Abstract
Background
Out-of-hospital cardiac arrest carries a poor prognosis with survival less than 10% in many patient cohorts. Survival is inversely associated with duration of resuscitation as external chest compressions do not provide sufficient blood flow to prevent irreversible organ damage during a prolonged resuscitation. Extracorporeal membrane oxygenation (ECMO) instituted during cardiac arrest can provide normal physiological blood flows and is termed Extracorporeal Cardio-Pulmonary Resuscitation (ECPR). ECPR may improve survival when used with in-hospital cardiac arrests. This possible survival benefit has not been replicated in trials of out-of-hospital cardiac arrests, possibly because of the additional time it takes to transport the patient to hospital and initiate ECPR. Pre-hospital ECPR may shorten the time between cardiac arrest and physiological blood flows, potentially improving survival. It may also mitigate some of the neurological injury that many survivors suffer.
Methods
Sub30 is a prospective six patient feasibility study. The primary aim is to test whether it is possible to institute ECPR within 30 min of collapse in adult patients with refractory out of hospital cardiac arrest (OHCA). The secondary aims are to gather preliminary data on clinical outcomes, resource utilisation, and health economics associated with rapid ECPR delivery in order to plan any subsequent clinical investigation or clinical service. On study days a dedicated fast-response vehicle with ECPR capability will be tasked to out-of-hospital cardiac arrests in an area of London served by Barts Heart Centre. If patients suffer a cardiac arrest refractory to standard advanced resuscitation and meet eligibility criteria, ECPR will be started in the pre-hospital environment.
Discussion
Delivering pre-hospital ECPR within 30 min of an out-of-hospital cardiac arrest presents significant ethical, clinical, governance and logistical challenges. Prior to conducting an efficacy study of ECPR the feasibility of timely and safe application must be demonstrated first. Extensive planning, multiple high-fidelity multiagency simulations and a unique collaboration between pre-hospital and in-hospital institutions will allow us to test the feasibility of this intervention in London. The study has been reviewed, refined and endorsed by the International ECMO Network (ECMONet).
Trial registration
Clinicaltrials. gov NCT03700125, prospectively registered October 9, 2018.
Keywords: Cardiac arrest, Extracorporeal membrane oxygenation, Pre-hospital medical care, Extra-corporeal cardiopulmonary resuscitation
Background
In England, 91% of patients with out-of-hospital cardiac arrest die before leaving hospital.1 Within London, the London Ambulance Service (LAS) attempts to resuscitate more than 4000 out-of-hospital cardiac arrest (OHCA) patients annually. In 2017/18, despite achieving return of spontaneous circulation (ROSC) in 32.5% of patients, only 9.4% of patients with attempted resuscitation were ultimately discharged home.2 Thus, nearly two thirds of patients with initially successful resuscitation progress to death before leaving hospital, often due to significant ischaemia of their vital organs sustained during the cardiac arrest. External chest compressions may mitigate this injury producing typical aortic blood flow rates of 0.6 L min−1. m−2, but this approximates to less than one third of normal cardiac output and is often insufficient in prolonged cardiac arrest.
Extracorporeal membrane oxygenation (ECMO) is a circulatory support technology that can provide greater oxygenated blood flow (up to 2 L min−1. m−2) to patients’ vital organs whilst they are in cardiac arrest. When used in this setting it is described as Extracorporeal Cardio-pulmonary Resuscitation (ECPR). Under ECPR, cannulae are placed in the femoral vein and artery to allow venous blood to be pumped via an artificial membrane lung to the arterial system at a flow rate sufficient to pressurise the arterial system, thereby enabling potentially protective organ perfusion during ongoing cardiac arrest. The aim of ECPR is to prevent irreversible organ damage suffered during prolonged conventional resuscitation, providing time for diagnostic and therapeutic procedures that may restore cardiac function (e.g. percutaneous coronary intervention). Moreover, ECMO may favourably influence myocardial oxygenation by enhancing aortic root pressure, reducing ventricular filling pressures, and reducing the need for cardiac work. This further limits on-going myocardial injury and is an emerging concept (“door to unloading time”) in the management of ST elevation myocardial infarction complicated by cardiogenic shock.3
Data from a large North American cohort of patients with non-traumatic OHCA who were potentially suitable for ECPR, suggest strongly that the risk of ischaemic injury during conventional resuscitation rises quickly.4 Indeed, by 15–20 min of Cardiopulmonary Resuscitation (CPR), the rate of survivorship of patients with a good neurological outcome declines dramatically, whilst the proportion of patients who progress to death or survival with poor neurological function increases rapidly. The poor outcomes in patients following 20 min of cardiac arrest are also observed in the PARAMEDIC2 trial, which tested the use epinephrine against a placebo drug in OHCA. Only ∼2% of patients, who remained in cardiac arrest long enough to receive the study drug in Advanced Life Support (ALS>) algorithm, survived with a good neurological outcome.5 Taken together, the evidence suggests that initiating ECPR as soon as logistically feasible (ideally within 30 min of cardiac arrest) provides the most favourable probability of neurologically intact survival.6,7
There are several randomised controlled trials in progress examining ECPR in patients with OHCA outlined in Table 1. Most transfer the patient to hospital prior to initiating ECPR support, a scenario that is often associated with an average interval between collapse and ECPR support of 60 min or more.8, 9, 10 After this elapsed interval of conventional CPR, the likelihood of neurologically favourable survival has already substantially declined.11 A systematic review of observational evidence suggests that a longer period of conventional resuscitation prior to initiation of ECPR is independently associated with poor clinical outcomes.6 ECPR investigators in Paris attempt to expedite ECPR by delivering it in the pre-hospital setting, avoiding the time elapsed during transfer to hospital. In this group the mean duration of CPR prior to ECMO support is still 70.9 min despite modifying their dispatch processes.9 Before embarking on comparative efficacy trials of ECPR with conventional CPR, it is imperative to understand how to most effectively deliver ECPR in a rapid fashion that minimises the interval between collapse and ECMO. We hypothesise that through a unique collaboration with pre-hospital medical services in London, the ambulance service and ECMO clinicians that it is possible to achieve ECPR within 30 min of collapse. We have demonstrated this in high fidelity simulation that has focussed on:
-
•
integration of the ECPR team with the established pre-hospital services
-
•
identification and management of human factors that influence team performance
-
•
deployment of the ECPR team as a primary resource based on screening of calls to the emergency services aiming to reach the patient within 10 min of collapse
-
•
utilisation of a low threshold for deployment, accepting that many cases may not fulfil eligibility criteria, in order to quickly be on scene for suitable patients
-
•
placement of guidewires, early, into the femoral vessels in parallel with standard resuscitation; guidewire insertion does not commit a patient to ECMO support if ROSC is achieved conventionally
Table 1.
Studies of ECMO in patients suffering out of hospital cardiac arrest recorded in trial registries.
| Study | Geographic region | Comparison | Clinicaltrials.gov identifier | Status |
|---|---|---|---|---|
| APCAR2 | Paris | Pre-hospital vs in hospital ECMO | NCT02527031 | Recruiting |
| INCEPTION | Netherlands | In hospital ECMO vs. conventional CPR | NCT03101787 | Recruiting |
| Prague OHCA | Prague | In hospital ECMO vs. conventional CPR | NCT01511666 | Recruiting |
| ECPB4OHCA | Vienna | In hospital ECMO vs. conventional CPR | NCT01605409 | Recruiting |
| ARREST | Minnesota | In hospital ECMO vs. conventional CPR | NCT03880565 | In set up |
| EROCA | Michigan | In hospital ECMO vs. conventional CPR | NCT03065647 | Recruiting |
The study presented here aims to investigate whether it is possible to deliver ECPR within 30 min of collapse in a real-life setting as a prelude to any future efficacy study.
Methods/design
Aim
The primary study aim is to test the feasibility of establishing ECMO under ECPR conditions within 30 min of collapse in six adult patients with refractory OHCA. The secondary aims are to gather preliminary data on clinical outcomes, resource utilisation, and health economics associated with ultra-rapid ECPR delivery in order to plan a subsequent clinical investigation or clinical service.
Trial setting
The study will occur in Greater London in an area served by Barts Heart Centre, London’s Air Ambulance and London Ambulance Service. This service is operated by Barts Health NHS Trust, a study site. The air ambulance is supported financially by Barts Health NHS Trust, London’s Air Ambulance Charity and London Ambulance Service NHS Trust. The area covers approximately 10 million people that live and work within the M25 orbital motorway. An additional study site will be the London Ambulance Service NHS Trust, who will dispatch the cardiac arrest team from their Emergency Operations Centre.
Trial participants
Inclusion criteria
Participants will include patients with a known or visible age of 18–65 years who:
-
•
have a witnessed out-of-hospital cardiac arrest
-
•
have a presumed cardiac aetiology to their cardiac arrest
-
•
receive bystander chest compressions within 3 min
-
•
remain in cardiac arrest at 20 min following collapse or fail to sustain ROSC in the pre-hospital setting
A presumed cardiac aetiology may be suggested by a known history of coronary artery disease, suggestive clinical symptoms preceding cardiac arrest, or the absence of evidence suggesting a non-cardiac aetiology. Outcomes in out-of-hospital cardiac arrest of non-cardiac aetiology, (for example hypoxic, neurological, traumatic aetiology) are known to be poor and lack any current significant evidence of potential ECPR benefit. Out-of-hospital cardiac arrest encompasses both those who collapse prior to notification of emergency services and those who collapse witnessed by emergency services. Bystander confirmation of cardiac arrest can be inaccurate, and we have chosen to start the 20 min from the moment of confirmed collapse and loss of consciousness. Although this does not confirm a patient has entered a full cardiac arrest state, it does confirm a loss of cerebration and potential organ hypoperfusion that our intervention seeks to redress. For the purpose of this study, patients who collapse initially whilst in transit to hospital will not be included.
Exclusion criteria
Patients will not be enrolled in the following settings:
-
•
known or visibly advanced pregnancy (when resuscitative hysterotomy will be prioritised)
-
•absent signs of life (physical movements or spontaneous respiratory effort) AND evidence of ineffective chest compressions which will be suggested by:
-
−absence of electrical activity on the electrocardiogram OR
-
−an end tidal expired carbon dioxide level of less than 1.3 kPa (10 mmHg)
-
−
-
•
evidence from others present at the scene or patient examination that ECMO is unlikely to provide long-term benefit for the patient (e.g. advanced malignancy, severe frailty, terminal comorbid condition).
Trial design
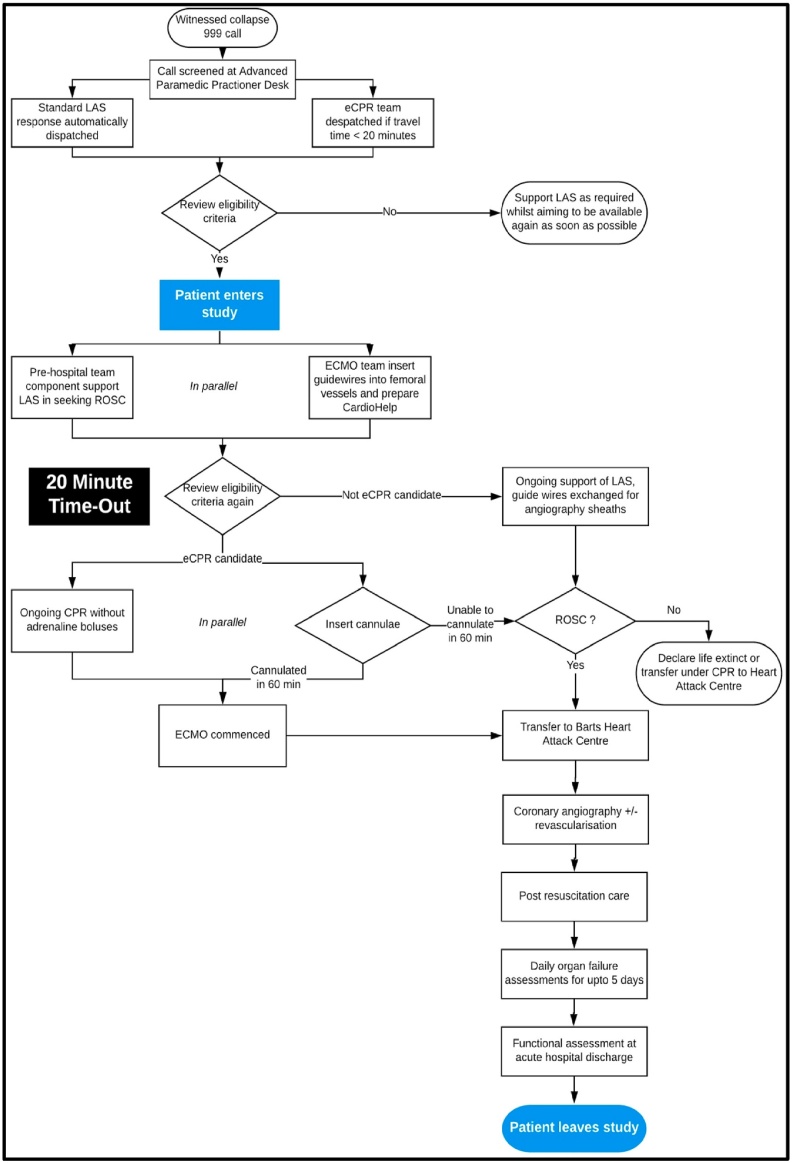
The study is an open-label, single arm feasibility study to assess the timeliness and safety of expedited ECMO delivery in a pre-hospital setting. The flowchart for the study is presented in Fig. 1.
Fig. 1.
Study flow chart.
Screening
ECMO support will be provided by an advanced cardiac arrest team that will be roaming in London in a fast response car. The study car will be dispatched to potential patients by the Advance Paramedic Practitioner desk in the Emergency Operations Centre at LAS. This desk conducts real-time screening of all calls to the emergency service that may be a cardiac arrest and dispatches additional resources to the scene. Specially trained paramedics listen to calls when indicated by initial triage questions (e.g. patient not breathing or breathing abnormally) or when requested by call handers. They utilise their clinical experience and other environmental clues (e.g. background noises of agonal breathing and caller comments) to identify potential patients in cardiac arrest. The desk will electronically dispatch the study vehicle if the estimated travel time is ideally less than 10 min but absolutely less than 20 min.
Since dispatch occurs on limited information, it is understood that the car will be dispatched to patients who will ultimately not be suitable for the study. This redundancy is expected and considered essential to identify suitable candidates in a timely manner.
Consent
Patients are unable to provide consent to enter the study at the point of since they are unconscious and in cardiac arrest. It is unlikely that a relative could be consulted to make an informed decision in the limited time available; particularly in the context of the distress of learning also that their relative has suffered a cardiac arrest. Therefore, patients will be enrolled under a waiver of consent as approved by the Research Ethics Committee. This was specifically discussed at a patient and public engagement event and was considered universally acceptable by attendees.
Valid advanced directives indicated by a Medic Alert bracelet or produced in writing will be honoured by both the ECMO study team and treating pre-hospital personnel. An advanced directive indicating a patient wishes not to receive blood products will not automatically preclude them from receiving ECMO.
Intervention
The ECMO study team will comprise one advanced paramedic, one senior physician in pre-hospital medicine, and two senior physicians in ECMO.
The advanced paramedic and consultant in pre-hospital medicine will be tasked with optimising the management of the cardiac arrest according to the UK Resuscitation Council Advanced Life Support guidelines, identifying reversible causes, managing the pre-hospital environment to ensure team safety and establishing collateral history to inform study eligibility.
The ECMO consultants will be tasked solely with preparing for and establishing ECMO support. Unless it is immediately evident that the patient would not fulfil eligibility criteria, they will prepare a sterile surgical area in the patient’s inguinal region and use real-time ultrasound guidance to insert guidewires percutaneously into the patient’s common femoral artery and femoral vein. If the patient achieves ROSC or is not suitable for the study these wires will be used to guide the insertion of arterial and central venous catheters that will be useful for the hospital care of the patient. In the context of the patient being suitable for the study then the wires will be used to guide the placement of the arterial and venous ECMO cannulae.
Twenty (20) minutes following collapse, the study inclusion and exclusion will be reviewed in a team “time out”. If a patient remains suitable for entry into the study then ECMO cannulae will be inserted. Once percutaneous dilation of the femoral vessels has commenced, the team will proceed to ECMO support, even if some form of ROSC is achieved, as many of these patients will remain in cardiogenic shock or fail to sustain this ROSC during the brief period from dilatation to cannulation. If cannulation cannot be achieved within 60 min, then further attempts will not be continued as functional outcomes are very poor after this time (4). At this time point, consideration will be given to terminate resuscitative interventions or transport the patient under on-going CPR to the nearest emergency department.
Target parameters for initial ECMO support and the on-going care of the patient on ECMO will be outlined in the Study Manual. The parameters of ECMO support will be driven by available preclinical and clinical data, endeavouring to balance restoring perfusion of the vital organs and minimising reperfusion injury. Patients will be transferred to the Heart Attack Centre at St. Bartholomew’s Hospital by the ECMO team and LAS.
Post-resuscitation care will be managed by standard institutional protocols utilised at St Bartholomew’s hospital that are consistent with best practices for post-cardiac arrest patients. These consider:
-
•
the need for coronary angiography
-
•
culprit vessel only revascularisation
-
•
anti-platelet therapy following percutaneous coronary intervention
-
•
use of intra-aortic balloon counter-pulsation or Impella
-
•
use of therapeutic hypothermia (initially using an external cooling jacket [CAERvest, BodyChillz Ltd, UK] and once in hospital, aiming to maintain the core temperature at 36 °C for 24 h and 37 °C for a subsequent 48 h)
-
•
haemodynamic management on ECMO
-
•
management of distal leg perfusion
-
•
anticoagulation management
-
•
weaning from VA-ECMO
-
•
neurological prognostication
-
•
general intensive care unit care
Primary outcome measure
The primary endpoint is the proportion of patients successfully established with pre-hospital ECPR within 30 min of collapse. The time of collapse will be defined as the time the call to the emergency services commences or the time that a patient was witnessed to suffer a cardiac arrest if this happens in the presence of the emergency services.
Secondary outcomes
The following secondary endpoints will be measured:
-
•
a complete timeline of pre-hospital events and milestones
-
•
number of patients not dispatched to as travel time too great/team unavailable
-
•
number of patients in whom it is attempted to start inserting guidewires but who do not meet study inclusion criteria at 20 min time out.
-
•
the number of patients successfully cannulated between 31 and 45 min
-
•
the number of patients successfully cannulated between 46 and 60 min
-
•
the proportion of patients who achieve ROSC prior to the 20 min timeout
-
•
the number of patients in refractory cardiac arrest at 20 min in whom ROSC is achieved prior to ECMO flow
-
•
the time interval between call to the emergency services and ECPR team arrival
-
•
the proportion of potentially supportable patients in whom guidewire placement is attempted
-
•
the organ dysfunction during the first five days of hospital stay
-
•
the survival to hospital discharge and 90 days
-
•
the neurological outcome at hospital discharge and 90 days (Cerebral Performance Category and modified Rankin scales)
-
•
the incidence of ECPR-related complications (failure to cannulate, vascular injury, site infection and distal leg ischaemia)
Details about resources used and health economic data will be collected to estimate the range of costs and affordability of any subsequent clinical trial or clinical service. These data will include:
-
•
ECMO equipment used
-
•
duration of ECMO support
-
•
duration of intensive care unit stay
-
•
maximum organ support on the Intensive Care Unit (ICU)
-
•
duration of acute hospital stay
-
•
duration of inpatient rehabilitation following acute hospital stay
Trial assessments
Assessments in the study are outlined in Table 2.
Table 2.
Assessments made in the study.
 |
Data collection and management
Pre-hospital data will be captured on a Case Report Form (CRF), which will be transferred to REDCap, a web-based electronic database which is hosted securely at Queen Mary University of London (QMUL). All patients, to whom the team are dispatched, will be recorded in the database. Hospital and follow-up data will be collected directly into REDCap on electronic CRFs. Copies of all source data will be stored manually. Only users approved by the Chief Investigator will have access to REDCap, and each user will be assigned specific user roles and rights. The Chief Investigator will have overall responsibility for data captured in the eCRF and be able to review, lock and electronically sign the completed eCRFs.
Statistical considerations
This is a single arm pilot study and no power calculation was undertaken. The sample size of six patients was selected as this felt to be sufficient to answer our primary question of whether the intervention could feasibly be delivered in a timely manner.
Clinical outcomes will be compared to a concurrent control group of patients who were routinely managed by London Ambulance Service on non-study days. These data are stored in a bespoke database. Only patients treated by the LAS over the duration of the SUB30 STUDY will be used in this comparative analysis. Patients will be matched based on their likelihood of receiving ECMO. A priori factors that will estimate this likelihood are:
-
•
Age (closest match to 5 years)
-
•
Sex
-
•
Occurrence of a witnessed cardiac arrest
-
•
Occurrence of bystander CPR
-
•
Initial cardiac rhythm
-
•
Elapsed interval to first LAS resource attendance on scene (closest match to 5 min)
-
•
Presumption of a cardiac aetiology to the arrest
-
•
No ROSC within 20 min of LAS attendance
Discussion
The Sub30 study presented a number of challenges that were considered during its inception and design.
Research governance
In the United Kingdom, research that does not involve an Investigation Medicinal Product is not governed by law. Nevertheless it is expected that best research practice should be maintained. Cognisant that participant mortality is likely to be high due to the severity of the underlying disease state and that adverse events may occur when applying a complex intervention in a challenging pre-hospital setting, the investigators elected to manage the study as if it fell within the higher degree of oversight defined in the UK Medicines for Human Use (Clinical Trials) Regulations 2004.
An independent Data Safety Monitoring Committee (DSMC) has been convened. The DSMC consists of three members expert in clinical risk, interventional cardiology, ECMO and intensive care medicine. The DSMC will review adverse events reported during this study and meet after the enrolment of every second patient. Adverse events will be defined in accordance with the Clinical Trial Regulation (EU No. 536/2014). Adverse events can be reported using trial proforma, emails to the trial team, use of the standard patient safety incident reporting systems at the study sites or anonymously to the investigators. The Chief and Principal investigators, and designees, will collate these reports in an electronic AE, hosted on the trial database. They will assess all AEs for seriousness, causality and expectedness. Expedited reporting to the Sponsor, Ethics Committee and DSMC is required for all adverse events that are serious, related to the intervention and unexpected.
A Trial Steering Committee (TSC) has been convened. The TSC consists of experts in resuscitation, clinical trials and advanced heart failure requiring mechanical support. They are supported by a patient and public representative. They will advise the investigators about the study following reports from the DSMC, trial statistician and a review of any new published data about the use of ECMO in out of hospital cardiac arrest.
The study will be monitored by the Cardiovascular Clinical Trials Unit. Monitoring will include source data verification in addition to assessing compliance with the protocol, Good Clinical Practice and trial Standard Operating Procedures. The study will be monitored after the enrolment of every second patient.
Design of the intervention
ECMO is usually instituted in an operating theatre or fluoroscopy room. Initiating ECMO in a pre-hospital environment is difficult due to the limited equipment available; environmental factors such as weather and ambient light; limited space; and family members and the public in the immediate vicinity. In the pre-hospital setting the ECMO team is likely to be working with other healthcare professionals that they have not met previously and undertaking a procedure that others are unfamiliar with. These and other human factors will influence whether the team can deliver the intervention in a time and safely manner.
We have spent three years repeatedly using high fidelity pre-hospital simulation with many other healthcare providers and in multiple scenarios to learn how to manage many of these adverse factors. We are preparing another manuscript to describe how repeated simulation and debrief can optimise clinical pathways before a patient is exposed to an intervention. Nevertheless, we believe this study provided unique challenges in training the team to deliver the intervention.
Ethical considerations
The study provided some specific ethical challenges that we shared with the Research Ethics Committee and Sponsor. These considerations included:
-
•
Emergency enrolment of patients who lack the capacity to provide consent. This has been discussed above and approval to waiver consent at enrolment was granted by the Research Ethics Committee.
-
•
Generalisability of the results to other geographical areas. The study is being undertaken in an urban area that has many patients suffering cardiac arrest and a well-established medicalised pre-hospital care system. Patient access to pre-hospital ECMO systems is likely to differ in other areas. This parallels the different provisions in rural areas for patient access to major trauma centres or emergency primary coronary intervention.
-
•
Potential redundancy of a highly trained team and service for a limited number of patients. This study provides a resource heavy team to attend a limited number of patients in cardiac arrest. If feasibility of the intervention is demonstrated, the design of a larger outcome study would include consideration of the health economics and potential additional patient cohorts that could benefit from such a service (such as patients in acute cardiogenic shock in emergency departments without access to rapid mechanical support).
-
•
ECMO may increase survival but with neurological injury. It is possible that ECMO may restore blood flow to organs such that the patient can survive, but they will have already suffered a significant hypoxic brain injury. We are aware of this risk and feel this underpins the need to do a well conducted study in this setting. Fortunately, this has seldom been the case in other reported cohorts of ECPR.10,12
-
•
Pre-hospital ECMO has the potential to impede standard Advanced Life Support algorithms. The training in the study has highlighted the need to optimise standard resuscitation as the foundation for improving clinical outcomes. Indeed, two members of the study team are specifically tasked with optimising Advanced Life Support and seeking ROSC before ECPR is initiated.
Trial status
Protocol version 3.4 (August 14, 2019).
Recruitment commenced on September 23, 2019 and was suspended half way through recruitment due to Covid-19. The second half of recruitment is expected to commence October 2020.
Ethical approval and consent to participate
The study received NHS Research ethics approval from the London – Harrow committee (19/LO/0035), Confidential Advisory Group approval (19/CAG/0024) and Health Research Authority approval. A waiver for consent was granted at the point of patient enrolment. All patients who regain mental capacity will be approached and consent requested for their continued participation in the study.
Consent for publication
Not applicable.
Sponsor
This study is sponsored by Barts Health Joint Research Management Office, Queen Mary Innovation Centre, 5 Walden Street, London E1 2 E F.
Availability of data and material
The dataset and samples created by this study will be available from the Chief Investigator on reasonable request and appropriate regulatory approvals.
Funding
The study is funded by Barts Charity and London’s Air Ambulance Charity. Maquet Cardiopulmonary GmbH have provided free of charge cannulae and ECMO disposables to support the study. Stryker have provided a loan Lucas 3.0 and Lifepak 15. BodyChillz Ltd have provided cooling CAERvests. Neither Barts Charity, London’s Air Ambulance Charity or Maquet Cardiopulmonary GmbH, Stryker or BodyChillz influenced the study design.
Authors’ contributions
SF and BS drafted the manuscript. All authors contributed to the design of the study, commented on the draft manuscript and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the support of the Joint Research Management Office, Barts Health NHS Trust as the Sponsor of the study. The study is managed and run by the Barts Cardiovascular Clinical Trials Unit a branch of the Barts Clinical Trials Unit (UKCRN registered unit identifier: 4). We also acknowledge the support from the members of the Trial Steering Committee (Prof Gavin Perkins [University of Warwick]; Dr Stephen Pettit [Royal Papworth Hospital]; Mr David Jeffrey [Patient representative]) and the Data Safety Monitoring Committee (Dr Elizabeth Haxby [Royal Brompton Hospital]; Prof Anthony Gershlick [University of Leicester]; Dr Georg Auzinger [Kings College Hospital]). We are grateful for support and endorsement of ECMONet.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2020.100029.
Contributor Information
Ben Singer, Email: b.singer@nhs.net.
Joshua C. Reynolds, Email: reyno406@msu.edu.
Gareth E. Davies, Email: gareth.davies5@nhs.net.
Fenella Wrigley, Email: Fenella.wrigley@lond-amb.nhs.uk.
Mark Faulkner, Email: mark.faulkner@lond-amb.nhs.uk.
Ben O’Brien, Email: ben.obrien@nhs.net.
Alastair G. Proudfoot, Email: alastair.proudfoot1@nhs.net.
Anthony Mathur, Email: a.mathur@qmul.ac.uk.
Thomas Evens, Email: tomevens@doctors.org.uk.
Jane Field, Email: field.jane@gmail.com.
Vivienne Monk, Email: v.monk@qmul.ac.uk.
Simon J. Finney, Email: simon.finney2@nhs.net.
List of abbreviations
- DSMC
Data Safety and Monitoring Committee
- ECMO
Extracorporeal Membrane Oxygenation
- ECPR
Extracorporeal Cardio-pulmonary Resuscitation
- LAS
London Ambulance Service
- OHCA
Out of Hospital Cardiac Arrest
- ROSC
Return of Spontaneous Circulation
- TSC
Trial Steering Committee
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.British Heart Foundation . 2015. Consensus paper on out-of-hospital cardiac arrest in England. (Revised 16 October 2015) [Google Scholar]
- 2.2018. London Ambulance Service NHS Trust Cardiac Arrest Annual Report. 2017/18. [Google Scholar]
- 3.Kapur N.K., Davila C.D. Timing, timing, timing: the emerging concept of the ’door to support’ time for cardiogenic shock. Eur Heart J. 2017;38(47):3532–3534. doi: 10.1093/eurheartj/ehx406. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds J.C., Grunau B.E., Elmer J. Prevalence, natural history, and time-dependent outcomes of a multi-center North American cohort of out-of-hospital cardiac arrest extracorporeal CPR candidates. Resuscitation. 2017;117:24–31. doi: 10.1016/j.resuscitation.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Perkins G.D., Chen J., Deakin C. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med. 2018;379(8):711–721. doi: 10.1056/NEJMoa1806842. [DOI] [PubMed] [Google Scholar]
- 6.Debaty G., Babz V., Durand M. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation. 2017;112:1–10. doi: 10.1016/j.resuscitation.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Fagnoul D., Combes A., De Backer D. Extracorporeal cardiopulmonary resuscitation. Curr Opin Crit Care. 2014;20(3):259–265. doi: 10.1097/MCC.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 8.Belohlavek J., Smid O., Franek O. Hyperinvasive approach prolongs the time window for favorable outcomes in refractory out-of-hospital cardiac arrest: a preliminary analysis of the “Prague OHCA Study”. Resuscitation. 2016;106:e18. [Google Scholar]
- 9.Lamhaut L., Hutin A., Puymirat E. A Pre-Hospital Extracorporeal Cardio Pulmonary Resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: an observational study and propensity analysis. Resuscitation. 2017;117:109–117. doi: 10.1016/j.resuscitation.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Yannopoulos D., Bartos J.A., Martin C. Minnesota resuscitation consortium’s advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J Am Heart Assoc. 2016;5(6) doi: 10.1161/JAHA.116.003732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wengenmayer T., Rombach S., Ramshorn F. Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR) Crit Care. 2017;21(1):157. doi: 10.1186/s13054-017-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia S., Drexel T., Bekwelem W. Early access to the cardiac catheterization laboratory for patients resuscitated from cardiac arrest due to a shockable rhythm: the Minnesota resuscitation consortium twin cities unified protocol. J Am Heart Assoc. 2016;5(1) doi: 10.1161/JAHA.115.002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset and samples created by this study will be available from the Chief Investigator on reasonable request and appropriate regulatory approvals.