Abstract
Central nervous system (CNS) innate immunity plays essential roles in infections, neurodegenerative diseases, and brain or spinal cord injuries. Astrocytes and microglia are the principal cells that mediate innate immunity in the CNS. Pattern recognition receptors (PRRs), expressed by astrocytes and microglia, sense pathogen-derived or endogenous ligands released by damaged cells and initiate the innate immune response. Toll-like receptors (TLRs) are a well-characterized family of PRRs. The contribution of microglial TLR signaling to CNS pathology has been extensively investigated. Even though astrocytes assume a wide variety of key functions, information about the role of astroglial TLRs in CNS disease and injuries is limited. Because astrocytes display heterogeneity and exhibit phenotypic plasticity depending on the effectors present in the local milieu, they can exert both detrimental and beneficial effects. TLRs are modulators of these paradoxical astroglial properties. The goal of the current review is to highlight the essential roles played by astroglial TLRs in CNS infections, injuries and diseases. We discuss the contribution of astroglial TLRs to host defense as well as the dissemination of viral and bacterial infections in the CNS. We examine the link between astroglial TLRs and the pathogenesis of neurodegenerative diseases and present evidence showing the pivotal influence of astroglial TLR signaling on sterile inflammation in CNS injury. Finally, we define the research questions and areas that warrant further investigations in the context of astrocytes, TLRs, and CNS dysfunction.
Keywords: Astrogliosis, Neuroprotection, Neuroinflammation, Infection, SARS-CoV-2, Cytokines, Alzheimer’s disease, Parkinson’s disease, Amyotrophic lateral sclerosis, Brain injury, Spinal cord injury, Pattern recognition receptors, COVID-19
1. Introduction
Astrocytes are the most abundant glial cells in the central nervous system (CNS) and display high functional heterogeneity (Khakh and Deneen, 2019, Matias et al., 2019). They contribute to many key physiological and pathophysiological mechanisms in the CNS (Sofroniew and Vinters, 2010, Valori et al., 2019). The physiology of astrocytes and their role in essential CNS functions have been summarized in comprehensive review articles and will not be discussed in detail in this review (Allen and Eroglu, 2017, Farhy-Tselnicker and Allen, 2018, Kreft et al., 2012, Santello et al., 2019, Verkhratsky and Nedergaard, 2018, Verkhratsky et al., 2019). Astrocytes provide energy and metabolic substrates to support neuronal activity (Brown and Ransom, 2007, Nortley and Attwell, 2017, Rouach et al., 2008). They maintain molecular homeostasis (Marina et al., 2018, Verkhratsky and Nedergaard, 2018), modulate neuronal excitability (Halassa et al., 2007, Mederos and Perea, 2019, Montana et al., 2004, Parpura et al., 1994, Verkhratsky et al., 2020) and synaptic development, transmission, and plasticity (Allen and Eroglu, 2017). Another important function of astrocytes is the maintenance of the blood-brain barrier (BBB). The BBB is primarily formed by densely aligned capillary endothelial cells. The tight junctions between the endothelial cells act as a physical barrier that limits the entry of pathogenic agents into the CNS. Astrocytes interact with the capillary endothelial cells through their perivascular endfeet and support the integrity of the BBB (Abbott et al., 2006). In addition, they produce and release vasoactive agents and regulate blood flow (Iadecola and Nedergaard, 2007).
In pathological conditions of the CNS, astrocytes undergo morphological, molecular, and functional changes in response to environmental stimuli and become reactive. Reactive astrocytes proliferate and significantly increase their number at the affected sites. These changes are collectively referred to as astrogliosis (Sofroniew, 2015). Reactive astrocytes exert both beneficial and detrimental functions (Escartin et al., 2019, Liddelow and Barres, 2017, Sofroniew, 2009). They contribute to the pathogenesis of neurodegenerative diseases (Sadick and Liddelow, 2019, Yamanaka and Komine, 2018), inflammatory CNS disorders (Ludwin et al., 2016), injuries (Yang et al., 2020b, Zhou et al., 2020), and neuropathic pain (Ji et al., 2019). They also confer neuroprotection (Liu et al., 2017).
In addition to microglia, astrocytes have emerged as key contributors to the innate immune response of the CNS to infections, neurodegenerative disorders, and injuries (Farina et al., 2007, Rivest, 2009). Pattern recognition receptors (PRRs), which play an essential role in mediating innate immune responses (Takeuchi and Akira, 2010), are also expressed by CNS cells, including astrocytes (Bsibsi et al., 2006, Bsibsi et al., 2002, Farina et al., 2005). Glial PRRs are key sensors of danger and essential facilitators of local neuroinflammation in CNS pathologies (Heneka et al., 2014). Pattern recognition receptors are involved in the crosstalk between glia and neurons and the interactions between infiltrating immune cells and cells intrinsic to the CNS. One of the best-characterized families of PRRs is the toll-like receptor (TLR) family (Akira, 2001, Kawai and Akira, 2011).
The goal of the present review is to highlight the role of TLRs in the innate immune response mounted by astrocytes to CNS infections, neurodegenerative diseases, and injuries.
2. Phenotypes and functions of reactive astrocytes in CNS pathology
Reactive astrocytes display different phenotypes and have been classified into A1 and A2 phenotypes (Liddelow and Barres, 2017, Liddelow et al., 2017). The A1 phenotype has pro-inflammatory, neurotoxic properties, whereas A2 astrocytes display neuroprotective characteristics. The number of A1 astrocytes is dramatically increased in neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS) (Liddelow et al., 2017). Interestingly, the number of A1 astrocytes also increases in healthy brains during normal aging (Clarke et al., 2018). However, single-cell sequencing in the cingulate cortex of HD brains indicated that astrocytes display multiple transcriptional phenotypes and that the expression of genes characteristic of A1, A2 and pan-astrocytes are simultaneously upregulated in the diseased brain (Al-Dalahmah et al., 2020). Based on the expression levels of genes which are considered astrocyte markers, namely GFAP (glial fibrillary acidic protein), SLC1A2 (solute carrier family 1 member 2; excitatory amino acid transporter 2) and MTs (metallothioneins) (Penkowa, 2006), astrocytes have been classified into at least three reactive states. This report also highlighted the presence of additional astrocyte phenotypes that did not fit precisely these three states and could be tentatively considered astrocytes in a transitional reactive state. It is worth noting that astrocyte heterogeneity is also observed in control brains. However, the proportion of astrocytes in each reactive state is different in controls than in HD brains. Thus, the phenotypic heterogeneity of astrocytes might extend beyond the A1 and A2 phenotypes.
Reactive astrogliosis is the fundamental cellular process that initiates the formation of a glial scar around lesions in traumatic CNS injuries (Sofroniew, 2009), AD (Wyss-Coray and Rogers, 2012), and multiple sclerosis (MS) (Ponath et al., 2018). The glial scar acts as a physical and chemical barrier to contain the cellular damage exerted by toxic molecules released from necrotic tissue, and restricts the infiltration of inflammatory cells from the periphery to the affected sites (Faulkner et al., 2004, Voskuhl et al., 2009). Ablation of the glial scar by selective removal of reactive astrocytes prolongs neuroinflammation and attenuates axonal regrowth following traumatic brain injury (TBI) (Bush et al., 1999) and spinal cord injury (SCI) (Anderson et al., 2016). Reactive astrocytes at the glial scar release neurotrophic and synaptogenic factors, and extracellular matrix (ECM) proteins to support neuronal survival, synapse formation, and synaptic plasticity (Sofroniew, 2009, Sofroniew, 2015). These findings indicate that the glial scar promotes protection and recovery following CNS injury. Paradoxically, the glial scar has also been considered as an impediment to axonal regeneration because cells at the glial scar, including reactive astrocytes, release effectors that inhibit axonal regrowth (Silver and Miller, 2004). Chondroitin sulfate proteoglycans (CSPGs) are among the inhibitors of axonal regeneration (McKeon et al., 1991, Smith and Strunz, 2005). Despite controversy, it is likely that the glial scar exerts both helpful and harmful effects depending on the context of CNS pathology.
The neuroprotective effects of reactive astrocytes are further supported by findings indicating that brain-derived neurotrophic factor (BDNF) released by astrocytes promotes oligodendrogenesis and remyelination in response to ischemic brain injury (Miyamoto et al., 2015). Moreover, glial cell-derived neurotrophic factor (GDNF) released by astrocytes supports dopaminergic and motor neuron survival (Li et al., 2012).
Another beneficial effect of reactive astrocytes is phagocytosis (Neumann et al., 2009). Even though microglia and macrophages are considered the primary cells that clear cellular debris in the diseased CNS, astrocyte-mediated phagocytosis also facilitates the repair and remodeling of the CNS in diseases and injury. In AD, reactive astrocytes take up and degrade the pathogenic amyloid β (Aβ) from the extracellular space (Koistinaho et al., 2004, Wyss-Coray et al., 2003). In synucleinopathies, including PD, reactive astrocytes take up and clear α-synuclein (α-syn). In the injured and ischemic brain, degenerated axons and apoptotic neurons are engulfed via phagocytosis by reactive astrocytes (Cheng et al., 1994, Lööv et al., 2012, Morizawa et al., 2017).
Reactive astrocytes also contribute to neuroinflammation via the release of cytokines, chemokines and other inflammatory mediators. The production of cytokines and chemokines is primarily initiated by stimulation of PRRs. Pathogen-derived or endogenous signals activate PRRs during infections, injuries, and diseases (Heiman et al., 2014, Azam et al., 2019, Olejnik et al., 2018). Pattern recognition receptor expression is increased in human astrocytes activated by pro-inflammatory signals (BsiBsi et al., 2006). In turn, stimulation of PRRs activates astrocytes, and modulates astrocyte polarization (Esen et al., 2004, Liu et al., 2016, Rosciszewski et al., 2018). The cytokines released by reactive astrocytes activate nearby glia, whereas the secreted chemokines facilitate the recruitment of peripheral immune cells. The overall outcome is the amplification of the local inflammatory reaction (Farina et al., 2007). Reactive astrocytes also release vasoactive endothelial growth factor (VEGF), which facilitates the infiltration of leukocytes by increasing the permeability of the blood–brain barrier (Argaw et al., 2009, Argaw et al., 2012). In addition, reactive astrocytes release reactive oxygen species (ROS) and glutamate which contribute to neuronal and oligodendrocyte toxicity and exacerbate neurodegeneration (Hamby et al., 2006, Takano et al., 2005).
In summary, it is likely that the beneficial and detrimental effects of astrocytes are due to their heterogeneity and the different phenotypes that they acquire depending on the triggers present at affected sites as well as the type, stage, and progression of the pathological condition. By modulating the innate immune and neuroinflammatory response of astrocytes as well as their polarization into a pro-inflammatory versus an anti-inflammatory phenotype, PRRs are likely to play a central role in the balance between the beneficial and detrimental influences of reactive astrocytes. This topic will be further discussed below.
3. TLRs modulate astrocyte function in vitro
The first discovered and best-characterized family of PRRs is the TLR family (Liu et al., 2020). A total of ten TLRs, TLR1-10, have been identified in humans whereas TLR1-13, with the exemption of TLR10 (Hasan et al., 2005), have been identified in mice (Kawai and Akira, 2006). TLR3, TLR7-9 and TLR13 are intracellular receptors expressed in the endosomal compartments. The other TLRs are located on the cell surface. TLRs recognize conserved molecular patterns in pathogens, also known as pathogen-associated molecular patterns (PAMPs), or endogenous ligands that are released by stressed, injured, or necrotic cells, collectively called danger-associated molecular patterns (DAMPs) (Heiman et al., 2014). The endosomal TLRs primarily recognize nucleic acids derived from viruses and bacteria, whereas cell surface TLRs recognize various bacterial components such as lipopolysaccharides (LPS; Poltorak et al., 1998), peptidoglycans (PGN), (Takeuchi et al., 1999), flagellin (Hayashi et al., 2001), and lipoproteins (Brightbill et al., 1999). In neurodegenerative diseases and CNS injuries, endogenous ligands induce TLR signaling. Relatively limited information is available with regard to endogenous activators of various TLRs. Self-mRNA released by necrotic cells (Karikó et al., 2004), mitochondrial DNA (mtDNA) released by damaged mitochondria (Bao et al., 2016, Collins et al., 2004, Gray et al., 1999, Heiman et al., 2014, Kawai and Akira, 2010, Oka et al., 2012), high mobility group box 1 (HMGB1), a nuclear chromatin protein produced by neurons and activated glia (Sun et al., 2017), heat shock proteins (HSPs) (Asea et al., 2002, Ohashi et al., 2000), α-syn released by neurons (Fellner et al., 2013, Kim et al., 2013), and Aβ peptides derived from the amyloid precursor protein (APP) (Liu et al., 2012) are among endogenous TLR ligands. Aβ-induced inflammatory response necessitates TLR4 (Hughes et al., 2020), and Aβ aggregates bind TLR4 (Yang et al., 2020a).
With the exemption of TLR3, all other TLRs utilize the myeloid differentiation primary response 88 (MyD88)-dependent signaling pathway. TLR3 uses a toll/interleukin-1 receptor (TIR)-domain-containing adapter-inducing interferon-β (TRIF)-dependent signaling pathway (Akira and Takeda, 2004, Kawai and Akira, 2011). TLR4 is the only TLR that utilizes both the MyD88-dependent and the TRIF-dependent signaling pathways. Activation of TLR signaling pathways leads to the transcription of inflammatory cytokines and type 1 interferons (IFNs) (Heiman et al., 2014).
In addition to innate immune cells, human and rodent CNS cells, including neurons and glia, express TLRs (Bowman et al., 2003, Bsibsi et al., 2006, Bsibsi et al., 2002, Kigerl et al., 2007, Lafon et al., 2006). Human astrocytes express TLR1-5 and TLR9, and mouse astrocytes express TLR1-9 (Carpentier et al., 2008, Carty and Bowie, 2011). The role of microglial TLRs in various CNS pathologies has been extensively studied (Anttila et al., 2017, Lehnardt, 2010, Rodríguez-Gómez et al., 2020, Sanchez-Guajardo et al., 2015, Younger et al., 2019). Despite some advances, the roles of TLRs in other CNS cells warrant further investigations.
A number of studies analyzed the modulation of astrocyte function by TLRs, in vitro. The ligands used were pathogen-derived components, endogenous TLR activators, or synthetic agonists and antagonists. The majority of these studies focused on the expression or release of various effectors, especially inflammatory mediators. Collectively, these investigations indicate that the profile of cytokine and chemokine expression or release by astrocytes is dependent on the TLR engaged and the ligand that binds a specific TLR (Bsibsi et al., 2006, Bsibsi et al., 2007, Esen et al., 2004, Farina et al., 2007, Krasowska-Zoladek et al., 2007). Astroglial TLR2 and TLR4 are most frequently associated with interleukin-1 (IL-1), IL-6, tumor necrosis factor-α (TNF-α), and C-C motif or C-X-C motif chemokine expression and release, in a ligand-dependent manner. In contrast, stimulation of TLR3 in human astrocyte cultures induces the expression of neuroprotective factors and anti-inflammatory cytokines (Bsibsi et al., 2006). In addition, a synthetic TLR9 agonist, oligodeoxynucleotide 1826 (ODN 1826), or an antagonist, ODN 2088, modulates the release of C-C motif and C-X-C motif chemokines by murine astrocytes, in vitro (Acioglu et al., 2016, Li et al., 2020a). Interestingly, astroglial TLR9 antagonism increases the release of C-C motif ligand 1 (CCL1), a major chemoattractant which promotes chemotaxis of peripheral macrophages in astrocyte-macrophage co-cultures (Li et al., 2020a). In addition, astroglial TLR9 antagonism modifies astrocyte-to-macrophage signals, and by doing so, promotes the polarization of peripheral macrophages into the beneficial M2 phenotype (Li et al., 2020a). TLR9 antagonism also alters astrocyte-to-neuron signals and abrogates the survival promoting effects of astrocytes on neurons (Acioglu et al., 2016).
In contrast to the aforementioned investigations, several studies reported that ligand binding to astroglial TLR does not induce pro-inflammatory cytokine release, in vitro, when contaminating microglia, which are often present in astrocyte cultures, are depleted (Barbierato et al., 2013, Facci et al., 2014). The studies concluded that a crosstalk between microglia and astrocytes is necessary to promote the TLR-induced inflammatory response by astrocytes. In agreement with this idea, transforming growth factor-β (TGF-β), which is released by microglia and macrophages in addition to astrocytes (Welser-Alves and Milner, 2013), enhances the TLR-elicited inflammatory response of astrocytes (Hamby et al., 2006). Alternatively, it has been proposed that the cytokines originate from the contaminating microglia, whose cytokine secretion might be increased in the presence of astrocytes (Saura, 2007, Solà et al., 2002). However, recent investigations resolved this issue by using 99% pure astrocyte cultures and corroborated the finding that astrocytes release pro-inflammatory cytokines in response to stimulation of TLR (Li et al., 2020a, Rodgers et al., 2020). Of note, even studies on microglia-depleted astrocyte cultures agree that astrocytes express functional TLRs, because they respond to TLR agonists by upregulating TLR expression (Marinelli et al., 2015).
Proliferation and migration of astrocytes, functions associated with cellular activation, are also modulated by TLR in vitro. Stimulation of rat astroglial TLR2 significantly enhances the migration of astrocytes in vitro. This was attributed to the upregulation of matrix metalloproteinase 9 (MMP-9), which degrades the ECM proteins and interacts with cell surface protein CD44 to facilitate the mobility of astrocytes (Dufour et al., 2010, Hsieh et al., 2010). Exposure of astrocyte cultures to the TLR2 and TLR4 agonist LPS promotes astrocyte proliferation via the release of TNF-α (Rodgers et al., 2020). In contrast, TLR3 has opposite effects on astrocyte proliferation. Activation of TLR3 in rat astrocyte cultures subjected to oxygen-glucose deprivation and reoxygenation (OGD/R), an in vitro model of ischemic/hypoxic injury, significantly reduces astrocyte proliferation (Li et al., 2015). Lastly, A TLR9 antagonist inhibits spinal cord astrocyte proliferation and migration (Li et al., 2019). These findings will be further discussed in section 7 in the context of SCI.
Taken together, these findings indicate that activation of TLRs modulates both immune and non-immune functions of astrocytes, and induces astrocyte reactivity. It is likely that the net consequence of astroglial TLR stimulation is context-dependent and influenced by the pathological environment in which the cells function. In the following sections, we will discuss the outcomes of astroglial TLR activation in CNS infections, injuries, and diseases. Our goal is to highlight the broad diversity of responses mounted by astrocytes in distinct pathologies and to underline the commonalities and divergences.
4. Role of astroglial TLRs in viral infections of the CNS
Viral infections of the CNS are infrequent but can cause severe neurological dysfunction and death (Swanson and McGavern, 2015). Neurotropic viruses, including Zika virus (ZIKV), tick-borne encephalitis virus (TBEV), West Nile virus (WNV), herpes simplex virus (HSV), and human immunodeficiency virus (HIV) infect the CNS using different routes and can lead to neuropathology and neural damage (Koyuncu et al., 2013, Ludlow et al., 2016, Potokar et al., 2019). ZIKV, a mosquito-borne RNA flavivirus, causes severe neurological complications including microcephaly and congenital brain anomalies when pregnant women are infected and the virus is transmitted to the fetus or the perinatal infant (Agumadu and Ramphul, 2018). HSV, an enveloped double-stranded DNA virus, usually infects humans at childhood and produces a life-long infection. It remains latent in neurons and can cause severe infection of the CNS when activated (Duarte et al., 2019). HIV is an enveloped single-stranded RNA virus which usually infects human immune cells and causes acquired immunodeficiency syndrome (AIDS) as a result of immune system failure. HIV infection of the brain impairs cognitive, behavioral, and motor functions known as AIDS dementia complex (ADC) in adults and HIV-related encephalopathy in children (del Palacio et al., 2012). The hallmarks of HIV-1-associated neuropathology include brain atrophy, white matter gliosis, and neuronal loss (Ketzler et al., 1990).
The early interactions between viruses that enter the CNS and the local cells determine the outcomes of infections. Astrocytes are one of the first cells that sense virus entry into the CNS (Lindqvist et al., 2016, van den Pol et al., 2017) and are also the primary target of certain viruses (Luo et al., 2019). The initial host defense relies on the effectiveness of the innate anti-viral response mounted by astrocytes and microglia (Barrat et al., 2019). The recognition of viruses by PRRs stimulates signaling pathways that lead to the coordinated activation of transcription factors, including interferon regulatory factor 3 (IRF3), which promote type 1 IFN transcription. Astrocytes are the main producers of IFN-β following infection of the CNS by various neurotropic viruses (Pfefferkorn et al., 2016). This early response limits the viral replication and the spread of the virus. The ability of astrocytes to build an effective anti-viral response has been attributed to the high basal expression of IFN-stimulated genes, which facilitate the rapid viral recognition and increased IFN production (Lindqvist et al., 2016). Interestingly, not only productively infected astrocytes but also abortively infected astrocytes can build innate immune responses (Pfefferkorn et al., 2016). Signaling through PRRs also causes the translocation of the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) from the cytoplasm into the nucleus and the initiation of cytokine and chemokine transcription (Heiman et al., 2014). The released cytokines and chemokines facilitate the recruitment of innate and adaptive effector cells that enhance the defense against the invading pathogen and restrict viral replication. Initially, the pro-inflammatory response contributes to the host defense. However, persistent inflammation causes CNS tissue damage (Claycomb et al., 2013).
A number of studies have also shown that astrocytes play a key role in viral storage, replication, and dissemination (Potokar et al., 2019). After peripheral inoculation of neonatal mice with ZIKV, the early targeting of astrocytes by the virus was followed by infection of neurons at a more advanced stage. The delayed neuronal infection was attributed to ZIKV release by infected astrocytes (van den Pol et al., 2017). Mouse and human astrocytes are also susceptible to HSV-1 infection, support the replication of the virus, serve as a viral reservoir, and spread the virus to other cells (Aravalli et al., 2005, Liu et al., 2013, Lokensgard et al., 2002). Even though astrocytes are not the most commonly targeted CNS cells by HIV-1, they can transiently support viral reproduction at the initial viral exposure. Inflammatory cytokines, such as TNF-α and IL-1, can reactivate the infected astrocytes and boost further viral replication (Tornatore et al., 1991). The mechanism of HIV entry into astrocytes remains elusive and appears to be different than what is observed in immune cells (Kramer-Hämmerle et al., 2005).
The essential role played by TLRs in mounting the innate immune response to infections is demonstrated by studies on TLR deficient mice which show increased susceptibility to infections (Akira et al., 2006). However, in some cases, TLR deficiency reduces the virus-induced disease severity and mortality because it abrogates the excessive production of inflammatory mediators which occurs as a result of the activation of TLR signaling (Gowen et al., 2006).
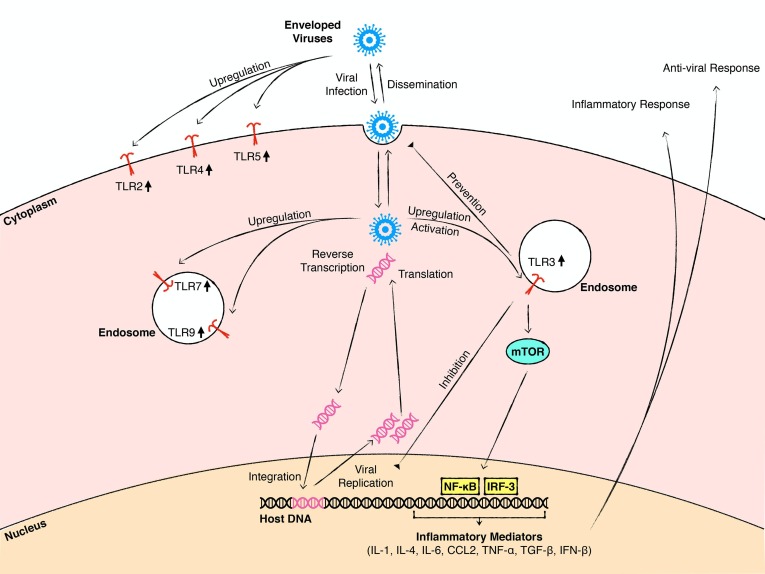
Among all members of the TLR family, TLR3 is considered to be the front-line defense receptor against viral infections, and the primary mediator of the cellular responses to viruses since it recognizes double-stranded RNA, a common by-product of viral replication (Alexopoulou et al., 2001). The clinical importance of TLR3 in viral infections of the CNS has been demonstrated by studies reporting that children with a negative dominant TLR3 allele are especially susceptible to HSV-1 encephalitis, whereas they do not show a general increase in vulnerability to other pathogens (Zhang et al., 2007). In agreement with these findings, peripheral inoculation of TLR3 deficient mice with HSV-2 and WNV results in an increase in the susceptibility of the CNS to HSV-2 and WNV infections, respectively (Daffis et al., 2008, Reinert et al., 2012), whereas enhancement of TLR3-mTORC2 (mammalian target of rapamycin complex 2) axis confers protection against HSV encephalitis (Sato et al., 2018) (Fig. 1 ).
Fig. 1.
A schematic summary of mechanisms by which astroglial TLRs contribute to viral infections of the CNS. Astrocytes sense and respond to various viruses that enter the CNS. In the early stage of viral infection, infected astrocytes mount an anti-viral response to protect the host. Exposure to viruses or viral components upregulates the expression of astroglial TLRs located at the cell membrane or endosomal compartments. The activation of TLR signaling, especially TLR3 signaling, facilitates the anti-viral response including prevention of viral dissemination, inhibition of viral replication, and production of anti-viral mediators. The TLR3-mTORC2 axis is among the pathways that mediate this response. TLR signaling also promotes the production and release of pro-inflammatory mediators and induces inflammatory responses in the CNS. These inflammatory responses can help the clearance of the pathogen but also cause degeneration and dysfunction of CNS cells. Astrocytes can also store, support the replication of viruses, and disseminate them to other CNS cells.
TLR3 deficient astrocytes show impaired type 1 IFN response following HSV-2 infection, even though the global type 1 IFN response in TLR3-/- mice is not different than that observed in wild type controls (Reinert et al., 2012). The impairment of type 1 IFN production by TLR3-/- astrocytes renders these cells more permissive to HSV-2 infection and viral replication compared to wild type astrocytes. Based on these findings, it has been postulated that subsequent to the entry of HSV-2 to the CNS, activation of TLR3 signaling in astrocytes controls the spread of the virus. The TLR3-mediated astroglial anti-viral response restricts the virus to the infected neurons and prevents the spread of the virus to other CNS cells (Reinert et al., 2012). Studies on primary mouse astrocyte cultures also show that HSV-1 infection induces the astroglial inflammatory response via effects on TLR3 and its downstream effector, NF-κB. (Liu et al., 2013) (Fig. 1).
Other viruses induce astroglial inflammatory responses via activation of TLR3. In primary human astrocyte cultures, ZIKV infection significantly increases the TLR3, IRF3 and NF-κB expression in a strain-dependent manner and promotes pro-inflammatory cytokine and chemokine release. Genetic silencing of TLR3 expression or pharmacological inhibition of TLR3 signaling reduces this pro-inflammatory response (Ojha et al., 2019). Activation of TLR3 by polyinosinic:polycytidylic acid (poly(I:C)) in HIV-infected human fetal astrocytes upregulates the expression of anti-viral mediators Viperin/cig5 and Indoleamine 2,3-dioxygenase (IDO) which, in turn, reduce the viral replication (Rivieccio et al., 2006, Suh et al., 2007). Inhibition of endosomal TLRs, including TLR3, enhances the permissiveness and accumulation of HIV in human fetal astrocytes (Kužnik et al., 2011) and promotes the onset of HIV-induced neurological disorders (Vijaykumar et al., 2008). Collectively, these studies highlight the central role played by astroglial TLR3 in the anti-viral and inflammatory response of astrocytes following viral infections (Fig. 1).
Information about the potential roles played by other astroglial TLRs in the host response to viral infections of the CNS is relatively limited. Exposure of cultured mouse or human astrocytes to different viruses or viral components upregulates TLR2, TLR4 and TLR5 levels (El-Hage et al., 2011, Villalba et al., 2012, Wang et al., 2012, Salaria et al., 2007, Serramía et al., 2016) which is paralleled by increased pro-inflammatory cytokine and chemokine production (El-Hage et al., 2008, Liu and Kumar, 2015, Ngwainmbi et al., 2014) (Fig. 1). However, it is not yet known whether the increased TLR2, TLR4, and TLR5 expression is directly linked to elevated inflammatory mediator production by infected astrocytes. TLR7 and TLR9 transcript levels significantly increase in ZIKV infected human astrocyte cultures (Hamel et al., 2017), but it is not yet known whether increased astroglial TLR7 and TLR9 expression contributes to the anti-viral response of astrocytes to ZIKV infection.
4.1. Coronaviruses, SARS-CoV-2, astrocytes and TLRs: a knowledge gap to be filled
The highly contagious severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) causes a cardiorespiratory disease identified as COVID-19, which has resulted in a global pandemic in 2020. Although the primary target of SARS-CoV-2 is the respiratory system, an increasing percentage of COVID-19 patients present neurological symptoms including anosmia, ageusia, encephalitis, seizures, and stroke (Baig and Sanders, 2020, Efe et al., 2020, Finsterer and Stollberger, 2020, Fotuhi et al., 2020, Gilani et al., 2020, Heneka et al., 2020, Liang et al., 2020, Liguori et al., 2020, Mahboob et al., 2020, Montalvan et al., 2020, Morgello, 2020, Moriguchi et al., 2020, Parsons et al., 2020, Saggese et al., 2020, Wu et al., 2020). A comprehensive understanding of the mechanisms by which SARS-CoV-2 causes neurological symptoms is currently lacking. It is not yet known whether the neurological deficits are the direct effects of SARS-CoV-2 on the CNS, the secondary outcome of the cytokine storm that the virus induces by over-activating the innate immune system, or due to other pathology such as excess blood clot formation in CNS blood vessels and cerebrovascular disease. However, reports showing the presence of viral RNA in the cerebrospinal fluid (CSF) of COVID-19 patients raised the possibility that SARS-CoV-2 is a neurotropic virus (Kabbani and Olds, 2020, Li et al., 2020b). Moreover, viral particles have been detected in the post-mortem brain of an individual affected by COVID-19, further supporting the idea that SARS-CoV-2 might infect the CNS (Paniz‐Mondolfi et al., 2020). Some potential routes of entry into the CNS have been discussed in different reports (Alam et al., 2020, Bostancıklıoğlu, 2020, Chigr et al., 2020, Montalvan et al., 2020). However, there is no consensus about the principal mode of entry due to the lack of information regarding this issue. In COVID-19 patients with severe disease, the levels of plasma GFAP and neurofilament light chain protein (NFL), markers of astrocyte and axonal damage, respectively, were increased, raising the possibility of astrocytic and neuronal injury (Kanberg et al., 2020).
Observations on the predecessors to SARS-CoV-2, namely, SARS-CoV and Middle East Respiratory Syndrome (MERS)-CoV, which caused the earlier SARS and MERS pandemics, also indicate the presentation of neurological symptoms by affected individuals (Lau et al., 2004, Saad et al., 2014, Umapathi et al., 2004, Xu et al., 2005). The presence of viral RNA in the brain and CSF of individuals with SARS further supports the notion of neurotropism (Lau et al., 2004, Xu et al., 2005). However, there is not enough evidence showing the presence of viral RNA in the CSF of MERS patients. SARS-CoV viral particles have been detected in hypothalamic and cortical neurons and this was paralleled by neurodegeneration (Gu et al., 2005). This study did not report the occurrence of viral particles in non-neuronal cells or detection of inflammatory cells in the infected CNS.
The major receptor for SARS-CoV and SARS-CoV-2 is the angiotensin-converting enzyme 2 (ACE2). Infection of human ACE2 transgenic mice with SARS-CoV causes a lethal disease. The virus spreads through trans-neuronal pathways (McCray et al., 2007), and neuronal infection is followed by neuronal death without manifestation of inflammation (Netland et al., 2008). The receptor for MERS-CoV is dipeptidyl peptidase 4 (DPP4). Infection of human DPP4 transgenic mice with MERS-CoV causes neuronal damage paralleled by infiltration of inflammatory cells (Li et al., 2016). Both ACE2 and DPP4 are expressed in murine astrocytes (Gallagher et al., 2006, Mentzel et al., 1996). However, it remains to be determined whether ACE2 or DPP4 are expressed in human astrocytes and whether SARS-CoV, SARS-CoV-2 or MERS-CoV infect or activate astrocytes.
Even though studies on the involvement of astrocytes in neurological symptoms and neuropathology associated with SARS-CoV, SARS-CoV-2, or MERS are scarce, investigations on other coronaviruses have been performed and could provide insights into potential mechanisms. Neurotropic strains of murine hepatitis coronavirus (MHV-A59) cause encephalomyelitis and chronic demyelinating disease in mice (Lavi et al., 1986, Gonzales et al., 2004). Infected astrocytes robustly upregulate the expression of class I major histocompatibility complex (MHC I), which could contribute to virus-induced pathology (Gilmore et al., 1994). Astrocytes also mount an anti-viral IFN-β response, in vitro (Wang et al., 1998). The extent of cytokine production by astrocytes correlates with neurovirulence (Li et al., 2004). The response of astrocytes is more robust than that of microglia, and ablation of IFN-α/β signaling in astrocytes leads to the uncontrolled viral spread in the CNS with fatal consequences (Hwang and Bergmann, 2018). A recent report questioned the effects of MHV-A59 on murine astrocyte cell lines (Lavi and Cong, 2020). The generation and classification of the astrocyte cell lines have been described before (Alliot and Pessac, 1984). The classification was based on distinct morphological characteristics and termed as type 1 (bearing several short processes), type 2 (bearing two processes), and type 3 (elongated cell body and no processes). Infection of type 1, but not type 2 or type 3 astrocytes, led to a robust production of pro-inflammatory cytokines. Given the heterogeneity of astrocytes, the studies suggest that different astrocyte subpopulations could respond to a coronavirus infection in a distinct manner. Whether TLRs play a role in the pro-inflammatory response mounted by astrocytes to MHV-A59 has not been investigated. It also remains to be determined whether the divergent responses are due to expression of different TLRs or other PRRs in distinct subtypes.
Nevertheless, the cytokine storm induced by SARS-CoV-2 raises the possibility of TLR involvement both in the immune system and, conceivably, in the CNS. In fact, in silico studies suggest that cell surface TLRs, and in particular TLR4, likely recognize molecular patterns derived from SARS-CoV-2 and initiate an inflammatory response (Choudhury and Mukherjee, 2020). Since spike proteins of other coronaviruses, such as SARS-CoV, are recognized by TLR2 (Dosch et al., 2009), it is plausible that SARS-CoV-2 also engages TLR2. Moreover, bioinformatics analyses identified single-strand RNA (ssRNA) sequences from SARS-CoV-2, which could be recognized by the endosomal TLR7/TLR8. The SARS-CoV-2 genome had more ssRNA fragments that could be sensed by these TLRs compared to SARS-CoV genome (Moreno-Eutimio et al., 2020). This could explain the high levels of pro-inflammatory cytokines found in the serum of COVID-19 patients (Huang et al., 2020).
If SARS-CoV-2 is neuroinvasive, further studies are necessary to determine whether astrocytes and TLRs play a role in the anti-viral response, neuroinflammation, the dissemination of the virus, and the damage to the CNS. However, it is possible to speculate experimentally testable mechanisms by which astroglial TLRs could play roles in SARS-CoV-2-infected CNS. Following the trans-neuronal entry of the virus into the CNS, the virus could infect and activate astrocytes by interacting with the ACE2 receptor. In addition, viral components such as the spike protein could stimulate TLR2 and TLR4, whereas viral RNA could stimulate TLR7 and TLR8. Simultaneous activation of multiple TLRs could result in a robust increase in cytokine transcription leading to a cytokine storm in the CNS. Some of the cytokines could induce neuronal and oligodendrocyte death and demyelination. They could also expand glial activation and increase TLR expression in CNS cells, exacerbating the cytokine storm and the release of neurotoxic effectors. Although our focus is on astrocytes, microglia could also become activated and they could play a central role in the demise of neurons and oligodendrocytes and the phagocytosis of myelin.
In view of the extent of the SARS-CoV-2 global pandemic, the diversity of symptoms that COVID-19 patients present, the high preponderance, variety and gravity of neurological deficits in severely affected individuals, and the lack of information regarding mechanisms by which the virus impacts the CNS, further investigations are urgently needed. It is necessary to determine whether SARS-CoV-2 is a neurotropic virus that can infect both neurons and glia, how CNS cells react to SARS-CoV-2 infection, in what way target cell function is altered, and whether TLRs and other PRRs are involved in mediating the effects of SARS-CoV-2 in the CNS.
5. Role of astroglial TLRs in bacterial infections of the CNS
Bacterial infections of the CNS lead to severe and permanent neurological deficits and life-threatening conditions (LaPenna and Roos, 2019). Evidence indicates that in addition to microglia, astrocytes contribute to bacterial infections of the CNS, by recognizing bacteria-derived molecules such as LPS and bacterial DNA (Geyer et al., 2019). Among all TLRs, the best studied in the context of bacterial infections of the CNS is TLR2. Following exposure to the gram-positive bacterium Staphylococcus aureus, primary mouse astrocytes upregulate TLR2 expression in an autocrine/paracrine manner through the release of TNF-α. In addition, the cells produce IL-1β, CCL2 and nitric oxide (NO), a potent antibacterial free radical (Phulwani et al., 2008), which activates astrocytes (Brahmachari et al., 2006). These effects are attenuated in TLR2-/- astrocytes, suggesting that they are mediated, at least partly, through astroglial TLR2 signaling (Esen et al., 2004, Phulwani et al., 2008). TLR2 also recognizes Streptococcus suis, a bacterium that can cause meningitis (Graveline et al., 2007). TLR2 signaling has been implicated in the activation of astrocytes in response to Streptococcus suis. Although primary murine astrocytes do not internalize Streptococcus suis, exposure to the bacteria upregulates astroglial TLR2 expression, in vitro. This is paralleled by astrocyte activation and induction of the pro-inflammatory response. Exposure to Streptococcus suis also increases TLR6 transcript levels, whereas TLR1 expression is not modulated. Both TLR1 and TLR6 form heterodimers with TLR2. Since TLR2/TLR1 and TLR2/TLR6 signaling elicits distinct cellular responses, the selective increase in TLR6 could shape the inflammatory reaction of astrocytes (Zheng et al., 2011). Astroglial TLR2 also appears to be involved in the infection of the CNS by Brucella, a gram-negative bacterium that causes neurobrucellosis with white matter lesions in periventricular and subcortical regions and neurological dysfunction (Guven et al., 2013). In addition to endothelial cells (Ferrero et al., 2011), Brucella infects and activates astrocytes and induces the release of cytokines, chemokines and matrix metalloproteinases (Delpino et al., 2012, Miraglia et al., 2013). In macaque monkeys infected with Brucella melitensis, TLR2 immunoreactivity and the proportion of astrocytes expressing TLR2 are increased in the white matter of subcortical areas. This is paralleled by astrocyte hypertrophy and cell arbor complexity (Lee et al., 2013). The significance of the changes observed in astrocytes in response to Brucella infections remains elusive.
Activation of astroglial TLR4 by bacterial LPS significantly enhances the secretion of inflammatory mediators in vitro (Carpentier et al., 2005, Krasowska-Zoladek et al., 2007, Kumar, 2019). Studies have also implicated astroglial TLR9 in the response to bacterial infections. In the mouse model of bacterial meningitis induced by Streptococcus pneumoniae, the levels of cathelin-related antimicrobial peptide (CRAMP) were significantly increased in the infected brains. CRAMP is primarily expressed by brain astrocytes, and stimulation of astrocyte cultures with a TLR9 agonist significantly upregulates CRAMP transcript levels whereas this effect is significantly attenuated in TLR9-/- astrocyte cultures (Brandenburg et al., 2013).
The aforementioned studies provide insights into the influence of astroglial TLR signaling in the response mounted to foreign pathogens. However, as stated before, TLRs are also activated by endogenous ligands in disease and injury and are principal inducers of the sterile inflammation frequently observed in neurodegenerative diseases and in CNS injuries. The following sections will discuss the beneficial and detrimental responses of astrocytes to activation of TLRs by endogenous pathogenic molecules relevant to AD, PD, ALS, and CNS injury.
6. Contribution of astroglial TLRs to neurodegenerative diseases
Neurodegenerative diseases are characterized by progressive neuronal loss. The most prevalent neurodegenerative diseases are AD and PD. ALS is also a progressive neurodegenerative disorder characterized by motor neuron death. The majority of AD, PD, and ALS cases are sporadic but a small percentage of cases exhibit familial origins due to mutations in specific genes (Bandres-Ciga et al., 2020, Mejzini et al., 2019, Tan et al., 2019, Van Cauwenberghe et al., 2016).
Both microglia and astrocytes play essential roles in neurodegenerative diseases, and interactions between these glial cells modulate CNS function (Cragnolini et al., 2020, Gleichman and Carmichael, 2020, Joshi et al., 2019, Liddelow et al., 2020). Astrogliosis is an important feature of AD, PD, and ALS (Dickson, 2018). Formation of toxic protein aggregates is a common feature of neurodegenerative diseases (Soto and Pritzkow, 2018, Strohm and Behrends, 2020). Although activated microglia are the principal glial cells that internalize and degrade pathogenic proteins, reactive astrocytes also contribute to the clearance of extracellular aggregates via phagocytosis and endocytosis (Acosta et al., 2017, Lee et al., 2015b, Nagele et al., 2003). Yet, the persistent accumulation of pathogenic proteins during the progression of the diseases can impair astrocyte function, and by doing so, it exacerbates the pathology (di Domenico et al., 2019, Gu et al., 2010, Piacentini et al., 2017).
Emerging evidence indicates that astroglial TLRs could play roles in neurodegenerative disorders. The majority of the studies that investigated the potential involvement of astroglial TLRs in neurodegenerative diseases have been performed in vitro, using enriched astrocyte cultures, astrocyte-neuron co-cultures, or induced pluripotent stem cell (iPSC)-derived astrocytes obtained from patients. Even though in vivo data are limited, in vitro investigations provide insights into cellular mechanisms, which can be tested in animal models of diseases. In this review, we will illustrate this issue by focusing on investigations that are relevant to AD, PD, and ALS.
6.1. Alzheimer’s disease
The most common symptom of AD is memory loss and cognitive deficits. AD symptoms usually start slowly, progress gradually, and worsen over time. As the disease progresses, individuals with AD may have language, orientation, mood, motivation, and behavioral deficits (Tarawneh and Holtzman, 2012). The etiology of AD is poorly understood. However, studies indicated that excessive deposition of extracellular Aβ, a protein derived from the proteolysis of APP, forming neuritic plaques is among the causes of the disease (LaFerla et al., 2007). In AD patients, reactive astrocytes are found in the peri-plaque of Aβ deposition in the brain and form a dense layer that isolates the nearby healthy tissue from the plaque (Sasaki et al., 2001, Sofroniew and Vinters, 2010). GFAP expression is increased in reactive astrocytes during AD, and the increase is paralleled by progression of the disease (Simpson et al., 2010). Therefore, it has been suggested that reactive astrocytes and astrogliosis are involved in the pathogenesis and pathophysiology of AD.
Exposure of rat astrocyte cultures to Aβ aggregates induces TNF-α production and reduces the viability of astrocytes. These effects can be inhibited by the use of TLR4 antagonists. Moreover, in astrocyte-neuron co-cultures, Aβ causes cell death, with the majority of dying cells being neurons (Fig. 2 ). Addition of TLR4 antagonists to co-cultures alleviates cell death. In these investigations, neuronal death was primarily attributed to Aβ-induced astrocyte neurotoxicity rather than direct toxic effects of Aβ on neurons, since Aβ did not induce neuron death when added to pure neuronal cultures. Nevertheless, the studies could not completely rule out direct effects of Aβ on neuronal viability as well as neuronal toxicity evoked by a small fraction of contaminating microglia (Hughes et al., 2020).
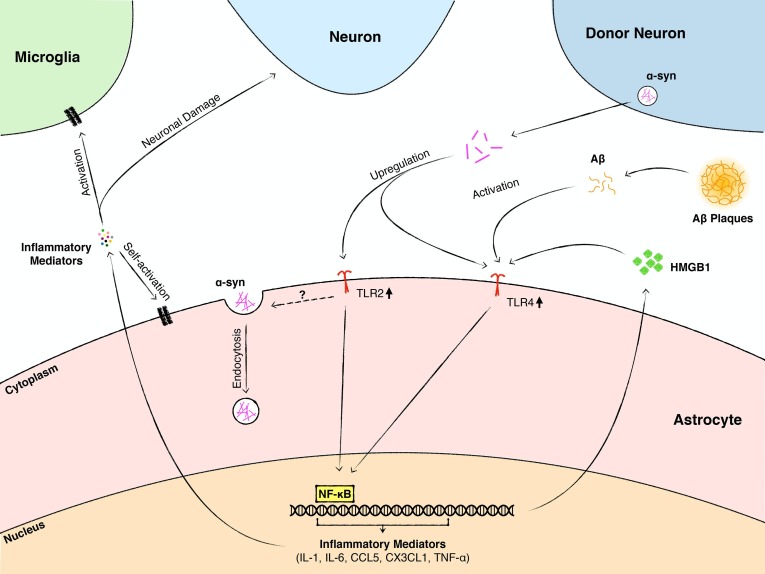
Fig. 2.
A schematic summary of mechanisms by which astroglial TLRs contribute to neurodegenerative diseases. In neurodegenerative disorders, such as AD and PD, the disease-associated peptides and proteins, including Aβ and α-syn, are significantly increased in the extracellular space, and also form inclusions within cells. Excessive deposition of Aβ in the extracellular space causes Aβ plaque formation. Aβ activates astrocytes through TLR4 signaling, induces the production of neurotoxic mediators, and causes neuronal death. α-syn is primarily released by neurons in pathological conditions. In the early stage of PD and other α-synucleinopathies, astrocytes take up extracellular α-syn through endocytosis, which is mediated by TLR2, but not TLR4. However, the mechanisms underlying TLR2-mediated internalization of α-syn are not known. Excessive α-syn upregulates the expression of astroglial TLR2 and TLR4, activates TLR2 and TLR4 signaling, and promotes astrocyte-mediated neuroinflammation through the production of inflammatory mediators. In ALS, nuclear chromatin protein HMGB1 is released by reactive astrocytes. It activates astroglial TLR4 signaling and fosters astrocyte-mediated neuroinflammation.
6.2. Parkinson’s disease
PD is characterized by the degeneration of ventral midbrain dopaminergic neurons leading to movement and non-motor related deficits, including cognition, behavior, and mood alterations (Goldman et al., 2018). Accumulation of PD-related proteins in neurons and non-neuronal cells is an indicator of disease progression (Booth et al., 2017).
α-Synuclein is a protein primarily released by neurons and implicated in PD pathology (Spillantini et al., 1997). The accumulation of α-syn-positive cytoplasmic inclusions in neurons is a hallmark of PD (Goedert et al., 2013). However, α-syn-containing inclusions are also observed in astrocytes, as indicated by the analysis of post-mortem tissue (Braak et al., 2007, Wakabayashi et al., 2000). It has been proposed that α-syn released by neurons is taken up by astrocytes via endocytosis and forms inclusions (Lee et al., 2010). Overexpression of α-syn in mouse astrocytes induces astrogliosis and disrupts normal astroglial function leading to neurodegeneration (Gu et al., 2010). When iPSC-derived astrocytes obtained from familial and sporadic PD patients were co-cultured with ventral midbrain dopaminergic neurons, they induced morphological changes indicative of neuronal degeneration. This was attributed to the secretion and transfer of α-syn from astrocytes to neurons although release of additional toxic molecules that affect neuronal survival could not be ruled out (di Domenico et al., 2019). These studies indicated that astrocytes play an active role in PD pathology and in the perpetuation of neuronal degeneration.
TLRs contribute to PD, and other α-synucleinopathies, by mediating the associated neuroinflammation and glial activation (Dzamko et al., 2017, Kim et al., 2018, La Vitola et al., 2018). Although the majority of the studies focused on neuronal and microglial TLR2 and TLR4 (Kouli et al., 2019), increasing evidence indicates that astroglial TLRs play a key role in PD pathology through modulation of astroglial inflammatory responses. Aggregated α-syn is a TLR2 ligand (Béraud et al., 2011, Kim et al., 2013), and exposure of primary rat astrocyte cultures to neuron-derived α-syn, in vitro, significantly increases TLR2 transcript levels and induces an inflammatory gene expression profile in astrocytes (Lee et al., 2010). The accumulation of α-syn in the cytoplasm of astrocytes is critical for the induction of the inflammatory response. In agreement with these findings, astroglial TLR2 expression is increased in transgenic mice overexpressing α-syn and which is paralleled by exacerbation of α-syn-related pathology, astrogliosis, microgliosis, and pro-inflammatory cytokine expression. These outcomes are attenuated by a TLR2 blocking antibody, in vivo (Kim et al., 2018). These investigations suggested that the accumulation of α-syn in astrocytes is the consequence of TLR2-mediated transfer of α-syn from neurons into astrocytes. In vitro studies demonstrated that activation of TLR2 by α-syn enhances the production of pro-inflammatory cytokines and chemokines including IL-1β, IL-6, TNF-α, CCL5, and CX3CL1 by astrocytes, whereas inhibition of astroglial TLR2 activation by blocking antibodies significantly decreases the pro-inflammatory response evoked by α-syn (Kim et al., 2018) (Fig. 2).
In addition to TLR2, TLR4 signaling has been implicated in α-syn-induced astrocyte activation and inflammatory responses. α-syn-induced release of TNF-α, CXCL1, and IL-6 is significantly reduced in TLR4-/- astrocytes compared to TLR4+/+ astrocytes. However, TLR4 deficiency does not affect α-syn uptake by astrocytes (Fellner et al., 2013). In agreement with this study, genetic deletion of astroglial TLR4 abrogates α-syn-induced activation of NF-κB and suppresses the transcription of pro-inflammatory cytokines and other inflammatory effectors. This study also corroborated that uptake of α-syn by TLR4-/- and TLR4+/+ astrocytes is not significantly different (Rannikko et al., 2015). Thus, α-syn induces the astroglial inflammatory reaction by acting through both TLR2 and TLR4 signaling (Fig. 2). However, the internalization of α-syn is likely mediated by TLR2, but not TLR4. In summary, astroglial TLR2 could play dual functions in PD and other synucleinopathies; a protective role by sequestrating and clearing extracellular α-syn and a detrimental role by promoting astrocyte-mediated inflammation.
6.3. Amyotrophic lateral sclerosis
ALS is an adult onset neurodegenerative disorder that selectively causes the loss of upper and lower motor neurons in the brain and spinal cord, leading to paralysis and death (van Es et al., 2017). The majority of ALS cases are sporadic, but approximately 10% of the cases are familial (Kiernan et al., 2011). Mutations in a number of genes have been identified in familial ALS, the best characterized being mutations in Cu/Zn superoxide dismutase 1 (SOD1) (Kaur et al., 2016). Transgenic mice expressing mutant human SOD1 (hSOD1) have been frequently used as animal models of familial ALS and have been instrumental in the discovery of cellular and molecular mechanisms involved in ALS pathology (Heiman-Patterson et al., 2011). These mice develop symptoms when they reach late adulthood and the disease progresses over time leading to death (Dal Canto and Gurney, 1997). Both astrocytes and microglia have been implicated in the progression of the disease and appear to play a fundamental role in both neurodegeneration and neuroprotection (Pehar et al., 2017).
Analysis of post-mortem tissue obtained from sporadic ALS patients showed a global increase in TLR2 and TLR4 transcript and protein levels in the spinal cord (Casula et al., 2011). Immunocytochemical co-localization studies indicated that TLR4, but not TLR2, is expressed in GFAP positive astrocytes in the white and grey matter of the cervical spinal cord obtained from ALS patients, whereas TLR2 is expressed primarily by microglia/macrophages. Neither TLR2 nor TLR4 is expressed in glia of control tissue. HMGB1, an agonist of both TLR2 and TLR4 (Martinotti et al., 2015), is expressed by glia, including astrocytes, of both control and ALS tissue. However, HMGB1 is primarily localized to the nucleus in control glia, and to the cytoplasm of glia of ALS spinal cord, suggesting that reactive astrocytes are one of the major sources of endogenous ligands for TLR2 and TLR4 in ALS (Casula et al., 2011). Studies on human mutant SOD1 expressing mice further corroborated the involvement of glial TLR4 in ALS-like disease. TLR4 is expressed in motor neurons, but not in glia, of control mice. However, TLR4 expression is observed in both reactive astrocytes and activated microglia of mutant hSOD1 mice at later stages of the disease. Genetic deletion of TLR4 in mutant hSOD1 mice improves neurological deficits and extends survival (Lee et al., 2015a). These studies support the notion that enhanced glial TLR4 signaling contributes to disease pathology.
7. Contribution of astroglial TLRs to CNS injuries: in vivo and in vitro studies
CNS injury includes TBI and SCI, as well as ischemic and hypoxic damage that can result from occlusion of arteries that supply blood to the CNS or from the damage of blood vessels following TBI or SCI. An initial tissue injury is often followed by progressive cell death, demyelination, and axonal degeneration, which exacerbates the damage.
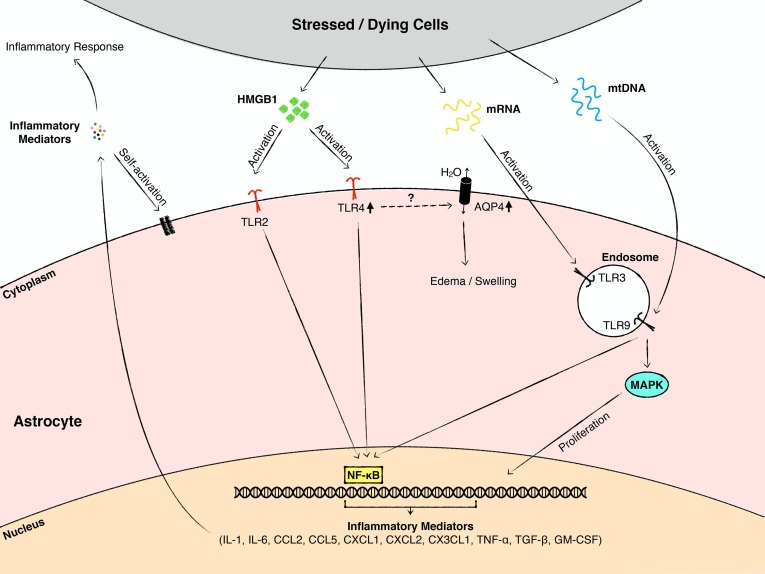
TLR2, TLR4, and TLR9 are the best studied TLRs in the context of CNS injury. However, specific information regarding the contribution of astroglial TLR signaling to CNS injury is scarce. In the healthy brain and spinal cord, the levels of astroglial TLR4 are very low and not readily detectable (Feng et al., 2017, Nishimura et al., 2013). However, following TBI (Jiang et al., 2018, Zan et al., 2018, Zhang et al., 2012), SCI (Nishimura et al., 2013), ischemic brain injury (Caso et al., 2007, Famakin et al., 2020), and spinal cord ischemia-reperfusion injury (Jing et al., 2020), the levels of astroglial TLR4 are dramatically upregulated. TLR4 positive astrocytes are found especially in the vicinity of the injury epicenter or in the penumbra following cerebral ischemia, but not at distant locations or in the unaffected contralateral side, indicating that the increase in TLR4 expression occurs in reactive astrocytes (Famakin et al., 2020, Jiang et al., 2018, Zhang et al., 2012). The rapid upregulation of astroglial TLR4 in response to injury suggests that it plays a role in local pathophysiological changes. Studies on intact rodents raise the possibility that HMGB1 is a trigger that upregulates TLR4 expression. Injection of recombinant HMGB1 into the uninjured cortex increases TLR4 expression in astrocytes surrounding the injection site, and exposure of cultured astrocytes to HMGB1 increases the expression of TLR4 (Famakin et al., 2020). However, it is worth noting that HMGB1 also acts through receptors other than TLR4 (Zhong et al., 2020), and some of its effects could be mediated, at least partly, through engagement of such receptors. In astrocyte cultures subjected to OGD/R, HMGB1 induces astrocyte swelling which is paralleled by an upregulation of Aquaporin-4 (AQP4) expression (Sun et al., 2017). This is an important effect because astrocyte swelling through AQP4 is a principal mechanism underlying CNS edema following injury. These effects of HMGB1 are partly mediated by TLR4/NF-κB signaling (Sun et al., 2017). Moreover, TLR4 modulates the viability of astrocytes following SCI. Pro-inflammatory M1 microglia/macrophages release cytotoxic molecules in response to injury which upregulate the expression of TLR4 and the necroptotic markers, receptor-interacting protein 3 (RIP3) and mixed lineage kinase domain-like protein (MLKL) in astrocytes, followed by astroglial necroptosis around the lesion. This effect is likely mediated through astroglial TLR4/MyD88 signaling, since astroglial MyD88 antagonism alleviates M1 microglia/macrophage-induced death of astrocytes (Fan et al., 2016) (Fig. 3 ).
Fig. 3.
A schematic summary showing the mechanisms by which astroglial TLRs contribute to CNS injury. Following TBI and SCI, cells and tissue at the affected site are progressively damaged. Stressed and necrotic cells release endogenous TLR ligands. HMGB1 activates TLR2 and TLR4, mRNA stimulates TLR3, and mtDNA promotes TLR9 signaling, respectively. This leads to the production of pro-inflammatory mediators, which further exacerbate the tissue damage and neuroinflammation. In addition, the activation of astroglial TLR4 enhances the expression of AQP4 and astrocyte swelling by mechanisms that remain elusive. Moreover, TLR9 is involved in the modulation of astrocyte proliferation in SCI.
TLR9 also plays a role in the modulation of astrocyte proliferation following SCI (Fig. 3). Astrocyte proliferation at the glial scar is significantly suppressed by intrathecal administration of a TLR9 antagonist to mice sustaining a SCI. As mentioned in Section 3, the TLR9 antagonist reduces the proliferation and migration of astrocytes in vitro, potentially through inhibition of the mitogen-activated protein kinase (MAPK) signaling pathway. These findings suggest that the effects of the TLR9 antagonist on astrocyte proliferation at the glial scar, could partly be due to the direct actions of the TLR9 antagonist on astrocytes (Li et al., 2019).
8. Conclusions and future directions
Astrocytes play both beneficial and detrimental roles in CNS pathology. Modulation of TLR signaling impacts various astrocyte functions, including proliferation, activation, and innate immune and inflammatory responses (Table 1 ). During infectious diseases, the major contribution of astroglial TLR signaling is the defense of the host, and the elimination of pathogens by mounting an anti-microbial and pro-inflammatory response. When persistent, the inflammatory reaction may cause neural damage. It is important to take into consideration the important fact that activation of TLR signaling could also promote the release of neuroprotective agents by astrocytes, under specific circumstances, and depending on the ligands and triggers present in the milieu. Thus, astrocytes could play paradoxical roles in CNS infections via involvement of TLRs and other PRRs. Understanding this dual role is critical since it could facilitate the design of therapeutic approaches that enhance the beneficial effects and attenuate the harmful ones. In addition, cooperation among different TLRs (Liu and Ding, 2016, Trinchieri and Sher, 2007) and the crosstalk between TLRs and other PRRs (Gąsiorowski et al., 2018, Oviedo-Boyso et al., 2014) are likely to be essential determinants of the overall astroglial response in CNS pathology. Future studies are needed in order to differentiate between the unique and complex influence of astroglial TLRs and the impact of other cells such as microglia and infiltrating macrophages or neutrophils, which also express functional TLRs (Sabroe et al., 2003). Such investigations could shed light into the cell type-specific mechanisms influencing the outcomes of CNS infections, and especially SARS-CoV-2, given the current pandemic. Interestingly, coronavirus RNA and antigen have previously been detected in active demyelinating plaques in MS (Murray et al., 1992). Therefore, it is important to investigate, not only the acute, but also the chronic effects of SARS-CoV-2 on CNS cells and determine the possible involvement of TLRs and other PRRs in this process.
Table 1.
Functions of astroglial TLRs.
| Experimental paradigm and related pathological condition | Functions mediated by astroglial TLRs | References |
|---|---|---|
| Infectious Diseases | ||
| Viral infection of rodents, in vivo and astrocyte cultures exposed to viruses or viral components | Reduction of viral permissiveness and replication, and promotion of the anti-viral and inflammatory response. | Rivieccio et al., 2006, Suh et al., 2007, Vijaykumar et al., 2008, El-Hage et al., 2008, El-Hage et al., 2011, Kužnik et al., 2011, Reinert et al., 2012, Liu et al., 2013, Ngwainmbi et al., 2014, Liu and Kumar, 2015, Pfefferkorn et al., 2016 |
| Bacterial infection of rodents, in vivo and astrocyte cultures exposed to bacterial components or bacteria, in vitro | Production of inflammatory mediators, cytokines, chemokines and growth factors | Esen et al., 2004, Phulwani et al., 2008, Zheng et al., 2011, Delpino et al., 2012, Miraglia et al., 2013, Carpentier et al., 2005, Krasowska-Zoladek et al., 2007, Kumar, 2019, Brandenburg et al., 2013 |
| Alzheimer’s Disease | ||
| Exposure of astrocyte cultures to Aβ | TNF-α production and reduced viability | Hughes et al., 2020 |
| Exposure of astrocyte-neuron co-cultures to Aβ | Neuronal death | Hughes et al., 2020 |
| Parkinson’s Disease | ||
| Exposure of astrocyte cultures to α-syn | Astrogliosis and neuroinflammation | Lee et al., 2010, Kim et al., 2018, Fellner et al., 2013 |
| Internalization of α-syn | Kim et al., 2018 | |
| ALS | ||
| Mouse model of fALS | Development of neurological deficits and survival | Lee et al., 2015a |
| Traumatic CNS injury | ||
| Animal model of SCI | Proliferation | Li et al., 2019 |
| Astrocyte cultures | Proliferation and migration | Li et al., 2019 |
| Astrocyte cultures | Modulation of cytokine and chemokine release | Acioglu et al., 2016, Li et al., 2019 |
| Astrocyte-neuron co-culture | Reduction of astrocyte-mediated neuronal survival | Acioglu et al., 2016 |
| In vitro model of ischemia | Astrocyte swelling/edema, Aquaporin modulation | Sun et al., 2017 |
| Animal model of SCI and astrocyte cultures | Astrocyte viability | Fan et al., 2016 |
In neurodegenerative diseases, pathogenic proteins that form aggregates induce astrocyte activation. Some of these proteins have been identified as endogenous TLR ligands and promote the pro-inflammatory response of astrocytes. The released chemokines and cytokines, in turn, influence the function of other cells in the CNS. Activation of glial TLR signaling can also protect neurons against toxicity elicited by protein aggregates since TLRs facilitate their internalization and clearance by astrocytes. In CNS injury, the specific contribution of astroglial TLRs to the functional and histopathological outcomes has not been adequately studied and necessitates further investigations.
In summary, despite the important role played by astrocytes in synaptic function, neuronal survival, and other neural mechanisms, and in spite of major advances in the understanding of innate immunity in the CNS, the specific contribution of astroglial TLRs to CNS pathology has received limited attention. Astroglial TLRs might be involved, not only in the modulation of inflammation associated with disease and injury, but in other key processes such as astrogliosis, astrocyte polarization and the crosstalk between astrocytes and other CNS cells. Future studies are needed to address these topics and to elucidate the underlying molecular mechanisms. It is necessary to investigate the role of astroglial TLRs by taking into account astrocyte heterogeneity and phenotypic plasticity in CNS disease and injury. Such investigations could identify astroglial TLRs as potential therapeutic targets.
Acknowledgments
This work was supported by New Jersey Commission on Spinal Cord Research Grants CSCR12IRG007 and CSCR17IRG007 and The Reynolds Family Spine Laboratory funds. This review was conceived as an overview of investigations on the contribution of astroglial TLRs to CNS infections, diseases and injuries. We apologize to those who published excellent reports related to this field but were not cited here.
References
- Abbott N.J., Rönnbäck L., Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Acioglu C., Mirabelli E., Baykal A.T., Ni L., Ratnayake A., Heary R.F., Elkabes S. Toll like receptor 9 antagonism modulates spinal cord neuronal function and survival: direct versus astrocyte-mediated mechanisms. Brain Behav. Immun. 2016;56:310–324. doi: 10.1016/j.bbi.2016.03.027. [DOI] [PubMed] [Google Scholar]
- Acosta C., Anderson H.D., Anderson C.M. Astrocyte dysfunction in Alzheimer disease: Astrocytes in Alzheimer's Disease. J. Neurosci. Res. 2017;95:2430–2447. doi: 10.1002/jnr.24075. [DOI] [PubMed] [Google Scholar]
- Agumadu V.C., Ramphul K. Zika virus: a review of literature. Cureus. 2018;10 doi: 10.7759/cureus.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S. Toll-like receptors and innate immunity. Adv. Immunol. 2001;78:1–56. doi: 10.1016/s0065-2776(01)78001-7. [DOI] [PubMed] [Google Scholar]
- Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Alam S.B., Willows S., Kulka M., Sandhu J.K. Severe acute respiratory syndrome coronavirus-2 may be an underappreciated pathogen of the central nervous system. Eur. J. Neurol. 2020 doi: 10.1111/ene.14442. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dalahmah O., Sosunov A.A., Shaik A., Ofori K., Liu Y., Vonsattel J.P., Adorjan I., Menon V., Goldman J.E. Single-nucleus RNA-seq identifies Huntington disease astrocyte states. Acta Neuropathol. Commun. 2020;8 doi: 10.1186/s40478-020-0880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Allen N.J., Eroglu C. Cell biology of astrocyte-synapse interactions. Neuron. 2017;96:697–708. doi: 10.1016/j.neuron.2017.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliot F., Pessac B. Astrocytic cell clones derived from established cultures of 8-day postnatal mouse cerebella. Brain Res. 1984;306:283–291. doi: 10.1016/0006-8993(84)90377-9. [DOI] [PubMed] [Google Scholar]
- Anderson M.A., Burda J.E., Ren Y., Ao Y., O’Shea T.M., Kawaguchi R., Coppola G., Khakh B.S., Deming T.J., Sofroniew M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila J.E., Whitaker K.W., Wires E.S., Harvey B.K., Airavaara M. Role of microglia in ischemic focal stroke and recovery: focus on Toll-like receptors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;79(Pt A):3–14. doi: 10.1016/j.pnpbp.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravalli R.N., Hu S., Rowen T.N., Palmquist J.M., Lokensgard J.R. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J. Immunol. 2005;175:4189–4193. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- Argaw A.T., Gurfein B.T., Zhang Y., Zameer A., John G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. PNAS. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw A.T., Asp L., Zhang J., Navrazhina K., Pham T., Mariani J.N., Mahase S., Dutta D.J., Seto J., Kramer E.G., Ferrara N., Sofroniew M.V., John G.R. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Invest. 2012;122:2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A., Rehli M., Kabingu E., Boch J.A., Baré O., Auron P.E., Stevenson M.A., Calderwood S.K. Novel signal transduction pathway utilized by extracellular HSP70: role of Toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Azam S., Jakaria M., Kim I.S., Kim J., Haque M.E., Choi D.K. Regulation of toll-Like receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: focus on TLR4 Signaling. Front. Immunol. 2019;10:1000. doi: 10.3389/fimmu.2019.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M., Sanders E.C. Potential neuroinvasive pathways of SARS‐CoV‐2: Deciphering the spectrum of neurological deficit seen in coronavirus disease‐2019 (COVID‐19) J. Med. Virol. 2020;92:1845–1857. doi: 10.1002/jmv.26105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandres-Ciga S., Diez-Fairen M., Kim J.J., Singleton A.B. Genetics of Parkinson's disease: an introspection of its journey towards precision medicine. Neurobiol. Dis. 2020;137:104782. doi: 10.1016/j.nbd.2020.104782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W., Xia H., Liang Y., Ye Y., Lu Y., Xu X., Duan A., He J., Chen Z., Wu Y., Wang X., Zheng C., Liu Z., Shi S. Toll-like Receptor 9 can be activated by endogenous mitochondrial DNA to induce podocyte apoptosis. Sci. Rep. 2016;6 doi: 10.1038/srep22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbierato M., Facci L., Argentini C., Marinelli C., Skaper S.D., Giusti P. Astrocyte-microglia cooperation in the expression of a pro-inflammatory phenotype. CNS Neurol. Disord.: Drug Targets. 2013;12:608–618. doi: 10.2174/18715273113129990064. [DOI] [PubMed] [Google Scholar]
- Barrat F.J., Crow M.K., Ivashkiv L.B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019;20:1574–1583. doi: 10.1038/s41590-019-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béraud D., Twomey M., Bloom B., Mittereder A., Ton V., Neitzke K., Chasovskikh S., Mhyre T.R., Maguire-Zeiss K.A. α-Synuclein alters toll-like receptor expression. Front. Neurosci. 2011;5:80. doi: 10.3389/fnins.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth H.D.E., Hirst W.D., Wade-Martins R. The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci. 2017;40:358–370. doi: 10.1016/j.tins.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostancıklıoğlu M. SARS-CoV2 entry and spread in the lymphatic drainage system of the brain. Brain Behav. Immun. 2020;87:122–123. doi: 10.1016/j.bbi.2020.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C.C., Rasley A., Tranguch S.L., Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- Braak H., Sastre M., Del Tredici K. Development of α-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson's disease. Acta Neuropathol. 2007;114:231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- Brahmachari S., Fung Y.K., Pahan K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J. Neurosci. 2006;26:4930–4939. doi: 10.1523/JNEUROSCI.5480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg L.-O., Jansen S., Albrecht L.-J., Merres J., Gerber J., Pufe T., Tauber S.C. CpG oligodeoxynucleotides induce the expression of the antimicrobial peptide cathelicidin in glial cells. J. Neuroimmunol. 2013;255:18–31. doi: 10.1016/j.jneuroim.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Brightbill H.D., Libraty D.H., Krutzik S.R., Yang R.B., Belisle J.T., Bleharski J.R., Maitland M., Norgard M.V., Plevy S.E., Smale S.T., Brennan P.J., Bloom B.R., Godowski P.J., Modlin R.L. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- Brown A.M., Ransom B.R. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–1271. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- Bsibsi M., Ravid R., Gveric D., van Noort J.M. Broad expression of toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Bsibsi M., Persoon-Deen C., Verwer R.W.H., Meeuwsen S., Ravid R., Van Noort J.M. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- Bsibsi M., Bajramovic J.J., Van Duijvenvoorden E., Persoon C., Ravid R., Van Noort J.M., Vogt M.H.J. Identification of soluble CD14 as an endogenous agonist for Toll-like receptor 2 on human astrocytes by genome-scale functional screening of glial cell derived proteins. Glia. 2007;55:473–482. doi: 10.1002/glia.20473. [DOI] [PubMed] [Google Scholar]
- Bush T.G., Puvanachandra N., Horner C.H., Polito A., Ostenfeld T., Svendsen C.N., Mucke L., Johnson M.H., Sofroniew M.V. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Canto M.C.D., Gurney M.E. A low expressor line of transgenic mice carrying a mutant human Cu,Zn superoxide dismutase (SOD1) gene develops pathological changes that most closely resemble those in human amyotrophic lateral sclerosis. Acta Neuropathol. 1997;93:537–550. doi: 10.1007/s004010050650. [DOI] [PubMed] [Google Scholar]
- Carpentier P.A., Begolka W.S., Olson J.K., Elhofy A., Karpus W.J., Miller S.D. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- Carpentier P.A., Duncan D.S., Miller S.D. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav. Immun. 2008;22:140–147. doi: 10.1016/j.bbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty M., Bowie A.G. Evaluating the role of Toll-like receptors in diseases of the central nervous system. Biochem. Pharmacol. 2011;81:825–837. doi: 10.1016/j.bcp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Caso J.R., Pradillo J.M., Hurtado O., Lorenzo P., Moro M.A., Lizasoain I. Toll-like receptor 4 Is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- Casula M., Iyer A.M., Spliet W.G.M., Anink J.J., Steentjes K., Sta M., Troost D., Aronica E. Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience. 2011;179:233–243. doi: 10.1016/j.neuroscience.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Cheng H.W., Jiang T., Brown S.A., Pasinetti G.M., Finch C.E., Mcneill T.H. Response of striatal astrocytes to neuronal deafferentation: an immunocytochemical and ultrastructural study. Neuroscience. 1994;62:425–439. doi: 10.1016/0306-4522(94)90377-8. [DOI] [PubMed] [Google Scholar]
- Chigr F., Merzouki M., Najimi M. Comment on “The neuroinvasive potential of SARS‐CoV‐2 may play a role in the respiratory failure of COVID‐19 patients”. J. Med. Virol. 2020;92:703–704. doi: 10.1002/jmv.25960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A., Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS‐CoV‐2 spike glycoprotein with ACE‐2 receptor homologs and human TLRs. J. Med. Virol. 2020;92:2105–2113. doi: 10.1002/jmv.25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L.E., Liddelow S.A., Chakraborty C., Münch A.E., Heiman M., Barres B.A. Normal aging induces A1-like astrocyte reactivity. PNAS. 2018;115:E1896–E1905. doi: 10.1073/pnas.1800165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb K.I., Johnson K.M., Winokur P.N., Sacino A.V., Crocker S.J. Astrocyte regulation of CNS inflammation and remyelination. Brain Sci. 2013;3:1109–1127. doi: 10.3390/brainsci3031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L.V., Hajizadeh S., Holme E., Jonsson I.-M., Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J. Leukoc. Biol. 2004;75:995–1000. doi: 10.1189/jlb.0703328. [DOI] [PubMed] [Google Scholar]
- Cragnolini A.B., Lampitella G., Virtuoso A., Viscovo I., Panetsos F., Papa M., Cirillo G. Regional brain susceptibility to neurodegeneration: what is the role of glial cells? Neural Regen. Res. 2020;15:838. doi: 10.4103/1673-5374.268897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffis S., Samuel M.A., Suthar M.S., Gale M., Jr., Diamond M.S. Toll-like receptor 3 has a protective role against west nile virus infection. JVI. 2008;82:10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpino M.V., Barrionuevo P., Macedo G.C., Oliveira S.C., Genaro S.D., Scian R., Miraglia M.C., Fossati C.A., Baldi P.C., Giambartolomei G.H. Macrophage-elicited osteoclastogenesis in response to Brucella abortus infection requires TLR2/MyD88-dependent TNF-α production. J. Leukoc. Biol. 2012;91:285–298. doi: 10.1189/jlb.04111185. [DOI] [PubMed] [Google Scholar]
- di Domenico A., Carola G., Calatayud C., Pons-Espinal M., Muñoz J.P., Richaud-Patin Y., Fernandez-Carasa I., Gut M., Faella A., Parameswaran J., Soriano J., Ferrer I., Tolosa E., Zorzano A., Cuervo A.M., Raya A., Consiglio A. Patient-specific iPSC-derived astrocytes contribute to non-cell-autonomous neurodegeneration in Parkinson's disease. Stem Cell Rep. 2019;12:213–229. doi: 10.1016/j.stemcr.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson D.W. Neuropathology of Parkinson disease. Parkinsonism Relat. Disord. 2018;46(Suppl 1):S30–S33. doi: 10.1016/j.parkreldis.2017.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch S.F., Mahajan S.D., Collins A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142:19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte L.F., Farias M.A., Alvarez D.M., Bueno S.M., Riedel C.A., Gonzalez P.A. Herpes simplex virus type 1 infection of the central nervous system: insights into proposed interrelationships with neurodegenerative disorders. Front. Cell. Neurosci. 2019;13:46. doi: 10.3389/fncel.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour A., Zucker S., Sampson N.S., Kuscu C., Cao J. Role of matrix metalloproteinase-9 dimers in cell migration: design of inhibitory peptides. J. Biol. Chem. 2010;285:35944–35956. doi: 10.1074/jbc.M109.091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamko N., Gysbers A., Perera G., Bahar A., Shankar A., Gao J., Fu Y., Halliday G.M. Toll-like receptor 2 is increased in neurons in Parkinson's disease brain and may contribute to α-synuclein pathology. Acta Neuropathol. 2017;133:303–319. doi: 10.1007/s00401-016-1648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efe I.E., Aydin O.U., Alabulut A., Celik O., Aydin K. COVID-19−associated encephalitis mimicking glial tumor. World Neurosurg. 2020;140:46–48. doi: 10.1016/j.wneu.2020.05.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N., Bruce-Keller A.J., Knapp P.E., Hauser K.F. CCL5/RANTES gene deletion attenuates opioid-induced increases in glial CCL2/MCP-1 immunoreactivity and activation in HIV-1 tat-exposed mice. J. Neuroimmune Pharmacol. 2008;3:275–285. doi: 10.1007/s11481-008-9127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N., Podhaizer E.M., Sturgill J., Hauser K.F. Toll-like receptor expression and activation in astroglia: differential regulation by HIV-1 Tat, gp120, and morphine. Immunol. Invest. 2011;40:498–522. doi: 10.3109/08820139.2011.561904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C., Guillemaud O., Carrillo‐de Sauvage M.-A. Questions and (some) answers on reactive astrocytes. Glia. 2019;67:2221–2247. doi: 10.1002/glia.23687. [DOI] [PubMed] [Google Scholar]
- Esen, N., Tanga, F. Y., DeLeo, J. A., & Kielian, T., 2004. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J Neurochem, 88, 746-758. DOI:10.1046/j.1471-4159.2003.02202.x. [DOI] [PubMed]
- Facci L., Barbierato M., Marinelli C., Argentini C., Skaper S.D., Giusti P. Toll-like receptors 2, -3 and -4 prime microglia but not astrocytes across central nervous system regions for ATP-dependent interleukin-1β release. Sci. Rep. 2014;4:6824. doi: 10.1038/srep06824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famakin B.M., Tsymbalyuk O., Tsymbalyuk N., Ivanova S., Woo S.K., Kwon M.S., Gerzanich V., Simard J.M. HMGB1 is a potential mediator of astrocytic TLR4 signaling activation following acute and chronic focal cerebral ischemia. Neurol. Res. Int. 2020;2020:1–9. doi: 10.1155/2020/3929438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Zhang K., Shan L., Kuang F., Chen K., Zhu K., Ma H., Ju G., Wang Y.-Z. Reactive astrocytes undergo M1 microglia/macrohpages-induced necroptosis in spinal cord injury. Mol. Neurodegeneration. 2016;11 doi: 10.1186/s13024-016-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy-Tselnicker I., Allen N.J. Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev. 2018;13 doi: 10.1186/s13064-018-0104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C., Krumbholz M., Giese T., Hartmann G., Aloisi F., Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J. Neuroimmunol. 2005;159:12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Farina C., Aloisi F., Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Faulkner J.R., Herrmann J.E., Woo M.J., Tansey K.E., Doan N.B., Sofroniew M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L., Irschick R., Schanda K., Reindl M., Klimaschewski L., Poewe W., Wenning G.K., Stefanova N. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia. 2013;61:349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Gao J., Cui Y., Li M., Li R., Cui C., Cui J. Neuroprotective effects of resatorvid against traumatic brain injury in rat: involvement of neuronal autophagy and TLR4 signaling pathway. Cell. Mol. Neurobiol. 2017;37:155–168. doi: 10.1007/s10571-016-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero M.C., Bregante J., Delpino M.V., Barrionuevo P., Fossati C.A., Giambartolomei G.H., Baldi P.C. Proinflammatory response of human endothelial cells to Brucella infection. Microbes Infect. 2011;13:852–861. doi: 10.1016/j.micinf.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Finsterer J., Stollberger C. Causes of hypogeusia/hyposmia in SARS‐CoV2 infected patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25903. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M., Mian A., Meysami S., Raji C.A. Neurobiology of COVID-19. JAD. 2020;76:3–19. doi: 10.3233/JAD-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P.E., Chappell M.C., Ferrario C.M., Tallant E.A. Distinct roles for ANG II and ANG-(1–7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am. J. Physiol.-Cell Physiol. 2006;290:C420–C426. doi: 10.1152/ajpcell.00409.2004. [DOI] [PubMed] [Google Scholar]
- Gąsiorowski K., Brokos B., Echeverria V., Barreto G.E., Leszek J. RAGE-TLR crosstalk sustains chronic inflammation in neurodegeneration. Mol. Neurobiol. 2018;55:1463–1476. doi: 10.1007/s12035-017-0419-4. [DOI] [PubMed] [Google Scholar]
- Geyer S., Jacobs M., Hsu N.J. Immunity against bacterial infection of the central nervous system: an astrocyte perspective. Front. Mol. Neurosci. 2019;12:57. doi: 10.3389/fnmol.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilani S., Roditi R., Naraghi M. COVID-19 and anosmia in Tehran, Iran. Med. Hypotheses. 2020;141:109757. doi: 10.1016/j.mehy.2020.109757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore, W., Correale, J., & Weiner, L. P., 1994. Coronavirus induction of class I major histocompatibility complex expression in murine astrocytes is virus strain specific. J Exp Med, 180, 1013-1023. DOI:10.1084/jem.180.3.1013. [DOI] [PMC free article] [PubMed]
- Gleichman A.J., Carmichael S.T. Glia in neurodegeneration: drivers of disease or along for the ride? Neurobiol. Dis. 2020;142:104957. doi: 10.1016/j.nbd.2020.104957. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M.G., Del Tredici K., Braak H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- Goldman J.G., Vernaleo B.A., Camicioli R., Dahodwala N., Dobkin R.D., Ellis T., Galvin J.E., Marras C., Edwards J., Fields J., Golden R., Karlawish J., Levin B., Shulman L., Smith G., Tangney C., Thomas C.A., Tröster A.I., Uc E.Y., Coyan N., Ellman C., Ellman M., Hoffman C., Hoffman S., Simmonds D. Cognitive impairment in Parkinson’s disease: a report from a multidisciplinary symposium on unmet needs and future directions to maintain cognitive health. npj Parkinson's Dis. 2018;4 doi: 10.1038/s41531-018-0055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D.M., Fu L.i., Li Y., Das Sarma J., Lavi E. Coronavirus-induced demyelination occurs in the absence of CD28 costimulatory signals. J. Neuroimmunol. 2004;146:140–143. doi: 10.1016/j.jneuroim.2003.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen B.B., Hoopes J.D., Wong M.H., Jung K.H., Isakson K.C., Alexopoulou L., Flavell R.A., Sidwell R.W. TLR3 deletion limits mortality and disease severity due to phlebovirus infection. J. Immunol. 2006;177:6301–6307. doi: 10.4049/jimmunol.177.9.6301. [DOI] [PubMed] [Google Scholar]
- Graveline, R., Segura, M., Radzioch, D., & Gottschalk, M., 2007. TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int Immunol, 19, 375-389. DOI:10.1093/intimm/dxm003. [DOI] [PubMed]
- Gray M.W., Burger G., Lang B.F. Mitochondrial evolution. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- Gu, J., Gong, E., Zhang, B., Zheng, J., Gao, Z., Zhong, Y., Zou, W., Zhan, J., Wang, S., Xie, Z., Zhuang, H., Wu, B., Zhong, H., Shao, H., Fang, W., Gao, D., Pei, F., Li, X., He, Z., Xu, D., … Leong, A. S., 2005. Multiple organ infection and the pathogenesis of SARS. J Exp Med, 202, 415–424 DOI:10.1084/jem.20050828. [DOI] [PMC free article] [PubMed]
- Gu X.L., Long C.X., Sun L., Xie C., Lin X., Cai H. Astrocytic expression of Parkinson's disease-related A53T α-synuclein causes neurodegeneration in mice. Mol Brain. 2010;3:12. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guven, T., Ugurlu, K., Ergonul, O., Celikbas, A. K., Gok, S. E., Comoglu, S., Baykam, N., & Dokuzoguz, B., 2013. Neurobrucellosis: clinical and diagnostic features. Clin Infect Dis, 56, 1407-1412. DOI:10.1093/cid/cit072. [DOI] [PubMed]
- Halassa M.M., Fellin T., Haydon P.G. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol. Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Hamby M.E., Hewett J.A., Hewett S.J. TGF-β1 potentiates astrocytic nitric oxide production by expanding the population of astrocytes that express NOS-2. Glia. 2006;54:566–577. doi: 10.1002/glia.20411. [DOI] [PubMed] [Google Scholar]
- Hamel R., Ferraris P., Wichit S., Diop F., Talignani L., Pompon J., Garcia D., Liégeois F., Sall A.A., Yssel H., Missé D. African and Asian Zika virus strains differentially induce early antiviral responses in primary human astrocytes. Infection, Genet. Evol. 2017;49:134–137. doi: 10.1016/j.meegid.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Hasan U., Chaffois C., Gaillard C., Saulnier V., Merck E., Tancredi S., Guiet C., Brière F., Vlach J., Lebecque S., Trinchieri G., Bates E.E.M. Human TLR10 Is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J. Immunol. 2005;174:2942–2950. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- Hayashi F., Smith K.D., Ozinsky A., Hawn T.R., Yi E.C., Goodlett D.R., Eng J.K., Akira S., Underhill D.M., Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Heiman A., Pallottie A., Heary R.F., Elkabes S. Toll-like receptors in central nervous system injury and disease: a focus on the spinal cord. Brain Behav. Immun. 2014;42:232–245. doi: 10.1016/j.bbi.2014.06.203. [DOI] [PubMed] [Google Scholar]
- Heiman-Patterson T.D., Sher R.B., Blankenhorn E.A., Alexander G., Deitch J.S., Kunst C.B., Maragakis N., Cox G. Effect of genetic background on phenotype variability in transgenic mouse models of amyotrophic lateral sclerosis: a window of opportunity in the search for genetic modifiers. Amyotrophic Lateral Sclerosis. 2011;12:79–86. doi: 10.3109/17482968.2010.550626. [DOI] [PubMed] [Google Scholar]
- Heneka M.T., Kummer M.P., Latz E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014;14:463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- Heneka M.T., Golenbock D., Latz E., Morgan D., Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alz. Res. Therapy. 2020;12 doi: 10.1186/s13195-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H.L., Wang H.H., Wu C.Y., Tung W.H., Yang C.M. Lipoteichoic acid induces matrix metalloproteinase-9 expression via transactivation of PDGF receptors and NF-kappaB activation in rat brain astrocytes. Neurotox. Res. 2010;17:344–359. doi: 10.1007/s12640-009-9111-4. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y.i., Zhang L.i., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L.i., Xie J., Wang G., Jiang R., Gao Z., Jin Q.i., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C., Choi M.L., Yi J.H., Kim S.C., Drews A., George-Hyslop P.S., Bryant C., Gandhi S., Cho K., Klenerman D. Beta amyloid aggregates induce sensitised TLR4 signalling causing long-term potentiation deficit and rat neuronal cell death. Commun. Biol. 2020;3:79. doi: 10.1038/s42003-020-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang M., Bergmann C.C. Alpha/beta-Interferon (IFN-α/β) signaling in astrocytes mediates protection against viral encephalomyelitis and regulates IFN-gamma-dependent responses. J. Virol. 2018;92(10) doi: 10.1128/JVI.01901-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C., Nedergaard M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Ji R.R., Donnelly C.R., Nedergaard M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019;20:667–685. doi: 10.1038/s41583-019-0218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Wang Y., Liang X., Xing X., Xu X., Zhou C. Toll-like receptor 4 knockdown attenuates brain damage and neuroinflammation after traumatic brain Injury via inhibiting neuronal autophagy and astrocyte activation. Cell. Mol. Neurobiol. 2018;38:1009–1019. doi: 10.1007/s10571-017-0570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing N., Fang B., Li Z., Tian A. Exogenous activation of cannabinoid-2 receptor modulates TLR4/MMP9 expression in a spinal cord ischemia reperfusion rat model. J. Neuroinflammation. 2020;17 doi: 10.1186/s12974-020-01784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A.U., Minhas P.S., Liddelow S.A., Haileselassie B., Andreasson K.I., Dorn G.W., II, Mochly-Rosen D. Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat. Neurosci. 2019;22:1635–1648. doi: 10.1038/s41593-019-0486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbani N., Olds J.L. Does COVID19 infect the brain? If so, smokers might be at a higher risk. Mol. Pharmacol. 2020;97:351–353. doi: 10.1124/molpharm.120.000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanberg N., Ashton N.J., Andersson L.-M., Yilmaz A., Lindh M., Nilsson S., Price R.W., Blennow K., Zetterberg H., Gisslén M. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95:e1754–e1759. doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- Karikó K., Ni H., Capodici J., Lamphier M., Weissman D. mRNA is an endogenous ligand for toll-like receptor 3. J. Biol. Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- Kaur S.J., McKeown S.R., Rashid S. Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis. Gene. 2016;577:109–118. doi: 10.1016/j.gene.2015.11.049. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Ketzler S., Weis S., Haug H., Budka H. Loss of neurons in the frontal cortex in AIDS brains. Acta Neuropathol. 1990;80:92–94. doi: 10.1007/BF00294228. [DOI] [PubMed] [Google Scholar]
- Khakh B.S., Deneen B. The emerging nature of astrocyte diversity. Annu. Rev. Neurosci. 2019;42:187–207. doi: 10.1146/annurev-neuro-070918-050443. [DOI] [PubMed] [Google Scholar]
- Kiernan M.C., Vucic S., Cheah B.C., Turner M.R., Eisen A., Hardiman O., Burrell J.R., Zoing M.C. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- Kigerl K.A., Lai W., Rivest S., Hart R.P., Satoskar A.R., Popovich P.G. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J. Neurochem. 2007;102:37–50. doi: 10.1111/j.1471-4159.2007.04524.x. [DOI] [PubMed] [Google Scholar]
- Kim C., Ho D.H., Suk J.E., You S., Michael S., Kang J., Joong Lee S., Masliah E., Hwang D., Lee H.J., Lee S.J. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Spencer B., Rockenstein E., Yamakado H., Mante M., Adame A., Fields J.A., Masliah D., Iba M., Lee H.J., Rissman R.A., Lee S.J., Masliah E. Immunotherapy targeting toll-like receptor 2 alleviates neurodegeneration in models of synucleinopathy by modulating α-synuclein transmission and neuroinflammation. Mol. Neurodegener. 2018;13:43. doi: 10.1186/s13024-018-0276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho M., Lin S., Wu X., Esterman M., Koger D., Hanson J., Higgs R., Liu F., Malkani S., Bales K.R., Paul S.M. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nat. Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- Kouli A., Horne C.B., Williams-Gray C.H. Toll-like receptors and their therapeutic potential in Parkinson's disease and α-synucleinopathies. Brain Behav. Immun. 2019;81:41–51. doi: 10.1016/j.bbi.2019.06.042. [DOI] [PubMed] [Google Scholar]
- Koyuncu O., Hogue I., Enquist L. Virus infections in the nervous system. Cell Host Microbe. 2013;13:379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Hämmerle S., Rothenaigner I., Wolff H., Bell J.E., Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Krasowska-Zoladek A., Banaszewska M., Kraszpulski M., Konat G.W. Kinetics of inflammatory response of astrocytes induced by TLR 3 and TLR4 ligation. J. Neurosci. Res. 2007;85:205–212. doi: 10.1002/jnr.21088. [DOI] [PubMed] [Google Scholar]
- Kreft M., Bak L.K., Waagepetersen H.S., Schousboe A. Aspects of astrocyte energy metabolism, amino acid neurotransmitter homoeostasis and metabolic compartmentation. ASN Neuro. 2012;4 doi: 10.1042/AN20120007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. Toll-like receptors in the pathogenesis of neuroinflammation. J. Neuroimmunol. 2019;332:16–30. doi: 10.1016/j.jneuroim.2019.03.012. [DOI] [PubMed] [Google Scholar]
- Kužnik A., Benčina M., Švajger U., Jeras M., Rozman B., Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 2011;186:4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- La Vitola P., Balducci C., Cerovic M., Santamaria G., Brandi E., Grandi F., Caldinelli L., Colombo L., Morgese M.G., Trabace L., Pollegioni L., Albani D., Forloni G. Alpha-synuclein oligomers impair memory through glial cell activation and via Toll-like receptor 2. Brain Behav. Immun. 2018;69:591–602. doi: 10.1016/j.bbi.2018.02.012. [DOI] [PubMed] [Google Scholar]
- LaFerla F.M., Green K.N., Oddo S. Intracellular amyloid-β in Alzheimer's disease. Nat. Rev. Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Lafon, M., Megret, F., Lafage, M., & Prehaud, C., 2006. The innate immune facet of brain: human neurons express TLR-3 and sense viral dsRNA. J Mol Neurosci, 29, 185-194. DOI:10.1385/JMN:29:3:185. [DOI] [PubMed]
- LaPenna P.A., Roos K.L. Bacterial infections of the central nervous system. Semin. Neurol. 2019;39:334–342. doi: 10.1055/s-0039-1693159. [DOI] [PubMed] [Google Scholar]
- Lau, K. K., Yu, W. C., Chu, C. M., Lau, S. T., Sheng, B., & Yuen, K. Y., 2004. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis, 10, 342–344. DOI:10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed]
- Lavi E., Cong L. Type I astrocytes and microglia induce a cytokine response in an encephalitic murine coronavirus infection. Exp. Mol. Pathol. 2020;115:104474. doi: 10.1016/j.yexmp.2020.104474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi E., Gilden D.H., Highkin M.K., Weiss S.R. The organ tropism of mouse hepatitis virus A59 in mice is dependent on dose and route of inoculation. Lab. Anim. Sci. 1986;36:130–135. [PubMed] [Google Scholar]
- Lee K.M., Chiu K.B., Sansing H.A., Didier P.J., Ficht T.A., Arenas-Gamboa A.M., Roy C.J., Maclean A.G. Aerosol-induced brucellosis increases TLR-2 expression and increased complexity in the microanatomy of astroglia in rhesus macaques. Front. Cell. Infect. Microbiol. 2013;3:86. doi: 10.3389/fcimb.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y., Lee J.D., Phipps S., Noakes P.G., Woodruff T.M. Absence of toll-like receptor 4 (TLR4) extends survival in the hSOD1G93A mouse model of amyotrophic lateral sclerosis. J. Neuroinflammation. 2015;12 doi: 10.1186/s12974-015-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Seo B.R., Koh J.Y. Metallothionein-3 modulates the amyloid-β endocytosis of astrocytes through its effects on actin polymerization. Mol. Brain. 2015;8:84. doi: 10.1186/s13041-015-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Suk J.E., Patrick C., Bae E.J., Cho J.H., Rho S., Hwang D., Masliah E., Lee S.J. Direct transfer of α-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Cudaback E., Breyer R.M., Montine K.S., Keene C.D., Montine T.J. Eicosanoid receptor subtype‐mediated opposing regulation of TLR‐stimulated expression of astrocyte glial‐derived neurotrophic factor. FASEB J. 2012;26:3075–3083. doi: 10.1096/fj.11-200279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Fu L., Gonzales D.M., Lavi E. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J. Virol. 2004;78:3398–3406. doi: 10.1128/jvi.78.7.3398-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Ni L., Eugenin E.A., Heary R.F., Elkabes S. Toll-like receptor 9 antagonism modulates astrocyte function and preserves proximal axons following spinal cord injury. Brain Behav. Immun. 2019;80:328–343. doi: 10.1016/j.bbi.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Li L., Ni L., Heary R.F., Elkabes S. Astroglial TLR9 antagonism promotes chemotaxis and alternative activation of macrophages via modulation of astrocyte-derived signals: implications for spinal cord injury. J. Neuroinflammation. 2020;17 doi: 10.1186/s12974-020-01748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Wohlford-Lenane C., Perlman S., Zhao J., Jewell A.K., Reznikov L.R., Gibson-Corley K.N., Meyerholz D.K., McCray P.B., Jr Middle east respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J. Infect. Dis. 2016;213:712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xu X.-L., Zhao D., Pan L.N., Huang C.W., Guo L.J., Lu Q., Wang J. TLR3 ligand Poly IC attenuates reactive astrogliosis and improves recovery of rats after focal cerebral ischemia. CNS Neurosci. Ther. 2015;21:905–913. doi: 10.1111/cns.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Xu J., Chu M., Mai J., Lai N., Tang W., Yang T., Zhang S., Guan C., Zhong F., Yang L., Liao G. Neurosensory dysfunction: a diagnostic marker of early COVID-19. Int. J. Infectious Dis. 2020;98:347–352. doi: 10.1016/j.ijid.2020.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S.A., Barres B.A. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Münch A.E., Chung W.-S., Peterson T.C., Wilton D.K., Frouin A., Napier B.A., Panicker N., Kumar M., Buckwalter M.S., Rowitch D.H., Dawson V.L., Dawson T.M., Stevens B., Barres B.A. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S.A., Marsh S.E., Stevens B. Microglia and astrocytes in disease: dynamic duo or partners in crime? Trends Immunol. 2020;41:820–835. doi: 10.1016/j.it.2020.07.006. [DOI] [PubMed] [Google Scholar]
- Liguori C., Pierantozzi M., Spanetta M., Sarmati L., Cesta N., Iannetta M., Ora J., Mina G.G., Puxeddu E., Balbi O., Pezzuto G., Magrini A., Rogliani P., Andreoni M., Mercuri N.B. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav. Immun. 2020;88:11–16. doi: 10.1016/j.bbi.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist R., Mundt F., Gilthorpe J.D., Wölfel S., Gekara N.O., Kröger A., Överby A.K. Fast type I interferon response protects astrocytes from flavivirus infection and virus-induced cytopathic effects. J. Neuroinflammation. 2016;13 doi: 10.1186/s12974-016-0748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Ding J.L. The molecular mechanisms of TLR‐signaling cooperation in cytokine regulation. Immunol. Cell Biol. 2016;94:538–542. doi: 10.1038/icb.2016.18. [DOI] [PubMed] [Google Scholar]
- Liu, G., Zhang, H., Zhao, C., & Zhang, H., 2020. Evolutionary history of the Toll-like receptor gene family across vertebrates. Genome Biol Evol, 12, 3615–3634. DOI:10.1093/gbe/evz266. [DOI] [PMC free article] [PubMed]
- Liu Z., Guan Y., Sun X., Shi L., Liang R., Lv X., Xin W. HSV-1 activates NF-kB in mouse astrocytes and increases TNF-α and IL-6 expression via Toll-like receptor 3. Neurol. Res. 2013;35:755–762. doi: 10.1179/016164113X13703372991516. [DOI] [PubMed] [Google Scholar]
- Liu T., Han Q., Chen G., Huang Y., Zhao L.-X., Berta T., Gao Y.J., Ji R.-R. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain. 2016;157:806–817. doi: 10.1097/j.pain.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Kumar A. Differential signaling mechanism for HIV-1 Nef-mediated production of IL-6 and IL-8 in human astrocytes. Sci. Rep. 2015;5 doi: 10.1038/srep09867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Liu Y., Hao W., Wolf L., Kiliaan A.J., Penke B., Rübe C.E., Walter J., Heneka M.T., Hartmann T., Menger M.D., Fassbender K. TLR2 is a primary receptor for Alzheimer's amyloid-β peptide to trigger neuroinflammatory activation. J. Immunol. 2012;188(3):1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- Liu B., Teschemacher A.G., Kasparov S. Astroglia as a cellular target for neuroprotection and treatment of neuro-psychiatric disorders. Glia. 2017;65:1205–1226. doi: 10.1002/glia.23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokensgard J., Cheeran M.C-J., Hu S., Gekker G., Peterson P. Glial cell responses to herpesvirus infections: role in defense and immunopathogenesis. J. Infect. Dis. 2002;186(s2):S171–S179. doi: 10.1086/344272. [DOI] [PubMed] [Google Scholar]
- Loov, C., Hillered, L., Ebendal, T., & Erlandsson, A., 2012. Engulfing astrocytes protect neurons from contact-induced apoptosis following injury. PLoS One, 7, e33090. DOI:10.1371/journal.pone.0033090. [DOI] [PMC free article] [PubMed]
- Ludlow M., Kortekaas J., Herden C., Hoffmann B., Tappe D., Trebst C., Griffin D.E., Brindle H.E., Solomon T., Brown A.S., van Riel D., Wolthers K.C., Pajkrt D., Wohlsein P., Martina B.E.E., Baumgärtner W., Verjans G.M., Osterhaus A.D.M.E. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 2016;131:159–184. doi: 10.1007/s00401-015-1511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwin S.K., Rao V.T., Moore C.S., Antel J.P. Astrocytes in multiple sclerosis. Mult. Scler. 2016;22:1114–1124. doi: 10.1177/1352458516643396. [DOI] [PubMed] [Google Scholar]
- Luo, Z., Su, R., Wang, W., Liang, Y., Zeng, X., Shereen, M. A., Bashir, N., Zhang, Q., Zhao, L., Wu, K., Liu, Y., & Wu, J., 2019. EV71 infection induces neurodegeneration via activating TLR7 signaling and IL-6 production. PLoS Pathog, 15, e1008142. DOI:10.1371/journal.ppat.1008142. [DOI] [PMC free article] [PubMed]
- Mahboob S., Boppana S.H., Rose N.B., Beutler B.D., Tabaac B.J. Large vessel stroke and COVID-19: case report and literature review. eNeurologicalSci. 2020;20:100250. doi: 10.1016/j.ensci.2020.100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N., Turovsky E., Christie I.N., Hosford P.S., Hadjihambi A., Korsak A., Ang R., Mastitskaya S., Sheikhbahaei S., Theparambil S.M., Gourine A.V. Brain metabolic sensing and metabolic signaling at the level of an astrocyte. Glia. 2018;66:1185–1199. doi: 10.1002/glia.23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli C., Di Liddo R., Facci L., Bertalot T., Conconi M.T., Zusso M., Skaper S.D., Giusti P. Ligand engagement of Toll-like receptors regulates their expression in cortical microglia and astrocytes. J. Neuroinflammation. 2015;12 doi: 10.1186/s12974-015-0458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti S., Patrone M., Ranzato E. Emerging roles for HMGB1 protein in immunity, inflammation, and cancer. Immunotargets Ther. 2015;4:101–109. doi: 10.2147/ITT.S58064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I., Morgado J., Gomes F.C.A. Astrocyte heterogeneity: impact to brain aging and disease. Front. Aging Neurosci. 2019;11:59. doi: 10.3389/fnagi.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCray P.B., Jr., Pewe L., Wohlford-Lenane C., Hickey M., Manzel L., Shi L., Netland J., Jia H.P., Halabi C., Sigmund C.D., Meyerholz D.K., Kirby P., Look D.C., Perlman S. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. ViroI. 2007;81:813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon R.J., Schreiber R.C., Rudge J.S., Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederos S., Perea G. GABAergic‐astrocyte signaling: a refinement of inhibitory brain networks. Glia. 2019;67:1842–1851. doi: 10.1002/glia.23644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejzini R., Flynn L.L., Pitout I.L., Fletcher S., Wilton S.D., Akkari P.A. ALS genetics, mechanisms, and therapeutics: where are we now? Front. Neurosci. 2019;13:1310. doi: 10.3389/fnins.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzel S., Dijkman H.B., Van Son J.P., Koene R.A., Assmann K.J. Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice: J. Histochem. Cytochem. 1996;44:445–461. doi: 10.1177/44.5.8627002. [DOI] [PubMed] [Google Scholar]
- Miraglia M.C., Scian R., Samartino C.G., Barrionuevo P., Rodriguez A.M., Ibañez A.E., Coria L.M., Velásquez L.N., Baldi P.C., Cassataro J., Delpino M.V., Giambartolomei G.H. Brucella abortus induces TNF-α-dependent astroglial MMP-9 secretion through mitogen-activated protein kinases. J. Neuroinflammation. 2013;10:47. doi: 10.1186/1742-2094-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N., Maki T., Shindo A., Liang A.C., Maeda M., Egawa N., Itoh K., Lo E.K., Lok J., Ihara M., Arai K. Astrocytes promote oligodendrogenesis after white matter damage via brain-derived neurotrophic factor. J. Neurosci. 2015;35:14002–14008. doi: 10.1523/JNEUROSCI.1592-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvan V., Lee J., Bueso T., De Toledo J., Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin. Neurol. Neurosurg. 2020;194:105921. doi: 10.1016/j.clineuro.2020.105921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana V., Ni Y., Sunjara V., Hua X., Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J. Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Eutimio M.A., López-Macías C., Pastelin-Palacios R. Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes. Microbes Infect. 2020;22:226–229. doi: 10.1016/j.micinf.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgello S. Coronaviruses and the central nervous system. J. Neurovirol. 2020;26:459–473. doi: 10.1007/s13365-020-00868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y.u., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizawa Y.M., Hirayama Y., Ohno N., Shibata S., Shigetomi E., Sui Y., Nabekura J., Sato K., Okajima F., Takebayashi H., Okano H., Koizumi S. Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat. Commun. 2017;8:28. doi: 10.1038/s41467-017-00037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R.S., Brown B., Brain D., Cabirac G.F. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann. Neurol. 1992;31:525–533. doi: 10.1002/ana.410310511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagele R.G., D’Andrea M.R., Lee H., Venkataraman V., Wang H.Y. Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 2003;971:197–209. doi: 10.1016/s0006-8993(03)02361-8. [DOI] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H., Kotter M.R., Franklin R.J. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132(Pt 2):288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwainmbi J., De D.D., Smith T.H., El-Hage N., Fitting S., Kang M., Dewey W.L., Hauser K.F., Akbarali H.I. Effects of HIV-1 tat on enteric neuropathogenesis. J. Neurosci. 2014;34:14243–14251. doi: 10.1523/JNEUROSCI.2283-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Natsume A., Ito M., Hara M., Motomura K., Fukuyama R., Sumiyoshi N., Aoki I., Saga T., Lee H.J., Wakabayashi T., Kim S.U. Interferon-β delivery via human neural stem cell abates glial scar formation in spinal cord injury. Cell Transplant. 2013;22:2187–2201. doi: 10.3727/096368912X657882. [DOI] [PubMed] [Google Scholar]
- Nortley R., Attwell D. Control of brain energy supply by astrocytes. Curr. Opin. Neurobiol. 2017;47:80–85. doi: 10.1016/j.conb.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Ohashi K., Burkart V., Flohé S., Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Ojha, C. R., Rodriguez, M., Karuppan, M. K. M., Lapierre, J., Kashanchi, F., & El-Hage, N., 2019. Toll-like receptor 3 regulates Zika virus infection and associated host inflammatory response in primary human astrocytes. PLoS One, 14, e0208543. DOI:10.1371/journal.pone.0208543. [DOI] [PMC free article] [PubMed]
- Oka T., Hikoso S., Yamaguchi O., Taneike M., Takeda T., Tamai T., Oyabu J., Murakawa T., Nakayama H., Nishida K., Akira S., Yamamoto A., Komuro I., Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejnik, J., Hume, A. J., & Mühlberger, E., 2018. Toll-like receptor 4 in acute viral infection: Too much of a good thing. PLoS Pathog, 14, e1007390. DOI:10.1371/journal.ppat.1007390. [DOI] [PMC free article] [PubMed]
- Oviedo-Boyso J., Bravo-Patiño A., Baizabal-Aguirre V.M. Collaborative action of Toll-like and NOD-like receptors as modulators of the inflammatory response to pathogenic bacteria. Mediators Inflamm. 2014;2014:432785. doi: 10.1155/2014/432785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacio M., Álvarez S., Muñoz‐Fernández M.Á. HIV‐1 infection and neurocognitive impairment in the current era. Rev. Med. Virol. 2012;22:33–45. doi: 10.1002/rmv.711. [DOI] [PubMed] [Google Scholar]
- Paniz‐Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J., Sordillo E.M., Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) J. Med. Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V., Basarsky T.A., Liu F., Jeftinija K., Jeftinija S., Haydon P.G. Glutamate-mediated astrocyte–neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J. Neurol. 2020;267:2799–2802. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehar M., Harlan B.A., Killoy K.M., Vargas M.R. Role and therapeutic potential of astrocytes in amyotrophic lateral sclerosis. Curr. Pharm. Des. 2017;23:5010–5021. doi: 10.2174/1381612823666170622095802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkowa, M. (2006). Metallothioneins are multipurpose neuroprotectants during brain pathology. FEBS J, 273, 1857-1870. DOI:10.1111/j.1742-4658.2006.05207.x. [DOI] [PubMed]
- Pfefferkorn C., Kallfass C., Lienenklaus S., Spanier J., Kalinke U., Rieder M., Conzelmann K.K., Michiels T., Staeheli P. Abortively infected astrocytes appear to represent the main source of Interferon-β in the virus-infected brain. J. Virol. 2016;90:2031–2038. doi: 10.1128/JVI.02979-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phulwani N.K., Esen N., Syed M.M., Kielian T. TLR2 expression in astrocytes is induced by TNF-α- and NF-κB-dependent pathways. J. Immunol. 2008;181:3841–3849. doi: 10.4049/jimmunol.181.6.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini R., Li Puma D.D., Mainardi M., Lazzarino G., Tavazzi B., Arancio O., Grassi C. Reduced gliotransmitter release from astrocytes mediates tau-induced synaptic dysfunction in cultured hippocampal neurons. Glia. 2017;65:1302–1316. doi: 10.1002/glia.23163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A., He X., Smirnova I., Liu M.Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Ponath G., Park C., Pitt D. The role of astrocytes in multiple sclerosis. Front. Immunol. 2018;9:217. doi: 10.3389/fimmu.2018.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potokar, M., Jorgacevski, J., & Zorec, R., 2019. Astrocytes in flavivirus infections. Int J Mol Sci, 20. DOI:10.3390/ijms20030691. [DOI] [PMC free article] [PubMed]
- Rannikko E.H., Weber S.S., Kahle P.J. Exogenous α-synuclein induces toll-like receptor 4 dependent inflammatory responses in astrocytes. BMC Neurosci. 2015;16:57. doi: 10.1186/s12868-015-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert L.S., Harder L., Holm C.K., Iversen M.B., Horan K.A., Dagnæs-Hansen F., Ulhøi B.P., Holm T.H., Mogensen T.H., Owens T., Nyengaard J.R., Thomsen A.R., Paludan S.R. TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. J. Clin. Invest. 2012;122:1368–1376. doi: 10.1172/JCI60893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat. Rev. Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Rivieccio M.A., Suh H.S., Zhao Y., Zhao M.L., Chin K.C., Lee S.C., Brosnan C.F. TLR3 ligation activates an antiviral response in human fetal astrocytes: a role for viperin/cig5. J. Immunol. 2006;177:4735–4741. doi: 10.4049/jimmunol.177.7.4735. [DOI] [PubMed] [Google Scholar]
- Rodgers K.R., Lin Y., Langan T.J., Iwakura Y., Chou R.C. Innate immune functions of astrocytes are dependent upon tumor necrosis factor-αlpha. Sci. Rep. 2020;10:7047. doi: 10.1038/s41598-020-63766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Gómez, J. A., Kavanagh, E., Engskog-Vlachos, P., Engskog, M., Herrera, A. J., Espinosa-Oliva, A. M., Joseph, B., Hajji, N., Venero, J. L., & Burguillos, M. A., 2020. Microglia: agents of the CNS pro-inflammatory response. Cells, 9(7). DOI:10.3390/cells9071717. [DOI] [PMC free article] [PubMed]
- Rosciszewski G., Cadena V., Murta V., Lukin J., Villarreal A., Roger T., Ramos A.J. Toll-like receptor 4 (TLR4) and triggering receptor expressed on myeloid cells-2 (TREM-2) activation balance astrocyte polarization into a proinflammatory phenotype. Mol. Neurobiol. 2018;55(5):3875–3888. doi: 10.1007/s12035-017-0618-z. [DOI] [PubMed] [Google Scholar]
- Rouach N., Koulakoff A., Abudara V., Willecke K., Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322(5907):1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Saad M., Omrani A.S., Baig K., Bahloul A., Elzein F., Matin M.A., Selim M.A.A., Mutairi M.A., Nakhli D.A., Aidaroos A.Y.A., Sherbeeni N.A., Al-Khashan H.I., Memish Z.A., Albarrak A.M. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int. J. Infectious Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabroe I., Read R.C., Whyte M.K.B., Dockrell D.H., Vogel S.N., Dower S.K. Toll-like receptors in health and disease: complex questions remain. J. Immunol. 2003;171:1630–1635. doi: 10.4049/jimmunol.171.4.1630. [DOI] [PubMed] [Google Scholar]
- Sadick J.S., Liddelow S.A. Don't forget astrocytes when targeting Alzheimer's disease. Br. J. Pharmacol. 2019;176:3585–3598. doi: 10.1111/bph.14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggese C.E., Del Bianco C., Di Ruzza M.R., Magarelli M., Gandini R., Plocco M. COVID-19 and stroke: casual or causal role? Cerebrovasc. Dis. 2020;49:341–344. doi: 10.1159/000509453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaria S., Badkoobehi H., Rockenstein E., Crews L., Chana G., Masliah E., Everall I.P., the HNRC Group Toll-like receptor pathway gene expression is associated with human immunodeficiency virus–associated neurodegeneration. J. Neurovirol. 2007;13:496–503. doi: 10.1080/13550280701558616. [DOI] [PubMed] [Google Scholar]
- Sanchez-Guajardo V., Tentillier N., Romero-Ramos M. The relation between α-synuclein and microglia in Parkinson's disease: recent developments. Neuroscience. 2015;302:47–58. doi: 10.1016/j.neuroscience.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Santello M., Toni N., Volterra A. Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 2019;22:154–166. doi: 10.1038/s41593-018-0325-8. [DOI] [PubMed] [Google Scholar]
- Sasaki N., Toki S., Chowei H., Saito T., Nakano N., Hayashi Y., Takeuchi M., Makita Z. Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer’s disease. Brain Res. 2001;888:256–262. doi: 10.1016/s0006-8993(00)03075-4. [DOI] [PubMed] [Google Scholar]
- Sato R., Kato A., Chimura T., Saitoh S.-I., Shibata T., Murakami Y., Fukui R., Liu K., Zhang Y., Arii J., Sun-Wada G.-H., Wada Y., Ikenoue T., Barber G.N., Manabe T., Kawaguchi Y., Miyake K. Combating herpesvirus encephalitis by potentiating a TLR3–mTORC2 axis. Nat. Immunol. 2018;19:1071–1082. doi: 10.1038/s41590-018-0203-2. [DOI] [PubMed] [Google Scholar]
- Saura, J., 2007. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation, 4, 26. DOI:10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed]
- Serramía M.J., Muñoz-Fernández M.Á., Álvarez S. HIV-1 increases TLR responses in human primary astrocytes. Sci. Rep. 2016;5 doi: 10.1038/srep17887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Miller J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Simpson J.E., Ince P.G., Lace G., Forster G., Shaw P.J., Matthews F., Savva G., Brayne C., Wharton S.B. Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol. Aging. 2010;31:578–590. doi: 10.1016/j.neurobiolaging.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Smith G.M., Strunz C. Growth factor and cytokine regulation of chondroitin sulfate proteoglycans by astrocytes. Glia. 2005;52:209–218. doi: 10.1002/glia.20236. [DOI] [PubMed] [Google Scholar]
- Sofroniew M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2015;7:a020420. doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solà C., Casal C., Tusell J.M., Serratosa J. Astrocytes enhance lipopolysaccharide-induced nitric oxide production by microglial cells: astrocytes enhance microglial activation. Eur. J. Neurosci. 2002;16:1275–1283. doi: 10.1046/j.1460-9568.2002.02199.x. [DOI] [PubMed] [Google Scholar]
- Soto C., Pritzkow S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 2018;21:1332–1340. doi: 10.1038/s41593-018-0235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Strohm L., Behrends C. Glia-specific autophagy dysfunction in ALS. Semin. Cell Dev. Biol. 2020;99:172–182. doi: 10.1016/j.semcdb.2019.05.024. [DOI] [PubMed] [Google Scholar]
- Suh H.S., Zhao M.L., Rivieccio M., Choi S., Connolly E., Zhao Y., Takikawa O., Brosnan C.F., Lee S.C. Astrocyte Indoleamine 2,3-Dioxygenase Is Induced by the TLR3 Ligand Poly(I:C): mechanism of induction and role in antiviral response. JVI. 2007;81:9838–9850. doi: 10.1128/JVI.00792-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Li M., Ma X., Feng H., Song J., Lv C., He Y. Inhibition of HMGB1 reduces rat spinal cord astrocytic swelling and AQP4 expression after oxygen-glucose deprivation and reoxygenation via TLR4 and NF-κB signaling in an IL-6-dependent manner. J. Neuroinflammation. 2017;14:231. doi: 10.1186/s12974-017-1008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson P.A., II, McGavern D.B. Viral diseases of the central nervous system. Curr. Opin. Virol. 2015;11:44–54. doi: 10.1016/j.coviro.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Kang J., Jaiswal J.K., Simon S.M., Lin J.- H.-C., Yu Y., Li Y., Yang J., Dienel G., Zielke H.R., Nedergaard M. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc. Natl. Acad. Sci. 2005;102:16466–16471. doi: 10.1073/pnas.0506382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Hoshino K., Kawai T., Sanjo H., Takada H., Ogawa T., Takeda K., Akira S. Differential Roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Tan, M., Malek, N., Lawton, M. A., Hubbard, L., Pittman, A. M., Joseph, T., Hehir, J., Swallow, D., Grosset, K. A., Marrinan, S. L., Bajaj, N., Barker, R. A., Burn, D. J., Bresner, C., Foltynie, T., Hardy, J., Wood, N., Ben-Shlomo, Y., Grosset, D. G., Williams, N. M. & Morris, H. R., 2019. Genetic analysis of Mendelian mutations in a large UK population-based Parkinson's disease study. Brain, 142, 2828-2844. DOI:10.1093/brain/awz191. [DOI] [PMC free article] [PubMed]
- Tarawneh, R., & Holtzman, D. M., 2012. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb Perspect Med, 2(5), a006148. DOI:10.1101/cshperspect.a006148. [DOI] [PMC free article] [PubMed]
- Tornatore C., Nath A., Amemiya K., Major E.O. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor-α and interleukin-1β. J. Virol. 1991;65:6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- Umapathi T., Kor A.C., Venketasubramanian N., Lim C.C.T., Pang B.C., Yeo T.T., Lee C.C., Lim P.L., Ponnudurai K., Chuah K.L., Tan P.H., Tai D.Y.H., Ang S.P.B. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J. Neurol. 2004;251:1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valori C.F., Guidotti G., Brambilla L., Rossi D. Astrocytes: emerging therapeutic targets in neurological disorders. Trends Mol. Med. 2019;25:750–759. doi: 10.1016/j.molmed.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Van Cauwenberghe C., Van Broeckhoven C., Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet. Med. 2016;18:421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A.N., Mao G., Yang Y., Ornaghi S., Davis J.N. Zika virus targeting in the developing brain. J. Neurosci. 2017;37:2161–2175. doi: 10.1523/JNEUROSCI.3124-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es M.A., Hardiman O., Chio A., Al-Chalabi A., Pasterkamp R.J., Veldink J.H., van den Berg L.H. Amyotrophic lateral sclerosis. The Lancet. 2017;390:2084–2098. doi: 10.1016/S0140-6736(17)31287-4. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A., Nedergaard M. Physiology of astroglia. Physiol. Rev. 2018;98:239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A., Parpura V., Vardjan N., Zorec R. Physiology of astroglia. Adv. Exp. Med. Biol. 2019;1175:45–91. doi: 10.1007/978-981-13-9913-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A., Untiet V., Rose C.R. Ionic signalling in astroglia beyond calcium. J. Physiol. 2020;598:1655–1670. doi: 10.1113/JP277478. [DOI] [PubMed] [Google Scholar]
- Vijaykumar T.S., Nath A., Chauhan A. Chloroquine mediated molecular tuning of astrocytes for enhanced permissiveness to HIV infection. Virology. 2008;381:1–5. doi: 10.1016/j.virol.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba M., Hott M., Martin C., Aguila B., Valdivia S., Quezada C., Zambrano Á., Concha M.I., Otth C. Herpes simplex virus type 1 induces simultaneous activation of Toll-like receptors 2 and 4 and expression of the endogenous ligand serum amyloid A in astrocytes. Med. Microbiol. Immunol. 2012;201:371–379. doi: 10.1007/s00430-012-0247-0. [DOI] [PubMed] [Google Scholar]
- Voskuhl R.R., Peterson R.S., Song B., Ao Y., Morales L.B.J., Tiwari-Woodruff S., Sofroniew M.V. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J. Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Hayashi S., Yoshimoto M., Kudo H., Takahashi H. NACP/α-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson's disease brains. Acta Neuropathol. 2000;99:14–20. doi: 10.1007/pl00007400. [DOI] [PubMed] [Google Scholar]
- Wang J.P., Bowen G.N., Zhou S., Cerny A., Zacharia A., Knipe D.M., Finberg R.W., Kurt-Jones E.A. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J. Virol. 2012;86:2273–2281. doi: 10.1128/JVI.06010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Haluskey J.A., Lavi E. Coronavirus MHV-A59 causes upregulation of interferon-β RNA in primary glial cell cultures. Adv. Exp. Med. Biol. 1998;440:451–454. doi: 10.1007/978-1-4615-5331-1_57. [DOI] [PubMed] [Google Scholar]
- Welser-Alves J.V., Milner R. Microglia are the major source of TNF-α and TGF-β1 in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochem. Int. 2013;63:47–53. doi: 10.1016/j.neuint.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray, T., & Rogers, J., 2012. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med, 2(1), a006346. DOI:10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed]
- Wyss-Coray T., Loike J.D., Brionne T.C., Lu E., Anankov R., Yan F., Silverstein S.C., Husemann J. Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat. Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Xu J., Zhong S., Liu J., Li L., Li Y., Wu X., Li Z., Deng P., Zhang J., Zhong N., Ding Y., Jiang Y. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K., Komine O. The multi-dimensional roles of astrocytes in ALS. Neurosci. Res. 2018;126:31–38. doi: 10.1016/j.neures.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Yang T., Dai Y., Chen G., Cui S. Dissecting the dual role of the glial scar and scar-forming astrocytes in spinal cord injury. Front. Cell. Neurosci. 2020;14:78. doi: 10.3389/fncel.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Wise L., Fukuchi K.I. TLR4 cross-talk with NLRP3 inflammasome and complement signaling pathways in Alzheimer’s Disease. Front. Immunol. 2020;11:724. doi: 10.3389/fimmu.2020.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger D., Murugan M., Rama Rao K.V., Wu L.J., Chandra N. Microglia receptors in animal models of traumatic brain injury. Mol. Neurobiol. 2019;56(7):5202–5228. doi: 10.1007/s12035-018-1428-7. [DOI] [PubMed] [Google Scholar]
- Zan J., Zhang H., Lu M.Y., Beng H.M., Zhong K.L., Sun X.O., Tan W. Isosteviol sodium injection improves outcomes by modulating TLRs/NF-κB-dependent inflammatory responses following experimental traumatic brain injury in rats. Neuroreport. 2018;29(10):794–803. doi: 10.1097/WNR.0000000000001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.Y., Jouanguy E., Ugolini S., Smahi A., Elain G., Romero P., Segal D., Sancho-Shimizu V., Lorenzo L., Puel A., Picard C., Chapgier A., Plancoulaine S., Titeux M., Cognet C., von Bernuth H., Ku C.L., Casrouge A., Zhang X.X., Barreiro L., Leonard J., Hamilton C., Lebon P., Heron B., Vallee L., Quintana-Murci L., Hovnanian A., Rozenberg F., Vivier E., Geissmann F., Tardieu M., Abel L., Casanova J.-L. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zhang Z.Y., Wu Y., Schluesener H.J. Immunolocalization of toll-like receptors 2 and 4 as well as their endogenous ligand, heat shock protein 70, in rat traumatic brain injury. NeuroImmunoModulation. 2012;19:10–19. doi: 10.1159/000326771. [DOI] [PubMed] [Google Scholar]
- Zheng H., Domínguez Punaro M.C., Segura M., Lachance C., Rivest S., Xu J., Houde M., Gottschalk M. Toll-like receptor 2 is partially involved in the activation of murine astrocytes by Streptococcus suis, an important zoonotic agent of meningitis. J. Neuroimmunol. 2011;234:71–83. doi: 10.1016/j.jneuroim.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Zhong H., Li X., Zhou S., Jiang P., Liu X., Ouyang M., Nie Y., Chen X., Zhang L., Liu Y., Tao T., Tang J. Interplay between RAGE and TLR4 Regulates HMGB1-induced inflammation by promoting cell surface expression of RAGE and TLR4. J.I. 2020;205:767–775. doi: 10.4049/jimmunol.1900860. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Shao A., Yao Y., Tu S., Deng Y., Zhang J. Dual roles of astrocytes in plasticity and reconstruction after traumatic brain injury. Cell Commun Signal. 2020;18(1) doi: 10.1186/s12964-020-00549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]