Abstract
Background
Human epidermal growth factor receptor 2 (HER2)-targeted therapies are successful in patients with HER2-positive malignancies; however, spatial and temporal heterogeneity of HER2 expression may prevent identification of optimal patients for these therapies.
Purpose
To determine whether imaging with the HER2-targeted PET tracer zirconium 89 (89Zr)-pertuzumab can depict HER2-positive metastases in women with HER2-negative primary breast cancer.
Materials and Methods
From January to June 2019, women with biopsy-proven HER2-negative primary breast cancer and biopsy-proven metastatic disease were enrolled in a prospective clinical trial (ClinicalTrials.gov NCT02286843) and underwent 89Zr-pertuzumab PET/CT for noninvasive whole-biopsy evaluation of potential HER2-positive metastases. 89Zr-pertuzumab–avid foci that were suspicious for HER2-positive metastases were tissue sampled and examined by pathologic analysis to document HER2 status.
Results
Twenty-four women (mean age, 55 years ± 11 [standard deviation]) with HER2-negative primary breast cancer were enrolled. Six women demonstrated foci at 89Zr-pertuzumab PET/CT that were suspicious for HER2-positive disease. Of these six women, three had biopsy-proven HER2-positive metastases, two had pathologic findings that demonstrated HER2-negative disease, and one had a fine-needle aspirate with inconclusive results.
Conclusion
Human epidermal growth factor receptor 2 (HER2)-targeted imaging with zirconium 89–pertuzumab PET/CT was successful in detecting HER2-positive metastases in women with HER2-negative primary breast cancer. This demonstrates the ability of targeted imaging to identify patients for targeted therapies that might not otherwise be considered.
© RSNA, 2020
Online supplemental material is available for this article.
See the editorial by Mankoff and Pantel in this issue.
Summary
Human epidermal growth factor receptor 2 (HER2)-targeted zirconium 89–pertuzumab PET/CT successfully helped to identify HER2-positive metastases in women with HER2-negative primary breast cancer.
Key Result
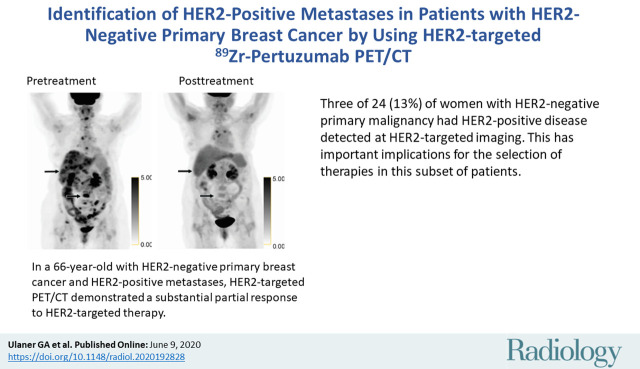
■ Three of 24 (13%) women with human epidermal growth factor receptor 2 (HER2)-negative primary malignancy had HER2-positive disease detected at HER2-targeted imaging. This has important implications for the selection of therapies in this subset of patients.
Introduction
Human epidermal growth factor receptor 2 (HER2), a critical marker in breast cancer, directly influences treatment. Approximately 20% of invasive ductal breast malignancies are classified as HER2 positive as a result of ERBB2 gene amplification and/or the subsequent overexpression of the HER2 protein on the surface of tumor cells (1). Patients with HER2-positive breast cancer are administered specific therapies targeted to HER2 that reduce the risk of death, whereas patients with HER2-negative breast cancer are not (2,3).
Increasing documentation of tumor heterogeneity both within and across lesions in a single patient has substantial therapeutic implications (4). Tissue samples indicate that HER2 expression may change between the primary breast malignancy and metastases (5–8). Inaccurate knowledge of receptor status in metastases because of tumor heterogeneity may lead to suboptimal selection of patients for HER2-targeted therapy. Indeed, 10%–15% of patients with HER2-negative primary breast cancer may still benefit from HER2-targeted treatment (9). It is currently unclear why some patients with HER2-negative breast cancer benefit from HER2-targeted treatments, or how to identify these patients.
We hypothesized that some women with HER2-negative primary malignancies develop HER2-positive metastases that could be identified by using HER2-targeted imaging. Such identification would be difficult by biopsy because only small samples from a limited number of lesions could be evaluated. However, specific radiotracers that identify HER2 could allow for whole-body evaluation of all identifiable lesions (10–13). Specifically, zirconium 89 (89Zr)-pertuzumab is a PET radiotracer that allows for visualization of HER2-positive lesions (14). Pertuzumab is a single-epitope monoclonal antibody that binds to HER2 at a single extracellular domain and inhibits receptor dimerization and downstream signaling pathways (15). Preclinical (16,17) and clinical (14) studies demonstrated the ability of pertuzumab radiolabeled with the positron-emitting radiometal 89Zr to allow targeted, noninvasive imaging of HER2 via PET imaging. We previously suggested the use of 89Zr-pertuzumab PET/CT as a method to help detect HER2-positive metastases in women with HER2-negative primary breast cancer (14). Here, we present the results of a prospective clinical trial evaluating the ability of 89Zr-pertuzumab PET/CT to depict HER2-positive metastases in women with HER2-negative primary breast cancer.
Materials and Methods
Study Participants
This single-center prospective study was performed at the Memorial Sloan-Kettering Cancer Center (New York, NY) with an institutional review board–approved study protocol (ClinicalTrials.gov; NCT02286843). All study participants provided Health Insurance Portability and Accountability Act authorization and written informed consent. The study was funded by the Department of Defense and National Institutes of Health. From January to June 2019, women undergoing treatment for metastatic HER2-negative primary breast cancer at Memorial Sloan-Kettering Cancer Center were identified as potential candidates as part of a convenience series. Inclusion criteria were biopsy-proven HER2-negative primary malignancy, biopsy-proven metastatic disease, at least five foci of demonstrable metastases at imaging (CT, MR, fluorodeoxyglucose [FDG] PET/CT) within 6 weeks of enrollment, age older than 18 years, and Eastern Cooperative Oncology Group performance score of 0–2. Exclusion criteria were creatinine greater than two times the upper limit of normal, aspartate aminotransferase or alanine aminotransferase greater than two times the upper limit of normal, life expectancy less than 3 months, pregnancy or lactation, and inability to undergo PET/CT because of weight limits. HER2 status was initially determined according to published 2013 American Society of Clinical Oncology and College of American Pathologists guidelines (18) and updated to the 2018 American Society of Clinical Oncology and College of American Pathologists guidelines (19) when available. HER2 immunohistochemistry results were categorized as follows: 0 or 1+, negative result; 2+, equivocal result; and 3+, positive result. Tissues with 2+ staining (equivocal) were assessed for HER2 amplification with fluorescence in situ hybridization (FISH) by using an FDA-approved probe set (HER2 IQFISH pharmDX; Dako, Santa Clara, Calif). No prior HER2-positive biopsy was allowed. Women with HER2-negative primary breast malignancy that met all inclusion criteria were offered enrollment on the protocol and research 89Zr-pertuzumab PET/CT.
89Zr-Pertuzumab
The 89Zr-pertuzumab was manufactured at the Memorial Sloan-Kettering Cancer Center Radiochemistry and Molecular Imaging Probes Core Facility in compliance with the requirements specified in the chemistry, manufacturing, and controls section of a U.S. Food and Drug Administration–acknowledged investigational new drug application (number 134411). The preparation process involved conjugating clinical grade pertuzumab (Perjeta; Genentech, South San Francisco, Calif) with a bifunctional chelator, p-SCN-Bn-desferrioxamine (Macrocylics, Plano, Tex), followed by radiolabeling with 89Zr, a positron-emitting radiometal with a 78.4-hour radioactive half-life. The bioconjugation and radiolabeling were performed by using a method that was previously described (20). We chose 89Zr as the radionuclide for this application on the basis of its advantageous physicochemical properties, including a physical half-life that complements the pharmacokinetic profile of antibodies and mild radiolabeling conditions (ambient temperature in 1 mol/L ammonium acetate buffer, pH 7.0) that help preserve the biochemical integrity and immunoreactivity of antibodies during the radiolabeling process (21,22).
The 89Zr-pertuzumab final drug product batches underwent quality control testing before batch release for patient administration to ensure conformance to the following acceptance specifications: radiochemical purity, as determined by radio-thin layer chromatography and size exclusion high-performance liquid chromatography; radio-immunoreactivity, as determined by using a live antigen-expressing cell binding assay; endotoxin content, as measured by Endosafe (Charles River Laboratories, Wilmington, Mass); sterilizing filter integrity, as measured by the bubble point method; pH as measured by pH strips; appearance as a clear and particle-free solution, as determined by visual inspection check; and radionuclidic identity verification, as measured by radioactive γ spectroscopy. Sterility testing by using the direct media inoculation method was performed after release.
Administration of 89Zr-Pertuzumab
All patients were administered the research 89Zr-pertuzumab tracer within 3 weeks of protocol enrollment. Vital signs were recorded before and after tracer administration. An intravenous line was established and flushed with 5% human serum albumin solution. A total of 48 mg of nonradiolabeled pertuzumab was then intravenously administered over 5 minutes. Cold pertuzumab was administered to help reduce nonspecific uptake of the subsequent radiolabeled pertuzumab (10). Then 74 MBq ± 10% of 89Zr-pertuzumab was intravenously administered in a mass of approximately 2 mg, to bring the total pertuzumab antibody mass to 50 mg for each participant. Participants were monitored for adverse effects on the day of and day after 89Zr-pertuzumab administration.
89Zr-Pertuzumab PET/CT and Image Analysis
Five or 6 days (to allow for scheduling around weekends) after administration of 89Zr-pertuzumab, participants underwent noncontrast agent–enhanced PET/CT from the skull apex to midthigh on a dedicated research scanner (GE Discovery PET/CT 710; GE Healthcare, Chicago, Ill) with an 80-mA CT component for attenuation correction, lesion localization, and correlation with CT findings. PET/CT images were reconstructed by using iterative reconstruction and displayed in multiplanar reconstructions with 3-mm axial section thickness by using PET Volume Computer Assisted Reading (GE Healthcare). 89Zr-pertuzumab PET/CT scans were interpreted by two different nuclear medicine experts (G.A.U. and J.A.C., with 15 and >30 years of experience with the use of research PET radiotracers, respectively). Both readers compared the 89Zr-pertuzumab PET/CT scans with prior imaging studies and access to prior biopsy results. Physiologic 89Zr-pertuzumab uptake was observed in the blood pool, liver, gallbladder, bowel, and kidney. Radiotracer uptake in areas that are not physiologic were graded both qualitatively and semiquantitatively. For qualitative scoring, we used a five-point scale, as follows: 1, definitely normal; 2, probably normal; 3, equivocal; 4, probably abnormal; and 5, definitely abnormal. Lesions scored 4 or 5 were documented as suspicious for HER2-positive malignancy. Readers evaluated the studies independently within 3 days of each other on separate workstations. Readers were blinded to the other reader, and data sheets were collected by a research assistant. Only those foci qualitatively scored as suspicious by both readers were considered suspicious foci and were considered for biopsy. Semiquantitative analysis of tracer uptake was performed by recording maximum standardized uptake value of lesions suspicious for cancer. Three-dimensional volumes of interest were placed in these areas and tracer uptake was quantified by using maximum standardized uptake value, calculated as follows: standardized uptake value = decay-corrected maximum region of interest activity (μCi/mL)/[injected dose (μCi)/body weight (g)].
Pathologic Confirmation of HER2 Status for 89Zr-Pertuzumab Foci Suspicious for Cancer at PET/CT
Image-guided biopsy was selected in concert with an oncologic interventional radiologist with greater than 10 years of experience to minimize risks to the participants while obtaining high-quality samples. Patients 4, 8, 14, and 18 underwent CT-guided core biopsies. Patient 11 underwent US-guided fine-needle aspiration. Patient 23 underwent a PET/CT-guided core biopsy. Biopsy specimens were evaluated by a board-certified breast pathology specialist (D.S.R., with >10 years of experience). Immunohistochemistry results were categorized according to American Society of Clinical Oncology guidelines (18). Carcinomas with 3+ immunohistochemistry or 2+ immunohistochemistry and concurrent positive result on HER2 FISH were classified as HER2-positive metastases.
HER2-targeted Therapy for HER2-Positive Metastases
Therapy was not a component of the clinical trial (detailed at ClinicalTrials.gov: NCT02286843). When HER2-positive metastases were confirmed at pathologic analysis, this information was provided to the treating oncologists. HER2-targeted therapy was then initiated at the discretion of the treating oncologists per standard prescribing guidelines.
Additional Data Collection and Analysis after Study Completion
After the completion of the prospective study, participant’s images were retrospectively evaluated to analyze the size of most 89Zr-pertumumab–avid metastasis, maximum standardized uptake value from FDG PET/CT studies within 1 month of trial enrollment (when available), and 89Zr-pertumumab lesion-to-local background and 89Zr-pertumumab lesion-to-mediastinal blood pool background measurements.
Results
Participant Characteristics
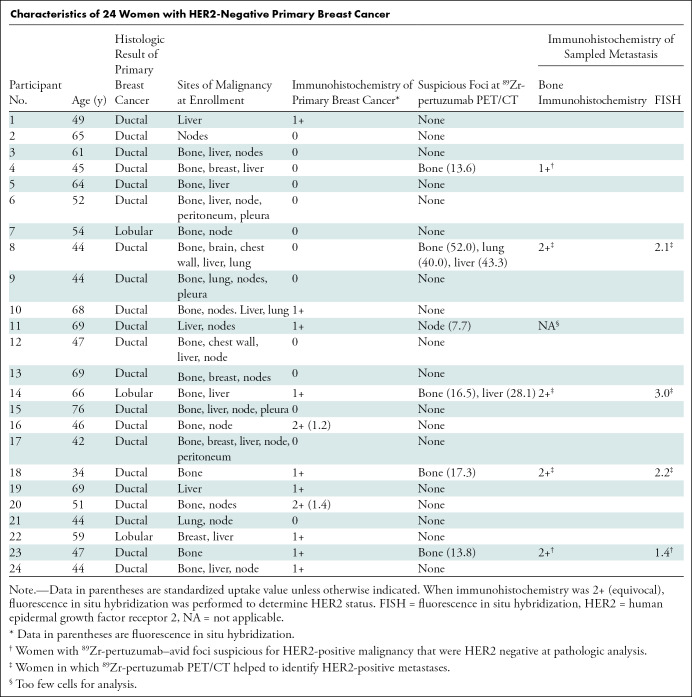
Patients at Memorial Sloan-Kettering Cancer Center with HER2-negative primary malignancy and biopsy-proven metastases were identified by research assistants or referring physicians as potential candidates for the protocol. Of the 65 possible candidates identified, 24 were excluded because of no foci of demonstrable metastases at imaging within 6 weeks of enrollment. No other inclusion or exclusion criteria caused exclusion of potential candidates. Of the 41 remaining potential candidates, 17 did not want to be part of the study. Twenty-four women (mean age, 55 years ± 11 [standard deviation]) with biopsy-proven HER2-negative primary breast cancer consented and they all completed the study protocol. Participant characteristics are listed in the Table.
Characteristics of 24 Women with HER2-Negative Primary Breast Cancer
Sites of Known Malignancy at the Time of Protocol Enrollment
CT, FDG PET/CT, and MRI helped to determine sites of known malignancy within 60 days of protocol enrollment. Diseases manifested as follows: known osseous disease in 18 women, hepatic disease and nodal disease in 15 women, breast disease in six women, lung disease in four women, pleura disease in three women, peritoneal disease in two women, and brain disease in one woman. Of the total 24 women, 19 had more than one organ system involved with disease. Sites of known malignancy are summarized in the Table.
89Zr-Pertuzumab PET/CT
All 24 women underwent 89Zr-pertuzumab PET/CT, were monitored for 30 minutes after tracer injection, and called the following day with no observed or reported adverse effects. Vital signs (recorded before and after injection) and clinical impact remained unchanged.
In six of the 24 women (25%), 89Zr-pertuzumab–avid foci considered suspicious for HER2-positive disease were identified. In five women, the organ system suspicious for HER2-positive malignancy was bone, two women had liver lesions suspicious for HER2-positive malignancy, and one each had lung and nodal lesions suspicious for HER2-positive malignancy. Two women had more than one organ system with lesions suspicious for HER2-positive malignancy. Sites of 89Zr-pertuzumab avidity suspicious for HER2-positive malignancy are summarized in the Table. Qualitative scoring by using the five-point scale is summarized in Table E1 (online).
Pathologic Evaluation of Sites of 89Zr-Pertuzumab Foci Suspicious for HER2-Positive Malignancy
Six women with 89Zr-pertuzumab foci suspicious for HER2-positive malignancy underwent tissue sampling. Of these six women, three underwent biopsy that proved HER2-positive metastases, two underwent pathologic analysis that demonstrated HER2-negative disease, and one underwent a fine-needle aspirate with inconclusive results (Table).
Participants 8, 14, and 18 demonstrated 89Zr-pertuzumab–avid foci and had biopsy-proven HER2-positive metastases. All three women had osseous lesions accessible to biopsy. Immunohistochemistry from the biopsy specimens in all three women was 2+ and therefore FISH was performed. All three women had FISH results consistent with HER2-positive disease. Figure 1 shows participant 14, a 66-year-old woman with HER2-negative (HER2 immunohistochemistry 1+) primary invasive lobular carcinoma. She was diagnosed with metastatic disease in 2016 and had undergone treatment with courses of palbociclib, letrozole, and capecitabine from 2016 to 2018. CT showed progression of hepatic metastases and stable osseous metastases in October 2018, and she was subsequently referred to the HER2-targeted imaging protocol. Research 89Zr-pertuzumab PET/CT performed in November 2018 showed multiple 89Zr-pertuzumab–avid osseous and hepatic foci suspicious for HER2-positive disease. Biopsy of an 89Zr-pertuzumab–avid osseous lesion underwent pathologic analysis and demonstrated a HER2 immunohistochemistry of 2+, then FISH with HER2 copy number 9.13, chromosome 17 centromere (CEP17) copy number 3.07, and a HER2-to-CEP17 ratio of 3.0, consistent with HER2-positive disease. Because of the diagnosis of HER2-positive metastases, she began a treatment regimen of docetaxel, trastuzumab, and pertuzumab. FDG PET/CT performed before initiation of docetaxel, trastuzumab, and pertuzumab and after 2 months of therapy demonstrated a substantial partial response (Fig 2).
Figure 1a:
![Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/86e3/7543717/27f78bf3fedc/radiol.2020192828.fig1a.jpg)
Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).
Figure 2a:
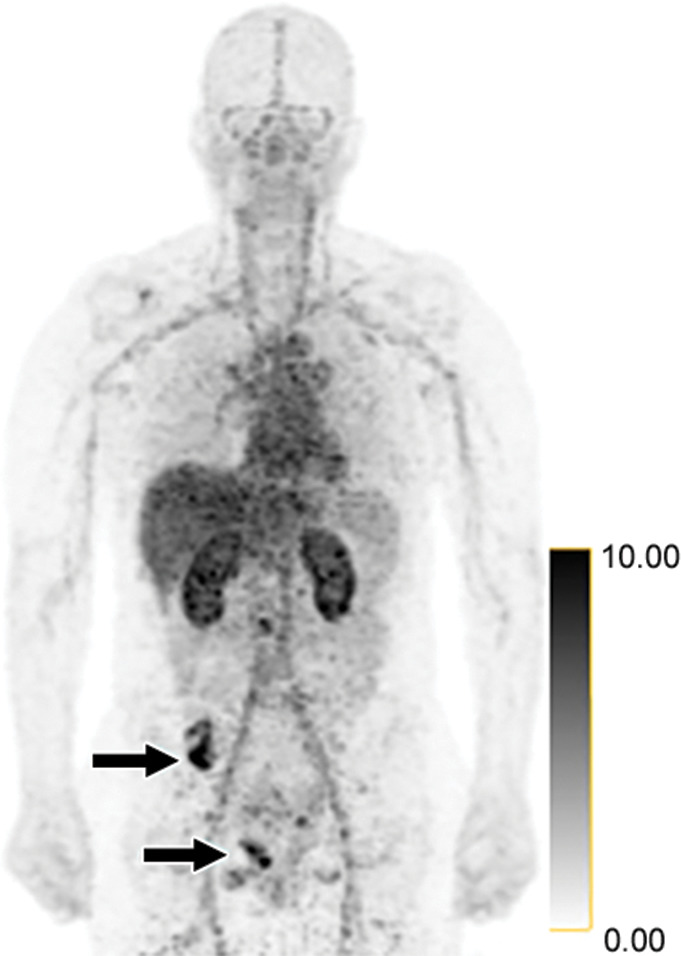
False-positive findings at zirconium 89 (89Zr)-pertuzumab PET/CT in a 47-year-old woman with human epidermal growth factor receptor 2 (HER2)-negative primary breast cancer. HER2 immunohistochemistry of the primary breast malignancy was 1+ (not shown), consistent with HER2-negative malignancy. (a) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates osseous foci in the right pelvis (arrows on osseous lesions; standardized uptake values, 13.8 and 11.9), suspicious for HER2-positive malignancy. (b) Axial CT and fused 89Zr-pertuzumab PET/CT images acquired from a PET/CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus (arrow). Pathologic analysis demonstrated HER2 immunohistochemistry 2+ and FISH 1.4 (not shown), consistent with HER2-negative disease.
Figure 1b:
![Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/86e3/7543717/043e4cf00f04/radiol.2020192828.fig1b.jpg)
Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).
Figure 1c:
![Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/86e3/7543717/1ded2e3b7916/radiol.2020192828.fig1c.jpg)
Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).
Figure 1d:
![Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/86e3/7543717/9f7a56e949be/radiol.2020192828.fig1d.jpg)
Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).
Figure 1e:
![Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/86e3/7543717/dcce29461063/radiol.2020192828.fig1e.jpg)
Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).
Figure 1f:
![Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/86e3/7543717/01774d2bcc11/radiol.2020192828.fig1f.jpg)
Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).
Figure 1g:
![Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/86e3/7543717/89f796909251/radiol.2020192828.fig1g.jpg)
Identification of human epidermal growth factor receptor 2 (HER2)-positive metastases in a 66-year-old woman with HER2-negative primary breast cancer by using HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT. (a) HER2 immunohistochemistry of the primary breast malignancy at 200× magnification was 1+ (faint staining, arrow), consistent with HER2-negative malignancy. (b) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates multiple osseous and hepatic foci, suspicious for HER2-positive malignancy (arrows show hepatic [standardized uptake value, 18.1] and osseous [standardized uptake value, 16.5] reference lesions). (c) Axial CT image in patient in prone position acquired from a CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus. (d) HER2 immunohistochemistry at 200× magnification of the biopsied lesion was 2+ (moderate staining, arrow); 2+ HER2 immunohistochemistry is considered equivocal and thus HER2 fluorescence in situ hybridization (FISH) was performed. (e) HE (CEP17) copy number 3.07, and HER2/CEP17 ratio of 3.0, consistent with HER2-positive disease. Given the new diagnosis of HER2-positive metastases, the woman began treatment with docetaxel, trastuzumab, and pertuzumab. (f) Maximum intensity projection image from fluorodeoxyglucose PET/CT performed before initiation of therapy and (g) after 2 months of therapy demonstrate a substantial partial response (arrows in f and g indicate hepatic [standardized uptake value, 4.5 before therapy, standardized uptake value equal to background after therapy] and osseous [standardized uptake value, 8.4 before therapy, standardized uptake value equal to background after therapy] reference lesions).
Figure 2b:
False-positive findings at zirconium 89 (89Zr)-pertuzumab PET/CT in a 47-year-old woman with human epidermal growth factor receptor 2 (HER2)-negative primary breast cancer. HER2 immunohistochemistry of the primary breast malignancy was 1+ (not shown), consistent with HER2-negative malignancy. (a) Maximum intensity projection image from research 89Zr-pertuzumab PET/CT demonstrates osseous foci in the right pelvis (arrows on osseous lesions; standardized uptake values, 13.8 and 11.9), suspicious for HER2-positive malignancy. (b) Axial CT and fused 89Zr-pertuzumab PET/CT images acquired from a PET/CT-guided biopsy of an 89Zr-pertuzumab–avid osseous focus (arrow). Pathologic analysis demonstrated HER2 immunohistochemistry 2+ and FISH 1.4 (not shown), consistent with HER2-negative disease.
Participant 4 and 23 demonstrated 89Zr-pertuzumab-avid foci; however, biopsy showed HER2-negative metastases. Figure 2 graphically depicts participant 23, a 47-year-old woman with HER2-negative (HER2 immunohistochemistry 1+) primary invasive ductal carcinoma. She was diagnosed with metastatic disease in 2017 and underwent treatment with multiple rounds of systemic therapy from 2017 to 2019. MRI in the spine and FDG PET/CT helped to demonstrate progression of osseous metastases in May 2019, and she was referred to the HER2-targeted imaging protocol. A research 89Zr-pertuzumab PET/CT performed in June 2019 demonstrated multiple 89Zr-pertuzumab–avid osseous foci suspicious for HER2-positive disease. Biopsy of an 89Zr-pertuzumab–avid osseous lesion underwent pathologic analysis and a HER2 immunohistochemistry of 2+ was shown, then FISH with HER2 copy number 3.2, CEP17 copy number 2.3, and HER2-to-CEP17 ratio of 1.4, consistent with HER2-negative disease. Thus, the pathologic analysis of the biopsy did not confirm HER2-positive disease on the 89Zr-pertuzumab PET/CT (Fig 2).
Participant 11 had a small 89Zr-pertuzumab–avid internal mammary node, in which fine-needle aspiration was attempted, but the tissue sample was not satisfactory for HER2 analysis. The participant declined repeat tissue sampling and subsequently died.
The remaining 19 participants did not have foci suspicious for HER2-positive disease at their research 89Zr-pertuzumab PET/CT and thus had no tissue sampling performed.
Additional Retrospective Data Collection After Prospective Study Completion
The site of the most 89Zr-pertuzumab–avid lesion was most commonly bone (n = 9), followed by liver (n = 4), node (n = 4), pleura (n = 2), breast (n = 2), chest wall (n = 1), and lung (n = 1). Size (when measurable) and corresponding FDG maximum standardized uptake value (when available) of the most 89Zr-pertuzumab–avid lesions are reported in Table E2 (online). All five 89Zr-pertuzumab–avid lesions with pathologic proof were osseous lesions, and thus not size measurable. Table E2 (online) also reports the standardized uptake value of the most 89Zr-pertuzumab–avid lesion, local background, and mediastinal background at 89Zr-pertuzumab PET/CT for each patient. For the three participants with 89Zr-pertuzumab–avid lesions proven at pathologic analysis to be HER2-positive metastases, 89Zr-pertuzumab lesion-to–local background ratios were 9.5, 3.6, and 3.3, whereas 89Zr-pertuzumab lesion-to–blood pool backgrounds were 3.9, 3.6, and 2.2. For the two participants with 89Zr-pertuzumab–avid lesions that were HER2-negative at pathologic analysis, the 89Zr-pertuzumab lesion-to–local background ratios were 3.9 and 5.5, whereas the 89Zr-pertuzumab lesion-to–blood pool backgrounds were 2.3 and 2.5. There was overlap between true-positive and presumably false-positive lesions for both tumor-to-background ratios.
Discussion
Tumor heterogeneity prevents optimal tumor therapy (4). Traditional analysis of tumor markers occurred by tissue sampling small portions of a small number of lesions. Our results showed that noninvasive, whole-body assessment of human epidermal growth factor receptor 2 (HER2) expression by HER2-targeted zirconium 89 (89Zr)-pertuzumab PET/CT can demonstrate HER2-positive metastases in women with HER2-negative primary breast cancers. Of 24 women with HER2-negative primary breast cancer who underwent 89Zr-pertuzumab PET/CT, six had HER2-positive foci. Of these six, three had biopsy-proven HER2-positive metastases, two had pathologic analysis demonstrating HER2-negative disease, and one had a fine-needle aspirate with inconclusive results. Thus, three of 24 (13%) study participants with HER2-neagtive primary disease underwent research HER2-targeted PET/CT that demonstrated HER2-positive metastases.
Whereas the number of women with 89Zr-pertuzumab–avid true-positive metastases in our pilot study was small (three of 24 women; 13%), the eventual impact of HER2-targeted imaging could be substantial. More than 900 000 women are living with metastatic breast cancer, and more than 50 000 women are diagnosed each year (23). Approximately 80% of these women have HER2-negative primary malignancies. If 13% of women with metastatic HER2-negative primary breast cancer harbor HER2-positive metastases, it would represent a current population of over 100 000 women. Thus, targeted HER2 imaging could substantially increase the number of patients eligible for and benefiting from HER2-targeted therapies.
Two of 24 women (8%) had 89Zr-pertuzumab–avid foci that were HER2-negative at pathologic analysis. This remains a limitation of HER2-targeted imaging. In women known to be HER2-positive, the likelihood of true-positive HER2-targeted PET will be high because of the high pretest probability. False-positive findings among women with HER2-negative disease likely relates to the lower pretest probability of finding HER2-positive disease in a given patient. Some discussion of the false-positive findings seems relevant. First, the HER2 immunohistochemistry of patients with the false-positive findings did change between the primary and biopsied metastasis. In one woman, a primary with HER2 immunohistochemistry of 1+ had a metastasis with a HER2 immunohistochemistry of 2+ and required FISH to determine whether the metastasis was HER2-positive or negative. Second, relatively high standardized uptake values at 89Zr-pertuzumab PET (values of 13.6 and 13.8) were proven at biopsy to be HER2 negative; therefore, it was not an issue of only mild avidity resulting in false-positive findings. A continued emphasis on decreasing false-positive findings will be necessary before the adoption of imaging tests such as these. We are greatly encouraged by the far lower false-positive rate in this trial of 89Zr-pertuzumab PET than in a previous study (24,25) of 89Zr-trastuzumab in which false-positive findings greatly outnumbered true-positive findings. Compared with trastuzumab, in vitro and in vivo models with 89Zr-trastuzumab demonstrated increased affinity for HER2 (16), even in the presence of trastuzumab; thus, the composition of the HER2 antibody may influence imaging results. Site-specific labeling of PET antibodies creates more homogeneous populations of PET antibodies and improved visualization of HER2-expressing tumors in models that are in vivo (26), which is an avenue of investigation we now translate to the clinic.
In addition to the false-positive studies, maximizing application of an expensive and intensive imaging study must be explored. An available U.S. Food and Drug Administration–approved serum test for HER2 predicts response to HER2-targeted therapies (27,28). If a serum test could help screen patients with HER2-negative primary malignancies for the presence of serum HER2, then HER2-targeted imaging could localize the HER2-positive disease and allow direct biopsy for pathologic confirmation. A two-step process such as this could increase the yield of HER2-targeted imaging in this patient population.
Our study used PET-guided biopsies, which may have multiple PET radiotracers to guide biopsies of CT occult lesions (28). This provided a mechanism of tissue sampling lesions otherwise difficult to localize, particularly bone lesions, because osseous malignancy is often more readily viewed at PET than at CT (30).
We took a conservative viewpoint: 89Zr-pertuzumab PET/CT examinations with foci that are suspicious for HER2-positive malignancy but with associated pathologic analyses negative for disease are false-positive imaging studies. However, because of tissue sampling error inherent with biopsy, this may not be correct. Thus, it is possible that the accuracy of 89Zr-pertuzumab PET/CT is underestimated in this study, and other end points such as response to HER2-targeted therapy could be explored in future studies.
Our study had some limitations, including its small sample size and lack of tissue sampling of multiple lesions suspicious and not suspicious for HER2-positive malignancy. Ethical and logistical reasons prevented biopsy of all 89Zr-pertuzumab foci and nonavid foci, which would more definitively document the sensitivity and specificity of the technique. HER2-mutant breast cancers, which recently have been more greatly appreciated (31,32), may not be identified by our HER2-targeted imaging. However, this limitation would occur infrequently because only about 3% of HER2-positive breast cancers are HER2-positive because of constitutively activating HER2 mutations.
In conclusion, we demonstrated the ability of human epidermal growth factor receptor 2 (HER2)-targeted zirconium 98–pertuzumab PET/CT to help identify HER2-positive metastases in women with HER2-negative primary breast cancer. HER2-targeted imaging requires further evaluation and optimization with larger studies. However, this noninvasive whole-body technique could help identify patients eligible for HER2-targeted therapies who may not otherwise be considered.
SUPPLEMENTAL TABLES
Disclosures of Conflicts of Interest: G.A.U. Activities related to the present article: grants received from Genentech. Activities not related to the present article: disclosed money paid to author by Sanofi; and grants from Sanofi, Novartis, and Puma biotechnology. Other relationships: disclosed no relevant relationships. J.A.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money to author’s institution for grant from Genentech. Other relationships: disclosed no relevant relationships. C.C.R. disclosed no relevant relationships. R.Y. disclosed no relevant relationships. V.H. disclosed no relevant relationships. D.S.R. disclosed no relevant relationships. K.J. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for consultancy from Novartis, Spectrum Pharmaceuticals, ADC Therapeutics, Pfizer, BMS, Abbvie, AstraZeneca; disclosed money to author’s institution for grants from Novartis, Clovis Oncology, Genentech, AstraZeneca, ADC Therapeutics, Novita Pharmaceuticals; disclosed money to author for development of educational presentations; money to author for travel expenses from Novartis, ADC Therapeutics, BMS, AstraZeneca, Jounce Therapeutics, Taiho Oncology; money paid to author from Synthon. Other relationships: disclosed no relevant relationships. S.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for consultancy from Novartis, Eli Lilly, Sermonix, Context Therapeutics, Revolution Medicine, BMS, Paige AI, Chugai; disclosed money to author’s institution for grants/grants pending from Daiichi-Sankyo; disclosed money to author for travel expenses from Novartis, Lilly, and BMS. Other relationships: disclosed no relevant relationships. D.M.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author from Lilly/Loxo Oncology, Chugai, Boehringer Ingelheim, AstraZeneca, Pfizer, Bayer, Genentech/Roche, Kinnate/Fount; and grants from AstraZeneca, Bayer. Other relationships: disclosed no relevant relationships B.M.Z. disclosed no relevant relationships. S.K.L. disclosed no relevant relationships. J.S.L. disclosed no relevant relationships.
G.A.U. supported by the Department of Defense Breast Cancer Research Program Breakthrough Award (BC132676); G.A.U., B.Z., J.S.L. supported by National Institutes of Health (R01 CA204167); J.S.L. supported by National Institutes of Health (R35 CA232130); G.A.U. supported by Genentech. The authors gratefully acknowledge the Memorial Sloan-Kettering Cancer Center Radiochemistry and Molecular Imaging Probe Core (National Institutes of Health Cancer Center Support Grant [P30 CA008748]) for additional support.
Abbreviations:
- CEP17
- chromosome 17 centromere
- FDG
- fluorodeoxyglucose
- FISH
- fluorescence in situ hybridization
- HER2
- human epidermal growth factor receptor 2
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235(4785):177–182. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344(11):783–792. [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353(16):1673–1684. [DOI] [PubMed] [Google Scholar]
- 4.McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 2015;27(1):15–26 [Published correction appears in Cancer Cell 2015;28(1):141.]. [DOI] [PubMed] [Google Scholar]
- 5.Niikura N, Liu J, Hayashi N, et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol 2012;30(6):593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoefnagel LD, van de Vijver MJ, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res 2010;12(5):R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang HJ, Han SW, Oh DY, et al. Discordant human epidermal growth factor receptor 2 and hormone receptor status in primary and metastatic breast cancer and response to trastuzumab. Jpn J Clin Oncol 2011;41(5):593–599. [DOI] [PubMed] [Google Scholar]
- 8.Priedigkeit N, Hartmaier RJ, Chen Y, et al. Intrinsic Subtype Switching and Acquired ERBB2/HER2 Amplifications and Mutations in Breast Cancer Brain Metastases. JAMA Oncol 2017;3(5):666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med 2008;358(13):1409–1411. [DOI] [PubMed] [Google Scholar]
- 10.Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 2010;87(5):586–592. [DOI] [PubMed] [Google Scholar]
- 11.Henry KE, Ulaner GA, Lewis JS. Human Epidermal Growth Factor Receptor 2-Targeted PET/Single- Photon Emission Computed Tomography Imaging of Breast Cancer: Noninvasive Measurement of a Biomarker Integral to Tumor Treatment and Prognosis. PET Clin 2017;12(3):269–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry KE, Ulaner GA, Lewis JS. Clinical Potential of Human Epidermal Growth Factor Receptor 2 and Human Epidermal Growth Factor Receptor 3 Imaging in Breast Cancer. PET Clin 2018;13(3):423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebhart G, Lamberts LE, Wimana Z, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol 2016;27(4):619–624. [DOI] [PubMed] [Google Scholar]
- 14.Ulaner GA, Lyashchenko SK, Riedl C, et al. First-in-Human Human Epidermal Growth Factor Receptor 2-Targeted Imaging Using 89Zr-Pertuzumab PET/CT: Dosimetry and Clinical Application in Patients with Breast Cancer. J Nucl Med 2018;59(6):900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol 2020;17(1):33–48. [DOI] [PubMed] [Google Scholar]
- 16.Marquez BV, Ikotun OF, Zheleznyak A, et al. Evaluation of (89)Zr-pertuzumab in Breast cancer xenografts. Mol Pharm 2014;11(11):3988–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massicano AVF, Lee S, Crenshaw BK, et al. Imaging of HER2 with [89Zr]pertuzumab in Response to T-DM1 Therapy. Cancer Biother Radiopharm 2019;34(4):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31(31):3997–4013. [DOI] [PubMed] [Google Scholar]
- 19.Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018;36(20):2105–2122. [DOI] [PubMed] [Google Scholar]
- 20.Vosjan MJ, Perk LR, Visser GW, et al. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat Protoc 2010;5(4):739–743. [DOI] [PubMed] [Google Scholar]
- 21.Holland JP, Sheh Y, Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nucl Med Biol 2009;36(7):729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deri MA, Zeglis BM, Francesconi LC, Lewis JS. PET imaging with ⁸⁹Zr: from radiochemistry to the clinic. Nucl Med Biol 2013;40(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA 2013;309(8):800–805. [DOI] [PubMed] [Google Scholar]
- 24.Ulaner GA, Hyman DM, Ross DS, et al. Detection of HER2-Positive Metastases in Patients with HER2-Negative Primary Breast Cancer Using 89Zr-Trastuzumab PET/CT. J Nucl Med 2016;57(10):1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulaner GA, Hyman DM, Lyashchenko SK, et al. 89Zr-Trastuzumab PET/CT for Detection of Human Epidermal Growth Factor Receptor 2-Positive Metastases in Patients With Human Epidermal Growth Factor Receptor 2-Negative Primary Breast Cancer. Clin Nucl Med 2017;42(12):912-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivier D, Fung K, Rodriguez C, et al. The Influence of Glycans-Specific Bioconjugation on the FcγRI Binding and In vivo Performance of 89Zr-DFO-Pertuzumab. Theranostics 2020;10(4):1746–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen ER, Sørensen PD, Jakobsen EH, Madsen JS, Brandslund I. Serum HER-2 predicts response and resistance to trastuzumab treatment in breast cancer. Clin Chem Lab Med 2013;51(7):1483–1492. [DOI] [PubMed] [Google Scholar]
- 28.Lee CK, Davies L, Gebski VJ, et al. Serum Human Epidermal Growth Factor 2 Extracellular Domain as a Predictive Biomarker for Lapatinib Treatment Efficacy in Patients With Advanced Breast Cancer. J Clin Oncol 2016;34(9):936–944. [DOI] [PubMed] [Google Scholar]
- 29.Cornelis FH, Durack JC, Pandit-Taskar N, et al. Long-Half-Life 89Zr-Labeled Radiotracers Can Guide Percutaneous Biopsy Within the PET/CT Suite Without Reinjection of Radiotracer. J Nucl Med 2018;59(3):399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 2009;50(Suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulaner GA, Saura C, Piha-Paul SA, et al. Impact of FDG PET Imaging for Expanding Patient Eligibility and Measuring Treatment Response in a Genome-Driven Basket Trial of the Pan-HER Kinase Inhibitor, Neratinib. Clin Cancer Res 2019;25(24):7381–7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyth LM, Piha-Paul SA, Won HH, et al. Efficacy and Determinants of Response to HER Kinase Inhibition in HER2-Mutant Metastatic Breast Cancer. Cancer Discov 2020;10(2):198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.