal perfusion by increasing systemic vascular resistance, a vicious cycle can be formed by tricking the body to sense that there is reduced renal perfusion, leading to an inappropriate upregulation of ACE to return kidney perfusion back to normal via Ang II (Fig. 2). Concomitantly, an upregulation of ACE2 is expected by virtue of its role as a physiologic antagonist of Ang II, by catabolizing Ang II to Ang (1–7) to reduce vasoconstriction, reduce sodium and water retention, and provide reno- and cardio-protective effects (Tikellis and Thomas, 2012; Turner, 2015; Tallant et al., 2006; Miziuri and Ohashi, 2015). Hence, the reduction in prostaglandin E2 and I2 syntheses is the main mechanism by which NSAIDs directly contribute to increased ACE2 expression, which can be utilized by SARS-CoV-2 (Fig. 2).
On the other hand, a question arises on whether this mechanism is relevant to SARS-CoV-2 infection, since SARS-CoV-2 is mainly a respiratory virus and the RAAS is mainly regulated by the kidneys. Previous studies have shown that the RAAS is implicated in acute pulmonary injury, pulmonary hypertension, and pulmonary fibrosis (Kuba et al., 2006). Likewise, RAAS is indeed activated in idiopathic pulmonary arterial hypertension (De Man et al., 2012), and the expression of angiotensin receptor 1, ACE, and ACE2 is upregulated in the lungs as a response to lung pathology (Kuba et al., 2006; Morrell et al., 1995; Morrell and Stenmark, 2013). Hence, ACE2 may also be increased in pulmonary tissues secondary to ACE-induced promotion of the RAAS. Further, it has been shown that COX-2 and PGE2 mediate an antiviral response in lung epithelial cells infected with human coronavirus, and their expression results in decreased coronavirus replication (Lin et al., 2016). Similarly, PGD2, a prostaglandin known to be expressed in the brain and airways, mediate an anti-inflammatory response that may protect against acute respiratory distress and acute lung injury (Murata et al., 2013). Hence, the use of NSAIDs not only decreases PGE2- and PGI2-mediated regulation of kidney function, but also PGD2-mediated protection against acute lung injury and PGE2-mediated antiviral responses, which may lead to an enhanced coronavirus replication in the lung epithelia. A review of clinical evidence has shown that ibuprofen and naproxen improved the symptoms of influenza, and naproxen combined with clarithromycin and oseltamivir reduced both the rate and the duration of hospitalization of patients with influenza pneumonia. However, no evidence was provided for COVID-19 (Yousefifard et al., 2020). The observed benefits in influenza infection may be due to the reduction in airway inflammation which is common in colds, and which is also observed in severe influenza. However, regarding viral entry and replication, this clinical evidence does not directly support nor reject any of the mechanisms discussed, especially with regards to SARS-CoV-2 infection. A similar systematic review of various NSAIDs, corticosteroids, and specific immunosuppressive or immune-stimulating drugs have shown no strong evidence supporting the use of NSAIDs for COVID-19; however, indomethacin was noted to have shown direct antiviral activity against SARS-CoV and canine coronavirus (CCoV) in vitro (Russell et al., 2020). The resulting benefit is not expected to contradict the mechanisms discussed; rather, it must have been a function of the structure of indomethacin, which is reflective of the authors’ comment that the results were unexpected since indomethacin is a COX inhibitor (Amici et al., 2006).
8. Corticosteroids for coronavirus infections
A retrospective cohort study published in 2005 showed that patients in Hong Kong infected with SARS-CoV, which were given corticosteroids to manage acute respiratory distress, experienced higher rates of adverse outcomes despite favorable baseline characteristics. Further, analysis showed that SARS patients treated with corticosteroids (hydrocortisone, methylprednisolone) were at a 20.7-fold higher risk of ICU admissions or mortality, independent of age and severity of disease (Auyeung et al., 2005). This study alone, albeit based on SARS-CoV, showed that corticosteroid use may in fact increase the risk of unfavorable outcomes for coronavirus-infected patients. However, data supporting the validity of this conclusion on other coronavirus infections are indeed warranted. To note, a systematic review and meta-analysis of 15 studies on the management of coronavirus pneumonia showed that patients with severe coronavirus symptoms were more likely to require corticosteroid therapy (RR 1.56; CI 1.28–1.90), but that they were also twice as likely to contract bacterial infection (RR 2.08; CI 1.54–2.81) and were twice as likely to succumb to the disease (RR 2.11; CI 1.13–3.94) (Yang et al., 2020). However, this systematic review and meta-analysis was not only limited to COVID-19 (2 studies), but included other coronavirus infections such as those caused by SARS-CoV (11 studies) and MERS-CoV (2 studies), which may allow for differences in the prognosis and clinical outcomes of COVID-19 patients. Another systematic review and meta-analysis of 11 studies on corticosteroid use in coronavirus infections showed a statistically significant delay in virus clearing of 3.78 days (CI 1.16–6.41), but with no significant changes in hospitalization duration, use of mechanical ventilation, or survival (Li et al., 2020b). In these two studies, the results may have differed mainly because the included studies were mostly retrospective in nature. Hence, baseline characteristics and management may not have been the same within a singular study, and this may have affected the associations of the analysis. Further, the analyses included SARS−COV, SARS−COV-2, and MERS−COV infections, mainly because of a scarcity in COVID-19 data. Because of the different clinical pictures, cellular receptors, and expected prognosis for these coronavirus infections, associations may also be significantly altered. A third systematic review and meta-analysis included SARS−COV, SARS−COV-2, and MERS−COV infections, but also data from influenza and community-acquired pneumonia. This study showed that corticosteroid use may in fact benefit COVID-19 and non−COVID-19 patients with ARDS (RR 0.72; CI 0.55−0.93), and may reduce mortality from CAP (RR 0.70; CI 0.50−0.98). In contrast, an increase in mortality was observed for corticosteroid use among COVID-19 patients without ARDS (HR 2.30; CI 1.00–5.29) (Ye et al., 2020), implying variations in effect based on severity. A major drawback of this study is the low to very low quality of evidence afforded by the studies as per the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach, which was mainly due to the nature of the studies included (mostly retrospective cohort studies). In addition, the inclusion of outcomes from non-coronavirus infections may have significantly confounded and affected the analysis of outcomes. On the other hand, a systematic review showed that corticosteroids may help reduce immunopathological damage and may improve outcomes when given high-dose with a quinolone early in the infection, but these results were specific for SARS-CoV and not for SARS-CoV-2 (Russell et al., 2020). To resolve the contradicting reports, randomized controlled trials of corticosteroid use and its benefits or risks to COVID-19 outcomes are needed, especially if more accurate results are to be generated. To date, there seems to be emerging clinical evidence that caution must be exercised in the use of corticosteroids for coronavirus-infected patients. However, due to the varying and conflicting results of their effects on clinical outcome, more specific and better designed studies are required for a better evaluation of current recommendations.
Notwithstanding, the observed risks are supported experimentally and pharmacologically as will be discussed. First, note that corticosteroids are divided into the mineralocorticoids and the glucocorticoids. Mineralocorticoids comprise a group of proinflammatory hormones that primarily act on the kidney to modulate renal-sodium balance and blood pressure by activating mineralocorticoid receptors (MR) (Shibata and Fujita, 2011) (Fig. 3 ). It is thought to be proinflammatory, acting through the upregulation of inflammatory cytokines to influence T and B lymphocytes, monocytes, neutrophils, and dendritic cells to mount an immune response. This interaction subsequently promotes inflammation and increased levels of reactive oxygen species (ROS) following the activation of hormonal response elements (HREs) inside the nucleus (Fig. 4 ) (Barnes, 2005). In pathological conditions, the mineralocorticoid receptors (MRs) can remain activated regardless of Ang II levels, interfering with normal salt and water retention and electrolyte regulation. This eventually leads to the exacerbation of hypertension, which if not promptly addressed, can lead to progressive organ injury and finally organ failure.
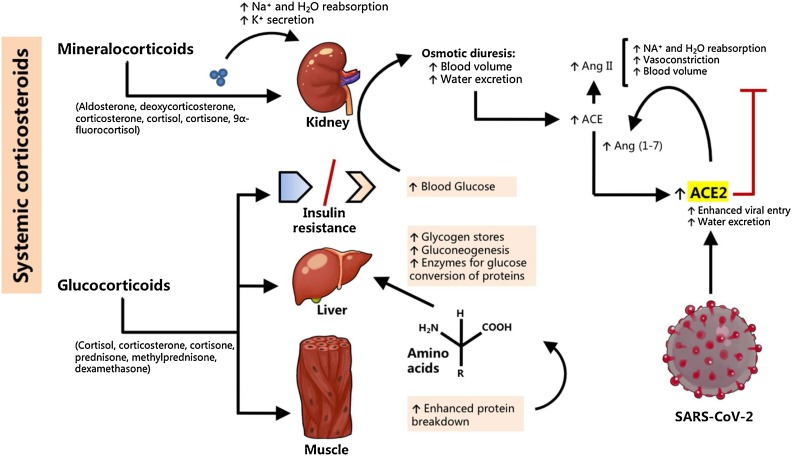
Fig. 3.
Corticosteroids may facilitate SARS-CoV-2 entry by affecting blood glucose levels and kidney function. Corticosteroids can be categorized into the mineralocorticoids and the glucocorticoids. The mineralocorticoids, which mimic the actions of aldosterone, affect kidney function by increasing the Na+ and H2O reabsorption. Because this mechanism does not promote Ang II formation, it is not expected to contribute majorly to increased ACE2 expression. On the other hand, corticosteroids affect the liver by increasing its capacity for gluconeogenesis. Muscles enhance protein breakdown into amino acids, which are carried to the liver for conversion into glucose. Further, glucocorticoids promote the actions of glycogen, and inhibit the actions of insulin. This gluconeogenic action coupled with insulin resistance leads to hyperglycemia in susceptible persons, which result in osmotic diuresis that can substantially reduce plasma volume. The body compensates by upregulating ACE to increase Ang II levels, thereby increasing Na+ and H2O reabsorption and vasoconstricting blood vessels. An expected increase in ACE2 to counteract increased Ang II action is proposed to be the mechanism of how corticosteroids, precisely glucocorticoids, can facilitate ACE2-mediated SARS-CoV-2 entry to cells and viral replication in COVID-19.
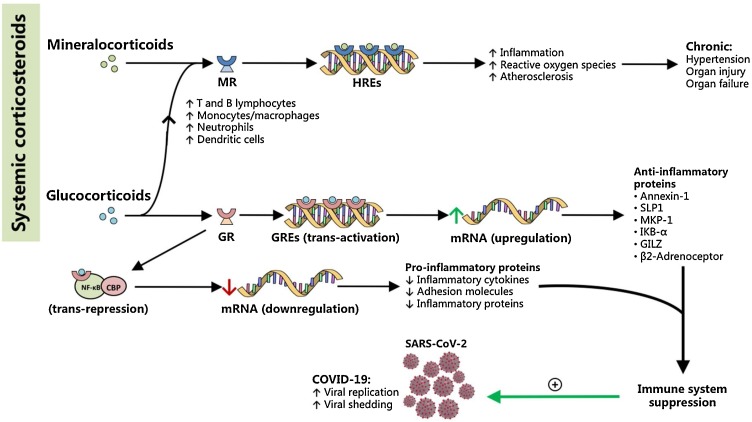
Fig. 4.
Corticosteroids mediate pro-inflammatory and anti-inflammatory pathways directed at the aggravation of COVID-19. Mineralocorticoids bind to nuclear mineralocorticoid receptors (MR) to interact with hormonal response elements (HREs), and are thought to mediate pro-inflammatory cascades that ultimately increase oxidative stress and contribute to pathologic inflammation and atherosclerosis. Chronic mineralocorticoid levels are thought to cause end-organ damage resulting in hypertension and end-organ failure. Meanwhile, glucocorticoids mediate anti-inflammatory pathways that result in the immunosuppressive actions of most corticosteroid drugs in medicine. Glucocorticoids bind to their nuclear glucocorticoid receptors (GR), but also to MR, and affect gene transcription via two important mechanisms: (1) transactivation, which is the direct interaction of the glucocorticoid-GR complex with genetic material, or (2) trans-repression, in which the complex interacts with other proteins such as CREB-binding protein (CBP) and NF-κB. Trans-activation leads to an upregulation of anti-inflammatory proteins, while trans-repression leads to decreased expression of inflammatory proteins. Together, these pathways converge to result in immunosuppression, which may aggravate COVID-19 by promoting SARS-CoV-2 replication and shedding, especially in the early stages of the disease. In effect, glucocorticoids as well as more specific therapies (i.e. monoclonal antibodies) may be better reserved for complications such as acute respiratory distress or acute lung inflammation where immunosuppression can be lifesaving.
Glucocorticoids on the other hand affect insulin metabolism, hepatic synthesis, and muscle protein catabolism by increasing amino acid production, and increase the availability of glycogen stores to buffer blood glucose concentration. Insulin resistance triggers an increase in blood glucose, which causes plasma volume depletion secondary to osmotic diuresis (Fig. 3). In response to resulting plasma volume reduction, the juxtaglomerular cells of the kidneys will produce renin to act upon liver angiotensinogen to form Ang I, followed by an ACE-mediated conversion to Ang II in an effort to activate the pathway called the renin-angiotensin system to return plasma volume back to normal. Subsequently, ACE2 can increase proportionally with Ang II, acting to produce Ang (1–7), which we hypothesize is the mechanism for glucocorticoid-enhanced SARS-CoV-2 virulence (Fig. 3). On the other hand, glucocorticoids suppress inflammation by binding to glucocorticoid response elements (GREs) inside the nucleus to interact with genes and affect gene transcription in three basic ways (trans-activation, trans-repression, and cis-repression) to suppress inflammation or to prevent an immune response from occurring altogether (Barnes, 2005)(Fig. 4).
9. Anti-Inflammatory drugs and their interaction with early SARS-CoV-2 entry
Recent guidelines released by the UK National Health Service (NHS) on the intake of ibuprofen for mild COVID-19 symptoms has drawn flak from some health experts as it may pose risks on increased severity (Gulland, 2020). Reduction of prostaglandin levels in the body, which is the main action of NSAIDs like ibuprofen, effectively reduces the immune response, and previous reports have shown no benefit of taking ibuprofen in symptom control and clinical outcome for respiratory infections, and have been shown to worsen the prognosis of community-acquired pneumonia (Little et al., 2013; Basille et al., 2017). In a randomized, placebo-controlled study for ibuprofen use in managing respiratory syncytial virus (RSV) infection, ibuprofen was not able to prevent lung consolidation and was shown to increase viral shedding (Walsh et al., 2016). From these studies, we can infer the classical mechanism by which anti-inflammatory drugs aggravate infection, which is to reduce the degree of immune response that the body can mount against a virus and to allow uncontrolled progression.
When a virus infects its host, it most often remains intracellular as viruses are obligate intracellular organisms, and extracellular viruses are most likely seen only during the lytic phase. When viruses infect a cell, antigen-presenting cells alert nearby immune cells through major histocompatibility complexes (MHCs) of the virus’ intracellular presence. Concomitantly, toll-like receptors (TLRs) recognize viral nucleic acids intracellularly and in effect promotes the cellular expression of interferons and inflammatory cytokines that act in an autocrine and paracrine fashion to induce an antiviral state in the host and in nearby, uninfected cells via the JAK/STAT pathway, and to alert the immune cells of an ongoing invasion (Lester and Li, 2014; Teijaro, 2016). Type I interferons have also been shown to promote prostaglandin synthesis (Fuse et al., 1982), the latter acting as a mediator between the thymus and the bone marrow to promote cellular and humoral types of immunity (Parker, 1972). A previous study has shown that positive-strand RNA viruses, to which the coronaviruses belong, induce the formation of the endoplasmic reticulum double membrane vesicles (ER-DMVs) that promote viral replication and protect viruses from immune detection, and treatment with interferon-beta significantly reduces these viral-induced membrane structures (Oudshoorn et al., 2016). Hence, suppression of prostaglandin synthesis by the use of NSAIDs may effectively reduce cell-mediated and humoral immunity against SARS-CoV-2, and may modulate immune cells towards the production of IL-10 for T-cell suppression and suppression of phagocytosis, particularly by PGE2 (Medeiros et al., 2012; Rodríguez et al., 2014). It is however noted that prostaglandins such as PGE2 act differently in different viral infections, and may promote viral replication and shedding in other viruses, such as herpes simplex virus (HSV), enterovirus 71, and cytomegalovirus, to name a few (Steer and Corbett, 2003; Sander et al., 2017). Hence, the effect of NSAIDs on different viral infections may differ, but for SARS-CoV-2 and for other viruses such as hepatitis B and human immunodeficiency virus (HIV), taking ibuprofen and other NSAIDs of similar action may aggravate viral replication. Additional risks by NSAIDs however lie in its ability to increase ACE2 expression by activating a gene called ADAM17, a metalloprotease that upregulates ACE2 by enabling ACE2 shedding, resulting in increased COVID-19 infection (Cure et al., 2020). From here, severity and susceptibility against COVID-19 is expected to increase as viral load relatively increases.
Only recently, a prothrombotic state has been associated with COVID-19 infection, and this has led to complicated clinical evaluations as COVID-19 is slowly added to the ever increasing list of differentials for thromboembolism. Studies included in a scoping review by Al-Ani et al. showed that venous thromboembolism (VTE) occurs in about 20 % of COVID-19 patients, with around 71.4 % of those who died from the disease being classified as having disseminated intravascular coagulation (DIC), according to one single-center study (Al-Ani et al., 2020). Similarly, a systematic review and meta-analysis of 3 of the 44 included studies showed VTE as a rather common complication of COVID-19 in 15 % of the 318 total patients from three different studies (Potere et al., 2020). These suggest that COVID-19, apart from being a mainly respiratory disease, is an ailment of the vasculature frequently complicating into VTE, which is expected from its likely tropism for the endothelium. Since NSAIDs, especially the selective COX-2 inhibitors, likely affect hemostatic balance by inhibiting prostacyclin synthesis without inhibiting COX-1-induced thromboxane production, a prothrombotic state is likely to occur especially among susceptible patients. This is the reason why rofecoxib was withdrawn from the market, after a clinical trial found an increased risk of myocardial infarction and stroke in participants after 18 months of use for colon cancer prevention, which is likely to be a class effect (Day and Graham, 2004). This points to a possible risk of VTE or other thromboembolic events in COVID-19 patients with multiple co-morbidities taking NSAIDs, although evidence is not yet clear. To the best of our knowledge, there is no direct evidence to date of NSAID use and induced thromboembolism among COVID-19 patients, but serious caution is advised because associations between thromboembolism and NSAID use have been well established in previous years, albeit in different contexts of disease. The reader is referred to this article for an overview (Rad et al., 2020).
Similarly, corticosteroids may enhance viral entry when provided for symptomatic treatment of SARS infection. As aforementioned, a 20.7-fold increase in the risk for adverse outcomes (ICU admission/mortality) was observed among patients in Hong Kong treated with corticosteroids for SARS, which was independent of age and disease severity (Auyeung et al., 2005). This may suggest the promotion of coronavirus replication secondary to immunosuppression, or of increased ACE2 expression secondary to osmotic diuresis, which we hypothesized earlier above (Fig. 4). It is important to note, however, that the proposed effects of corticosteroids and NSAIDs on SARS-CoV2 infection is applicable only for the early stages of the disease. During the late stages, such as in acute respiratory distress syndrome (ARDS) or acute airway inflammation, treatment with corticosteroids or other more targeted therapies, such as monoclonal antibodies, may be lifesaving. Recently, a large randomized clinical trial called the Randomised Evaluation of COvid-19 thERapY (RECOVERY) trial conducted the world’s largest clinical trial on COVID-19 treatments. In this study they selected 2104 COVID-19 patients and subjected them to low to moderate dose (6 mg/day) dexamethasone treatment, which then compared them to 4321 controls who received the standard care for coronavirus infection. As a result, the investigators discovered a significant decrease in patient deaths. Death among severely-ill COVID-19 patients on ventilators were reduced by 33 %, while death among patients requiring oxygen decreased by 20 % (University of Oxford, 2020). In contrast, there was no clinical significance seen in patients with less severe cases, suggesting that the dexamethasone might be more useful in patients experiencing cytokine-mediated inflammation. This shows the potential of corticosteroids to provide benefit in severe COVID-19; however, it is noted that great caution must be exercised when using dexamethasone, as it is neither indicated for mild cases nor for prophylaxis. High levels of cortisol may increase blood pressure, increase the risk of developing diabetes, and increase the risk of infection resulting from its immunosuppressive properties, and synthetic steroids are bound to have similar effects in susceptible patients. To this end, these drugs should be given with utmost caution and only if necessary. Other clinical evidence showing the effects of corticosteroids and other anti-inflammatory drugs specifically on COVID-19 infections is less clear. A systematic review of four studies done in China showed conflicting results, where two studies associated corticosteroid use with negative outcomes such as risk of ICU admission and longer durations of viral presence in oropharyngeal swabs and fecal samples, with one study showing no benefit to outcome, and another study showing a 62 % decline in mortality among severe COVID-19 patients with ARDS treated with methylprednisolone (Veronese et al., 2020), the latter being consistent with our previous statement. However, it is not clear in the review if corticosteroids are indeed detrimental for COVID-19, as the study they included, which showed a higher rate of ICU admissions among COVID-19 patients treated with corticosteroids, was only a case series and is at best suggestive (Veronese et al., 2020). Further, the administration of corticosteroids among the studies varied in terms of chronology, with one study being given before ARDS and the other during ARDS. Meanwhile, a rapid systematic review of 59 publications on inhaled corticosteroid (ICS) use in COVID-19 have found no sufficient evidence of benefit or harm on clinical outcomes (Halpin et al., 2020), which reflects the scarcity of COVID-19 clinical studies to date.
10. Anti-Inflammatory drugs versus paracetamol for mild COVID-19 symptoms
While the scientific community has yet to discover targeted therapies for the new deadly coronavirus strain, a number of drugs used for symptomatic management of COVID-19 have provided preliminary data on what to use and avoid. In particular, the World Health Organization (WHO) initially recommended the avoidance of ibuprofen and other non-steroidal anti-inflammatory drugs (NSAIDs) in managing COVID-19 symptoms following an advocacy from the French health minister against the use of anti-inflammatory drugs for patients with suspected COVID-19 infection, and instead recommended the use of paracetamol to treat fever. The claim was based on anecdotal reports of COVID-19 patients who developed severe symptoms after taking NSAIDs at the early phase of COVID-19. The WHO later recalled their recommendation after citing a lack of clinical evidence for avoiding NSAIDs in the management of COVID-19. However, health experts continued to advise the exercise of caution in using anti-inflammatory drugs for symptomatic management of COVID-19, as written in a correspondence published at the British Medical Journal (AFP, 2020; Day, 2020).
Alternatively, paracetamol may be given as first-line for the management of fever without targeting inflammation, due to its effectiveness and safety profile. Unlike the NSAIDs, paracetamol is a weak prostaglandin inhibitor, and in contrast to COX-1/COX-2 inhibition, it inhibits the COX-3 isoenzyme, which is a COX-1 splice variant abundant in CNS rather than in the peripheral tissues. This provides paracetamol with analgesic and antipyretic properties, but with little to no peripheral anti-inflammatory properties (Sharma and Mehta, 2014; Chandrasekharan et al., 2002). Further, paracetamol employs the serotonergic pathway to provide analgesia. By stimulating the descending serotonergic pathway, mediated by the 5-HT1A/1B receptors in the CNS and acting on afferent nerve fibers, paracetamol largely inhibits noxious stimuli (Sharma and Mehta, 2014; Roca-Vinardell et al., 2018; Gerriets and Nappe, 2020). In addition, paracetamol also demonstrates an opioid sparing effect of 20 % that may increase analgesia in patients under opioid analgesics (Remy et al., 2005). Hence, by virtue of these pathways, paracetamol may be a safer and more effective alternative for early symptomatic management of headache, fever and pain in COVID-19.
11. Conclusion
COVID-19 is a respiratory disease caused by the coronavirus SARS-CoV-2, and has caused a pandemic by infecting more than 31 million people in more than 190 countries to date, while claiming hundreds of thousands of lives as it impacts heavily on both economy and lifestyle. Initial clinical reports have shown correlations between adverse outcomes for COVID-19 and hypertension, which they attributed to increased expression of ACE2. This was followed shortly by anecdotal reports of increased symptom severity among patients taking anti-inflammatory drugs like ibuprofen early in the disease, which was later hypothesized to be associated with ACE2. In this paper, we provide concrete mechanisms of how NSAIDs and corticosteroids may aggravate SARS-CoV-2 infection in the early stages of disease, through their interaction with prostaglandin synthesis and the RAAS, apart from the conventional pathway of immunosuppression at a time when the body needs it the most. However, as evidence is often conflicting and scarce, more evidence is needed to gain a definitive understanding of COVID-19.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors do not have any conflicts of interest to disclose.
Acknowledgments
We would like to thank the healthcare workers and scientists in this pandemic for continually providing care to COVID-19 patients and for providing reports on the clinical and molecular aspects of this disease. Dr. Rafael A. Manalo and Emma Manalo are acknowledged for providing great inspiration in this work. Iris would also like to thank her family, especially Cherrie and Gilbert, and Paz Nepomuceno for supporting her career in Molecular Medicine.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.virusres.2020.198190.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Abate G., Memo M., Uberti D. Impact of COVID-19 on alzheimer’s disease risk: viewpoint for research action. InHealthcare. 2020;8(September(3)):286. doi: 10.3390/healthcare8030286. Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;(May 21) doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AFP Updated: WHO now doesn’t recommend avoiding ibuprofen For COVID-19 symptoms. ScienceAlert. 2020 https://www.sciencealert.com/who-recommends-to-avoid-taking-ibuprofen-for-covid-19-symptoms Web URL:Accessed: 19 March 2020. [Google Scholar]
- Al-Ani F., Chehade S., Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb. Res. 2020;192:152–160. doi: 10.1016/j.thromres.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano G., Guaraldi G., Fontana F., Ferrari A., Magistroni R., Mussini C. Covid-19 Working Group. The role of the renin-angiotensin system in severe acute respiratory syndrome-CoV-2 infection. Blood Purif. 2020:1–5. doi: 10.1159/000507914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici C., Di Caro A., Ciucci A., Chiappa L., Castilletti C., Martella V., Decaro N. Indomethacin has a potent antiviral activity against SARS coronavirus. Antivir. Ther. (Lond.) 2006;11:1021–1030. [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020 doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020:17. doi: 10.1016/S2213-8587(20)30238-2. July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung T.W., Lee J.S., Lai W.K., Choi C.H., Lee H.K. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J. Infect. 2005;51(2):98–102. doi: 10.1016/j.jinf.2004.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020 doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Barnes P.J. How corticosteroids control inflammation: quintiles prize lecture. Br. J. Pharmacol. 2005;2006(3):245–254. doi: 10.1038/sj.bjp.0706736. 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basille D., Plouvier N., Trouve C., Duhaut P., Andrejak C., Jounieaux V. Non-steroidal anti-inflammatory drugs may worsen the course of community-acquired pneumonia. Lung. 2017;195(2):201–208. doi: 10.1007/s00408-016-9973-1. [DOI] [PubMed] [Google Scholar]
- Bittner E.A., Martyn J.A.J. Pharmacology and Physiology in Anesthesia. 2nd ed. Elsevier; 2019. 21 -- neuromuscular physiology and pharmacology. ISBN 978-0-323-48110-6. [DOI] [Google Scholar]
- Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Path. 2014;11(2) doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushra R., Aslam N. An overview of clinical pharmacology of ibuprofen. Oman Med. J. 2010;25(3):155–161. doi: 10.5001/omj.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Severe outcomes among patients with coronavirus disease 2019 (COVID-19). United States, February 12-March 16, 2020. Morb Mort Weekly Rep. 2020 doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2020. Severe Outcomes Among Patients With Coronavirus Disease 2019 (COVID-19) – United States, February 12 – March 16, 2020. MMWR Morb Mortal Wkly Rep; pp. 343–346. 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Jf, Kok K., Zhu Z., Chu H., To Kk, Yuan S., Yuen K. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infec. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan N.V., Dai H., Roos K.L., Evanson N.K., Tomsik J. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc. Natl. Acad. Sci. 2002;99(21):13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Li X., Song Q., Hu C., Su F., Dai J., Ye Y., Huang J., Zhang X. Assessment of Hypokalemia and Clinical Characteristics in Patients With Coronavirus Disease 2019 in Wenzhou, China. JAMA Network Open. 2020;1(June (6)) doi: 10.1001/jamanetworkopen.2020.11122. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S.M., Lee Y.Y., Ha E., Yoon J.S., Won K.C., Lee H.W. The risk of diabetes on clinical outcomes in patients with coronavirus disease 2019: a retrospective cohort study. Diabetes Metab. J. 2020;44(3):405–413. doi: 10.4093/dmj.2020.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke N.E., Turner A.J. Angiotensin-converting enzyme 2: the first decade. Int. J. Hypertens. 2012 doi: 10.1155/2012/307315. January 1;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cure M.C., Kucuk A., Cure E. NSAIDs may increase the risk of thrombosis and acute renal failure in patients with COVID-19 infection. Therapie. 2020:1. doi: 10.1016/j.therap.2020.06.012. July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. doi: 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]
- Day R.O., Graham G.G. The vascular effects of COX-2 selective inhibitors. Aust. Prescr. 2004;27:142–145. [Google Scholar]
- De Man F., Tu L., Handoko L., Rain S., Ruiter G. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012;186(8):780–789. doi: 10.1164/rccm.201203-0411OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmarakby A.A., Bhatia K., Crislip R., Sullivan J.C. Hemodynamic responses to acute angiotensin II infusion are exacerbated in male versus female spontaneously hypertensive rats. Physiol. Rep. 2016;4(January(1)) doi: 10.14814/phy2.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emameh R.Z., Falak R., Bahreini E. Application of System Biology to Explore the Association of Neprilysin, Angiotensin-Converting Enzyme 2 (ACE2), and Carbonic Anhydrase (CA) in Pathogenesis of SARS-CoV-2. Biol. Proced. Online. 2020;22:11. doi: 10.1186/s12575-020-00124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Liu X., Pan W., Douglas M.W., Bao S. Epidemiology of 2019 novel coronavirus disease in Gansu Province, China, 2020. Emerg Infect Dis. 2020 doi: 10.3201/eid2606.200251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet. 2020 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Coronaviruses: An Overview of Their Replication and Pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- Fountain J.H., Lapin S.L. StatPearls. StatPearls Publishing; Treasure Island (FL): 2019. Physiology, renin angiotensin system. [internet]; [PubMed] [Google Scholar]
- Furuhashi M., Moniwa N., Mita T., Fuseya T., Ishimura S., Ohno K. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am. J. Hypertens. 2015;28(1):15–21. doi: 10.1093/ajh/hpu086. [DOI] [PubMed] [Google Scholar]
- Fuse A., Mahmud I., Kuwata T. Mechanism of simulation by human interferon of prostaglandin synthesis in human cell lines. Cancer Res. 1982;42(8) 3029-14. [PubMed] [Google Scholar]
- Gallagher P.E., Ferrario C.M., Tallant E.A. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2008;295(6):H2373–H2379. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari T.A., Vinh A., Jones E.S., Widdop R.E. Ganging up on angiotensin II type 1 receptors in vascular remodeling. Hypertension. 2012;60(July(1)):17–19. doi: 10.1161/HYPERTENSIONAHA.112.193375. [DOI] [PubMed] [Google Scholar]
- Gerriets V., Nappe T.M. StatPearls. StatPearls Publishing; Treasure Island (FL): 2020. Acetaminophen.https://www.ncbi.nlm.nih.gov/books/NBK482369/ Accessed: 19 March 2020.Web URL: [Google Scholar]
- Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., Gervasoni C., Ridolfo A.L., Rizzardini G., Antinori S. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin. Infect. Dis. 2020:26. doi: 10.1093/cid/ciaa330. Mach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulland A. The Telegraph. 2020. Experts question official NHS advice over ibuprofen and coronavirus.https://www.telegraph.co.uk/global-health/science-and-disease/experts-question-official-nhs-advice-ibuprofen-coronavirus/ Accessed: Web URL: 18 March 2020. [Google Scholar]
- Guo J., Huang Z., Lin L., Lv J. Coronavirus disease 2019 (covid‐19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J. Am. Heart Assoc. 2020;9(April (7)) doi: 10.1161/JAHA.120.016219. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin D.M.G., Singh D., Hadfield R.M. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.01009-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L., Lely A.T. Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(June(2)):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikmet F., Méar L., Edvinsson Å, Micke P., Uhlén M., Lindskog C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020;16(July(7)) doi: 10.15252/msb.20209610. e9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Hofmann-Winkler H., Pöhlmann S. Activation of Viruses by Host Proteases. Springer; 2018. Priming time: how cellular proteases arm coronavirus spike proteins. ISBN 978-3-319-75474-1. [DOI] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell. 2020 doi: 10.1016/j.molcel.2020.04.022. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P.M., Knipe D.M. 6th ed. Lippincott Williams & Williams; 2013. Fields Virology. ISBN 978-1-97-511254-511257. [Google Scholar]
- Hu B., Huang S., Yin L. The cytokine storm of COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.L., Li X., Meng Y., Xiao B., Ma Q. Upregulation of angiotensin-converting enzyme (ACE) 2 in hepatic fibrosis by ACE inhibitors. Clin. Exp. Pharmacol. Physiol. 2010;37(1):e1–e6. doi: 10.1111/j.1440-1681.2009.05302.x. [DOI] [PubMed] [Google Scholar]
- Igase M., Kohara K., Nagai T., Miki T., Ferrario C.M. Increased expression of angiotensin converting enzyme 2 in conjunction with reduction of neointima by angiotensin type 1 receptor blockade. Hypertens. Res. 2008;31(3):553–559. doi: 10.1291/hypres.31.553. [DOI] [PubMed] [Google Scholar]
- Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., Hall R., Poirier G., Ronco J.J., Tidswell M., Hardes K. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 2017;21(December(1)):1–9. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.-H. Renal effects of prostaglandins and cyclooxygenase-2 inhibitors. Electrolyte Blood Press. 2008;6(1):35–41. doi: 10.5049/EBP.2008.6.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Penninger J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr. Opin. Pharmacol. 2006;6(3):271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester S.N., Li K. Toll-lik.e receptors in antiviral innate immunity. J. Mol. Biol. 2014;426(6):1246–1264. doi: 10.1016/j.jmb.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J. Med. Virol. 2020;92(June(6)):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Chen C., Hu F., Wang J., Zhao Q., Gale R.P., Liang Y. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia. 2020;5:1–9. doi: 10.1038/s41375-020-0848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.-R., Wu Y.-H., Yen W.-C., Yang C.-M., Chiu D.T.-Y. Diminished COX-2/PGE2-mediated antiviral response due to impaired NOX/MAPK signaling in G6PD-knockdown lung epithelial cells. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0153462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little P., Moore M., Kelly J., Williamson I., Leydon G. Ibuprofen, paracetamol, and steam for patients with respiratory tract infections in primary care: pragmatic randomised factorial trial. BMJ. 2013;347:f6041. doi: 10.1136/bmj.f6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Xin H., Jiang X.Y., Wang Y.X., Zhang Y.S. Relationship between renal injury and the antagonistic roles of angiotensin-converting enzyme (ACE) and ACE2. Genet. Mol. Res. 2014;1(January (2)):2333–2342. doi: 10.4238/2014.April.3.5. 13. [DOI] [PubMed] [Google Scholar]
- Medeiros A., Peres-Busalaf C., Verdan F.F., Serezani C.H. Prostaglandin E2 and the suppression of phagocyte innate immune responses in different organs. Mediators Inflamm. 2012;2012 doi: 10.1155/2012/327568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miziuri S., Ohashi Y. ACE and ACE2 in kidney disease. World J. Nephrol. 2015;4(1):74–82. doi: 10.5527/wjn.v4.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuiri S., Ohashi Y. ACE and ACE2 in kidney disease. World J. Nephrol. 2015;6(Febuary (1)):74. doi: 10.5527/wjn.v4.i1.74. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montastruc F., Romano C., Montastruc J.-L., Silva S., Seguin T., Minville V. Pharmacological characteristics of patients infected with SARS-CoV-2 admitted to Intensive Care Unit in South of France. Therapie. 2020 doi: 10.1016/j.therap.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Del Pozo C.H., Prosper F., Romero J.P. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020 Apr:24. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell N.W., Stenmark K.R. The renin-angiotensin system in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2013;187(10):1138–1139. doi: 10.1164/rccm.201210-1872LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell N.W., Atochina E.N., Morris K.G., Danilov S.M., Stenmark K.R. Angiotensin converting enzyme expression is increased in small pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. J. Clin. Invest. 1995;96(4):1823–1833. doi: 10.1172/JCI118228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad J.J., Levy B.I. Interaction between RAAS inhibitors and ACE2 in the context of COVID-19. Nat. Rev. Cardiol. 2020 doi: 10.1038/s41569-020-0368-x. https://doi.org/10. 30;1038.March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Durango N., Fuentes C.A., Castillo A.E., González-Gómez L.M., Vecchiola A., Fardella C.E., Kalergis A.M. Role of the renin-angiotensin-aldosterone system beyond blood pressure regulation: molecular and cellular mechanisms involved in end-organ damage during arterial hypertension. Int. J. Mol. Sci. 2016;17(July(7)):797. doi: 10.3390/ijms17070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T., Aritake K., Tsubosaka Y., Maruyama T., Nakagawa T. Anti-inflammatory role of PGD2 in acute lung inflammation and therapeutic application of its signal enhancement. Proc Natl Acad Sci. 2013;110(13):5205–5210. doi: 10.1073/pnas.1218091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudshoorn D., van der Hoeven B., Limpens R.W., Beugeling C., Snijder E.J. Antiviral innate immune response interferes with the formation of replication-associated membrane structures induced by a positive-strand RNA virus. mBio. 2016;7(6):e01991–16. doi: 10.1128/mBio.01991-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C.W. The role of prostaglandins in the immune response, in: prostaglandins in cellular biology. Springer, Boston, MA; 1972. ISBN 978-1-4684-2844-5. [DOI] [Google Scholar]
- Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1–7 axis of the renin–angiotensin system in heart failure. Circ. Res. 2016;15(April(8)):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta F., Matera M.G., Cazzola M., Bianco A. Severe respiratory SARS-CoV2 infection: Does ACE2 receptor matter? Respir. Med. 2020 doi: 10.1016/j.rmed.2020.105996. April 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potere N., Valeriani E., Candeloro M., Tana M., Porreca E., Abbate A., Spoto S. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Crit Care. 2020;24:389. doi: 10.1186/s13054-020-03022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabelo L.A., Todiras M., Nunes-Souza V., Qadri F., Szijártó I.A., Gollasch M., Penninger J.M., Bader M., Santos R.A., Alenina N. Genetic deletion of ACE2 induces vascular dysfunction in C57BL/6 mice: role of nitric oxide imbalance and oxidative stress. PLoS One. 2016;12(April (4)) doi: 10.1371/journal.pone.0150255. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad A.A., Vardanyan R., Tas N.R. Ibuprofen and thromboembolism in SARS-CoV-2. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy C., Marret E., Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br. J. Anaesth. 2005;94(4):505–513. doi: 10.1093/bja/aei085. [DOI] [PubMed] [Google Scholar]
- Ren L.L., Wang Y.M., Wu Z.Q., Xiang Z.C., Guo L. Identification of a novel coronavirus causing severe pneumonia in humans: a descriptive study. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciotti E. FitzGerald GA. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Vinardell A., Berrocoso E., Llorca-Torralba M., García-Partida J.A., Gilbert-Rahola J., Mico J.A. Involvement of 5-HT1A/1B receptors in the antinociceptive effect of paracetamol in the rat formalin test. Neurobiol Pain. 2018;3:15–21. doi: 10.1016/j.ynpai.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez M., Domingo E., Municio C., Alvarez Y., Hugo E. Polarization of the innate immune response by prostaglandin E2: a puzzle of receptors and signals. Mol. Pharmacol. 2014;85(1):187–197. doi: 10.1124/mol.113.089573. [DOI] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;25 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell B., Moss C., George G., Santaolalla A., Cope A., Papa S., Van Hemelrijck M. Associations between mmune-suppressive and stimulating drugs and novel COVID-19 -- a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander W.J., O’Neill H.G., Pohl C.H. Prostaglandin E2 as a modulator of viral infections. Front. Physiol. 2017;8:89. doi: 10.3389/fphys.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmaier S.M., De Chaumont C., Balbi M., Terpolilli N.A., Kleinschnitz C., Gruber A., Plesnila N. The formation of microthrombi in parenchymal microvessels after traumatic brain injury is independent of coagulation factor XI. J. Neurotrauma. 2016;1(September (17)):1634–1644. doi: 10.1089/neu.2015.4173. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena C.M., Leandro A., Azul L., Seiça R., Perry G. Vascular oxidative stress: impact and therapeutic approaches. Front. Physiol. 2018;4(Deember 9):1668. doi: 10.3389/fphys.2018.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C.V., Mehta V. Paracetamol: mechanisms and updates. Contin. Educ. Anaesth. Crit. Care Pain. 2014;14(4):153–158. [Google Scholar]
- Shibata S., Fujita T. The kidneys and aldosterone/mineralocorticoid receptor system in salt-sensitive hypertension. Curr. Hypertens. Rep. 2011;13(2):109–115. doi: 10.1007/s11906-010-0175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegeleer A.D., Bronselaer A., Teo J.T., Byttebier G., De Tre G., Belmans L., Dobson R. The Effects of ARBs, ACEis, and Statins on Clinical Outcomes of COVID-19 Infection Among Nursing Home Residents. J. Am. Med. Dir. Assoc. 2020;21(7):909–914. doi: 10.1016/j.jamda.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer S.A., Corbett J.A. The role and regulation of COX-2 during viral infection. Viral Immunol. 2003;16(4):447–460. doi: 10.1089/088282403771926283. [DOI] [PubMed] [Google Scholar]
- Tallant E.A., Ferrario C.M., Gallagher P.E. Cardioprotective role for angiotensin-(1-7) and angiotensin converting enzyme 2 in the heart. Future Cardiol. 2006;2(3):335–342. doi: 10.2217/14796678.2.3.335. [DOI] [PubMed] [Google Scholar]
- Teijaro J.R. Type I interferons in viral control and immune regulation. Curr. Opin. Virol. 2016;16:31–40. doi: 10.1016/j.coviro.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch G.H., Allen T.J. Rodent models of streptozotocin‐induced diabetic nephropathy (Methods in Renal Research) Nephrology. 2007;12(June(3)):261–266. doi: 10.1111/j.1440-1797.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- Thaweerat W. Current evidence on pancreatic involvement in SARS-CoV-2 infection. Pancreatology. 2020:27. doi: 10.1016/j.pan.2020.05.015. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikellis C., Thomas M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012;2012 doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk C., Turk S., Temirci E.S., Malkan U.Y., Haznedaroglu I.C. In vitro analysis of the renin-angiotensin system and inflammatory gene transcripts in human bronchial epithelial cells after infection with severe acute respiratory syndrome coronavirus. J. Renin. Syst. 2020;21(2) doi: 10.1177/1470320320928872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A.J. The Protective Arm of the Renin Angiotensin System: Functional Aspects and Therapeutic Implications. Acad Press; 2015. Chapter 25 - ACE2 cell biology, regulation, and physiological functions. ISBN 978-0-12-801364-9. [DOI] [Google Scholar]
- University of Oxford . 2020. Low-cost Dexamethasone Reduces Death by up to One Third in Hospitalised Patients With Severe Respiratory Complications of COVID-19. RECOVERY Trial 2020.https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19 Web URL: [Google Scholar]
- Veronese N., Demurtas J., Yang L., Tonelli R., Barbagallo M., Lopalco P., Lagolio E. Use of corticosteroids in coronavirus disease 2019 pneumonia: a systematic review of the literature. Front. Med. 2020;(7):170. doi: 10.3389/fmed.2020.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrigkou E., Tsangaris I., Bonovas S., Tsantes A., Kopterides P. The evolving role of the renin–angiotensin system in ARDS. Crit. Care. 2017;21(1) doi: 10.1186/s13054-017-1917-5. 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukelic S., Griendling K.K. Angiotensin II, from vasoconstrictor to growth factor: a paradigm shift. Circ. Res. 2014;28(Febuary (5)):754–757. doi: 10.1161/CIRCRESAHA.114.303045. 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P., Behrens N., Chaigneau F.R.C., McEligot H., Agrawal K. A randomized placebo controlled trial of ibuprofen for respiratory syncytial virus infection in a bovine model. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0152913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A., Chiew C.J., Lee V.J. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect. Dis. 2020 doi: 10.1016/s1473-3099(20)30129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WorldOMeter . 2020. COVID-19 Coronavirus Outbreak.https://www.worldometers.info/coronavirus/ Web URL: Accessed: October 16, 2020. [Google Scholar]
- WorldOMeter . 2020. COVID-19 Coronavirus Outbreak, United States.https://www.worldometers.info/coronavirus/country/us/ Web URL: Accessed: October 16, 2020. [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;13(March (6483)):1260–1263. doi: 10.1126/science.abb2507. 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki J., Lores E., Ye M., Soler M.J., Batlle D. Kidney and lung ACE2 expression after an ACE inhibitor or an Ang II receptor blocker: Implications for COVID-19. J. Am. Soc. Nephrol. 2020;31:1941–1943. doi: 10.1681/ASN.2020050667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;24(Febuary (1)):1–5. doi: 10.1038/s41368-020-0074-x. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Liu J., Zhou Y., Zhao X., Zhao Q., Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J. Infect. 2020;1:E13–E20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatabe J., Yoneda M., Yatabe M.S., Watanabe T., Felder R.A., Jose P.A., Sanada H. Angiotensin III stimulates aldosterone secretion from adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinology. 2011;1(April (4)):1582–1588. doi: 10.1210/en.2010-1070. 152. [DOI] [PubMed] [Google Scholar]
- Ye Z., Wang Y., Colunga-Lozano L.E., Prasad M., Tangamornsuksan W., Rochwerg B., Yao L. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2020;192:E756–E767. doi: 10.1503/cmaj.200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefifard M., Zali A., Zarghi A., Neishaboori A.M., Hosseini M., Safari S. Non-steroidal anti-inflammatory drugs in management of COVID-19; a systematic review on current evidence. Int. J. Clin. Pract. 2020;27 doi: 10.1111/ijcp.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Li S., Niu S. ACE2 and COVID-19 and the resulting ARDS. Postgrad. Med. J. 2020 doi: 10.1136/postgradmedj-2020-137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.