Clinical Implications.
-
•
The clinical impact of coronavirus disease 2019 in primary immunodeficiency diseases varies from mild illness to death. In our center, humoral immunodeficiency patients with poor outcomes had preexisting autoimmune/inflammatory complications, lung disease, or additional comorbidities, and exhibited higher proinflammatory responses (IL-6, D-dimer).
Coronavirus disease 2019 (COVID-19) remains an ongoing pandemic, and data on the clinical impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with primary immunodeficiency diseases (PIDs) are limited.1, 2, 3 In Spring of 2020, New York City became the epicenter of the SARS-CoV-2 pandemic. Here, we report the clinical features and outcomes of COVID-19 in patients from a large PID center in New York City during this period.
Between January and July 2020, 16 patients followed at the Mount Sinai Hospital PID clinic tested positive for SARS-CoV-2. Of these, 12 were confirmed to have COVID-19 by nucleic acid amplification from nasopharyngeal/oropharyngeal swab specimens and 4 by serologic assay.4 Table I summarizes the demographic characteristics, PID diagnoses, and related comorbidities of patients in the cohort. Five of the 16 patients were female, and the median age was 44.5 years (interquartile range, 28-64 years). Nine patients had common variable immunodeficiency (CVID; nuclear factor kappa B subunit 1, n = 2; signal transducer and activator of transcription 3 gain-of-function, n = 1) and 3 patients had X-linked agammaglobulinemia (XLA) due to Bruton tyrosine kinase (BTK) mutations. Other PID diagnoses in the cohort included hypogammaglobulinemia, IgA-IgG2 deficiency, IFN-γ receptor 2 (IFNGR2) deficiency, and X-linked hyper-IgM syndrome (XHIGM) (n = 1 each). Seven patients had preexisting PID-associated autoimmune/inflammatory complications.
Table I.
Demographic characteristics, PID history, and disease characteristics of patients with PID presenting with confirmed COVID-19
| ID | Age (y) | Sex | Immune deficiency | PID-associated comorbidities | Comorbidities | Presenting symptoms | Presenting vital sign abnormalities | Duration (d) | CXR abnormalities | Coinfection(s) | Highest level of care | Treatment(s) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 82 | M | CVID | None | DM type 2, CAD | Cough, subjective fever, diarrhea | None | 30 | Yes | None | Not admitted | HCQ, azithromycin | Recovered |
| 2 | 61 | F | CVID (NFKB1) | None | None | Cough, subjective fever, chills, fatigue, weakness | Fever, hypoxia | 19 | Yes | None | Hospital ward (standard nasal cannula) | HCQ, azithromycin | Recovered |
| 3 | 38 | M | CVID (NFKB1) | Enteropathy | None | Cough, dyspnea, subjective fever, chills, weakness | Tachycardia | 24 | Yes | None | Hospital ward (standard nasal cannula) | HCQ, azithromycin | Recovered |
| 4 | 65 | F | CVID | None | OSA | Cough, dyspnea, subjective fever, fatigue, weakness | Hypoxia | 41 | Yes | None | Hospital ward (standard nasal cannula) | HCQ, azithromycin, convalescent plasma | Recovered |
| 5 | 38 | M | CVID (STAT3 GOF) | GLD, AIHA, ITP, lymphadenopathy | None | Cough, subjective fever, fatigue, headache | Fever | 6 | NA | None | Not admitted | None | Recovered |
| 6 | 49 | M | CVID | Granulomas, bronchiectasis | None | Cough, subjective fever | NA | 10 | Yes | None | Not admitted | None | Recovered |
| 7 | 56 | M | CVID | None | None | Cough, dyspnea, subjective fever, fatigue | NA | 21 | NA | None | Not admitted | HCQ, azithromycin | Recovered |
| 8 | 54 | F | CVID | ITP, bronchiectasis | None | Cough, dyspnea | Hypoxia | 29 | Yes | None | ICU (mechanical ventilation) | HCQ, azithromycin, steroid | Died |
| 9 | 76 | F | CVID | ILD | CKD, DM type 2, CAD | Cough, dyspnea, subjective fever, AMS, emesis, diarrhea | Fever, hypoxia | 16 | Yes | None | ICU (mechanical ventilation) | HCQ, azithromycin, steroid, investigational agent | Died |
| 10 | 39 | F | Hypogammaglobulinemia | None | Kidney transplant, h/o lymphoma | Cough, subjective fever, chills, myalgia, abdominal pain, fatigue, weakness | Fever, tachycardia, hypotension | 36 | Yes | Campylobacter enteritis | ICU (mechanical ventilation) | HCQ, azithromycin, convalescent plasma, steroid | Died |
| 11 | 75 | M | IgA-IgG2 deficiency | AIHA | DM type 2 | Cough, dyspnea, subjective fever, diarrhea | Hypoxia | 32 | Yes | None | ICU (mechanical ventilation) | HCQ, azithromycin | Died |
| 12 | 40 | M | XLA | None | None | Cough, dyspnea, subjective fever, weakness | Hypoxia | 34 | Yes | None | Hospital ward (standard nasal cannula) | Azithromycin, convalescent plasma | Recovered |
| 13 | 24 | M | XLA | Bronchiectasis | None | Cough, dyspnea, subjective fever, diarrhea | Fever | 31 | Yes | None | Hospital ward (no O2 support) | Azithromycin, convalescent plasma | Recovered |
| 14 | 10 | M | XLA | None | None | Cough, subjective fever, diarrhea, emesis, chest pain | None | 31 | Yes | None | Hospital ward (standard nasal cannula) | Convalescent plasma, investigational agent | Recovered |
| 15 | 21 | M | XHIGM | None | None | Cough, subjective fever, stomatitis, oral ulcers, diarrhea | Fever | 50 | No | Oral candidiasis | Hospital ward (no O2 support) | Steroids | Recovered |
| 16 | 1 | M | IFNGR2 Deficiency | None | None | Cough, subjective fever, diarrhea | Fever, tachypenia | Unknown | Yes | MAC | ICU (mechanical ventilation) | Steroids | Recovered |
AIHA, Autoimmune hemolytic anemia; DM, diabetes mellitus; CAD, coronary artery disease; CKD, chronic kidney disease; CXR, chest x-ray; F, female; GLD, granulomatous lung disease; HCQ, hydroxychloroquine; ICU, intensive care unit; ITP, immune thrombocytopenic purpura; M, male; MAC, mycobacterium avium complex; NA, not applicable/available; NFKB1, nuclear factor kappa B subunit 1; OSA, obstructive sleep apnea; STAT3 GOF, signal transducer and activator of transcription 3 gain-of-function.
COVID-19 was diagnosed in 12 patients by nucleic acid amplification from nasopharyngeal/oropharyngeal swab specimens and in 4 patients (patient 6, 7, 15, and 16) by serologic assay.
Table I presents the clinical parameters and course of COVID-19 in the study cohort. The most commonly recorded symptoms were cough (16 of 16), subjective fever (15 of 16), dyspnea (8 of 16), diarrhea (7 of 16), fatigue/weakness (7 of 16), and emesis (2 of 16). One patient presented with altered mental status as the primary complaint. One patient with XHIGM experienced fever, cough, severe oral ulcers, and stomatitis during the associated clinical course. At initial presentation to a health care setting, fever (>38°C) was recorded in 7 patients, hypoxia (Spo2 <92%) was recorded in 6 patients, and hypotension (systolic blood pressure <90 mm Hg) was recorded in 1 patient. The median duration of symptoms (time from symptom onset to resolution or death) was 29 days (interquartile range, 18-33 days). Chest X-ray on presentation was abnormal in 13 of 14 cases in which data were available. Co-infections identified during the COVID-19 hospitalization included Campylobacter enteritis in a patient with hypogammaglobulinemia, Mycobacterium avium complex lung disease in a patient with IFNGR2 deficiency, and oral candidiasis in a patient with XHIGM.
Twelve of 16 patients required hospitalization, 5 of which involved intensive care unit–level care. Oxygen supplementation was needed in 10 of 16 patients (standard nasal cannula, n = 5; mechanical ventilation, n = 5). Nine patients received hydroxychloroquine, 11 patients received azithromycin, and 5 patients received steroids during the treatment course. In addition, 5 patients received convalescent plasma under expanded access protocols. Two patients received investigational agents under clinical trials.
In all, 4 of 16 individuals died (CVID, n = 2; hypogammaglobulinemia, n = 1; IgA-IgG2 deficiency, n = 1) and 12 of 16 individuals recovered from COVID-19. Three of the 4 patients who died had preexisting PID-associated autoimmune/inflammatory complications; 2 of these individuals also had preexisting PID-associated chronic lung disease (bronchiectasis, n = 1; interstitial lung disease, n = 1). In addition, 1 patient was a kidney transplant recipient. The age of those who died ranged from 39 to 76 years.
Two of 9 patients with CVID required mechanical ventilation. Although all 3 patients with XLA in this series did not require mechanical ventilation and all recovered, the significance of this observation is difficult to interpret given the limited number of cases, the relatively young age of these individuals, and the utilization of convalescent plasma.
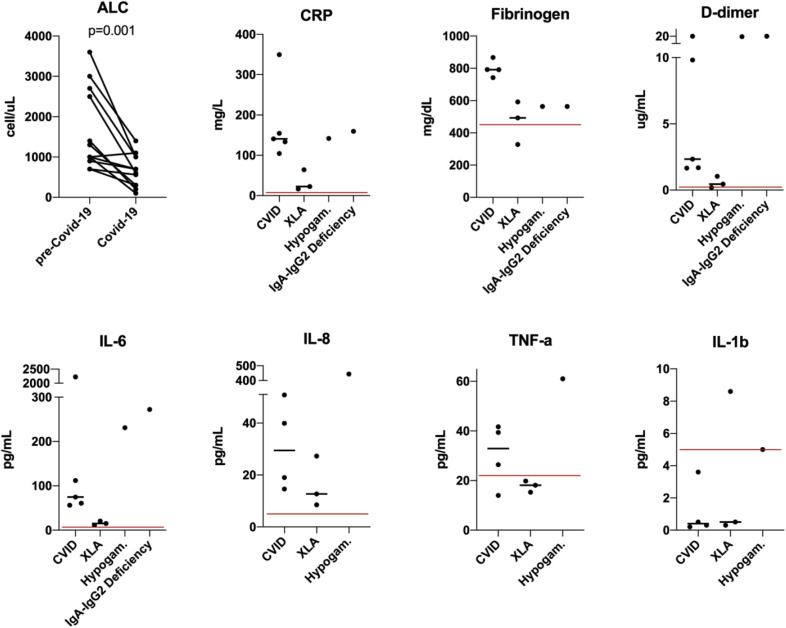
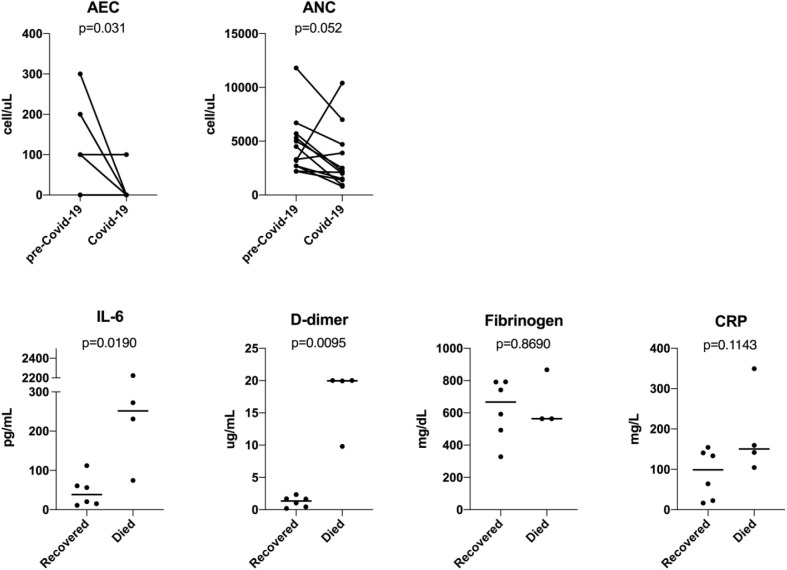
Figure 1 and Table E1 in this article's Online Repository at www.jaci-inpractice.org show immune parameters and inflammatory profiles of COVID-19 among antibody-deficient individuals. In patients with paired data from before and during the COVID-19 presentation, we noted significant declines in total lymphocyte count (median 1150 vs 650 cells/μL, P = .001, n = 12; lymphopenia <1000 cells/μL, n = 8) and eosinophil count (median 100 vs 0 cells/μL, P = .031, n = 12; see Figure E1 in this article's Online Repository at www.jaci-inpractice.org) during COVID-19. There was a trend toward lower neutrophil count (median 3900 vs 2150 cell/μL, P = .052, n = 12; neutropenia <1000 cells/μL, n = 2) during COVID-19 (Figure E1). Lymphopenia in COVID-19 has been associated with disease severity in patients without PIDs.5 Three of 4 individuals who died in this cohort had lymphopenia (100, 300, and 700 cells/μL). There was no statistically significant difference in nadir lymphocyte count between those who died and those who recovered (median 500 vs 850 cells/μL, respectively).
Figure 1.
Immune parameters and inflammatory profile of COVID-19 in antibody-deficient patients. Changes in absolute lymphocyte counts in patients with paired data are shown (n = 12, P value calculated using Wilcoxon matched-pairs signed rank test). Peak inflammatory markers and cytokine profiles during COVID-19 are also shown. ALC, Absolute lymphocyte count; Hypogam., hypogammaglobulinemia. Black solid lines reflect medians for the group, red lines reflect upper limits of normal for individual tests.
Figure E1.
Absolute eosinophil counts and absolute neutrophil counts of COVID-19 in antibody-deficient subjects with paired data (n = 12; P value calculated using Wilcoxon matched-pairs signed rank test). Comparison of peak IL-6, D-dimer, fibrinogen, and CRP between those who died and those who recovered. P value calculated using Mann-Whitney test. AEC, Absolute eosinophil count; ANC, absolute neutrophil count.
In individuals with humoral immunodeficiency with available data, systemic inflammatory markers were commonly elevated (10 of 10 patients had elevated C-reactive protein [CRP], 8 of 9 had elevated fibrinogen, 8 of 10 had elevated D-dimer). Similarly, peak serum IL-6 and IL-8 were commonly elevated (10 of 10 patients for IL-6 and 8 of 8 patients for IL-8). Serum TNF-α was elevated in 4 of 8 patients, whereas IL-1b was not commonly elevated (1 of 8 patients). Inflammatory markers including IL-6 and D-dimer were significantly higher in those who died compared with those who recovered (P = .0190 and .0095, respectively; Figure E1), whereas no significant differences were observed for CRP and fibrinogen (insufficient data points were available for meaningful comparison of IL8, TNF-α, and IL-1b).
BTK protein is involved in the signaling of the viral ssRNA-sensing Toll-like receptor pathway.6 Indeed, increased monocyte BTK activation has been shown during severe COVID-19, with the application of BTK inhibitors leading to reduced measures of inflammation and protection against pulmonary injury in initial studies.6 , 7 Here, we observed reduced measures of inflammation (CRP, fibrinogen, D-dimer), IL-6, IL-8, and TNF-α in individuals with XLA as compared with patients with CVID and other antibody-deficient patients during SARS-CoV-2 infection (Figure 1). However, this observation should be interpreted with caution given the limited number of cases, relatively young age of patients with XLA in this series, and the greater numbers of features associated with increased morbidity in the subjects with CVID.
Three patients demonstrated detectable serum SARS-CoV-2–specific IgG (CVID, n = 2; IFNGR2 deficiency, n = 1; patients 6 and 7 via qualitative immunoassays conducted at Clinical Laboratory Improvement Amendments–certified laboratories; patient 16 via ELISA as previously described,4 with anti–spike protein IgG titer of 1:960), which was not found in commercial immunoglobulin replacement products during the study period.4 In addition, 1 patient with XHIGM (patient 15) had detectable serum SARS-CoV-2 spike protein–specific IgM (titer 1:80) via established ELISA.4
In summary, the clinical impact of COVID-19 in PIDs varies from mild symptoms to death. The proportion of deaths in this series (25%) was greater than that in the general population with COVID-19 reported at New York City hospitals (10.2%),8 and similar to outcomes data reported in the kidney transplant population (28%).9 In this single-center experience, those who died had preexisting PID-associated or other comorbidities. Profound systemic inflammatory responses were evident in many antibody-deficient individuals during SARS-CoV-2 infection. Some patients with primary antibody defects were able to produce detectable SARS-CoV-2–specific humoral responses, though the duration and clinical significance of these responses are unknown, and the use of serology testing should not be universally relied upon for diagnosis in patients with humoral or combined immunodeficiency. Investigations of individuals with severe COVID-19 have recently revealed novel inborn errors of immunity10; studies of PIDs may provide further mechanistic clues to understand the pathophysiology of this illness. A multinational registry, previously established by the International Union of Immunology Sciences (https://iuis.org), will be crucial for understanding the full clinical and immunological impact of COVID-19 in PIDs.
Footnotes
Conflicts of interest: The authors report no conflicts of interest related to the work presented.
Online Repository
Table E1.
Immune parameters and inflammatory profile of COVID-19 in antibody-deficient subjects
| ID | Age (y) | Sex | Immune deficiency | ALC (pre-COVID) | ALC (nadir) | ANC (pre-COVID) | ANC (nadir) | AEC (pre-COVID) | AEC (nadir) | Peak CRP | Peak fibrinogen | Peak D-dimer | Peak IL-6 | Peak IL-8 | Peak TNF-α | Peak IL-1b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 82 | M | CVID | 500 | — | 4,600 | — | 100 | — | — | — | — | — | — | — | — |
| 2 | 61 | F | CVID (NFKB1) | 1,400 | 200 | 2,700 | 1,500 | 200 | 0 | 133.6 | 742 | 1.66 | 112 | 50.8 | 41.7 | 3.6 |
| 3 | 38 | M | CVID (NFKB1) | 700 | 300 | 2,200 | 1,400 | 0 | 0 | 154.4 | 792 | 1.69 | 60.7 | 19 | 14 | 0.5 |
| 4 | 65 | F | CVID | 3,000 | 1,400 | 3,300 | 3,900 | 100 | 0 | 140.8 | 791 | 2.34 | 56.3 | 14.6 | 26.4 | <0.3 |
| 5 | 38 | M | CVID (STAT3 GOF) | 700 | 560 | 2,200 | 2,100 | 100 | 0 | — | — | — | — | — | — | — |
| 6 | 49 | M | CVID | 1,000 | — | 1,200 | — | 0 | — | — | — | — | — | — | — | — |
| 7 | 56 | M | CVID | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 8 | 54 | F | CVID | 3,600 | 1,000 | 11,800 | 7,000 | 300 | 0 | 104.5 | 867 | 9.81 | 74.6 | 39.9 | 39.4 | 0.2 |
| 9 | 76 | F | CVID | 900 | 700 | 6,700 | 4,700 | 0 | 0 | 349.38 | — | >20 | 2223.4 | — | — | — |
| 10 | 39 | F | Hypogammaglobulinemia | 1,300 | 300 | 5,700 | 2,200 | 0 | 0 | 141.9 | 564 | 19.95 | 231 | 444 | 61 | 5 |
| 11 | 75 | M | IgA-IgG2 deficiency | 1,000 | 100 | 3,200 | 10,400 | 0 | 0 | 159.4 | 564 | >20 | 272.3 | — | — | — |
| 12 | 40 | M | XLA | 1,000 | 1,100 | 5,000 | 2,500 | 100 | 100 | 16.4 | 592 | 0.45 | 15.1 | 8.5 | 15.3 | 0.5 |
| 13 | 24 | M | XLA | 2,700 | 1,000 | 4,500 | 900 | 200 | 0 | 64 | 493 | 1.04 | 20.5 | 27.3 | 18.1 | 8.6 |
| 14 | 10 | M | XLA | 2,500 | 600 | 5,300 | 2,000 | 200 | 0 | 22.4 | 328 | <0.2 | 11.1 | 12.7 | 19.8 | <0.3 |
| 15 | 21 | M | XHIGM | 1,000 | 700 | 2,700 | 800 | 0 | 0 | — | — | — | — | — | — | — |
| 16 | 1 | M | IFN-γ receptor 2 deficiency | — | 6,800 | — | 9,100 | — | 100 | — | — | — | — | — | — | — |
AEC, Absolute eosinophil count; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; F, female; M, male.
Bolded number reflects abnormal values outside the limits of normal for each individual test.
References
- 1.Quinti I., Lougaris V., Milito C., Cinetto F., Pecoraro A., Mezzaroma I. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146:211–213.e4. doi: 10.1016/j.jaci.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mira E., Yarce O.A., Ortega C., Fernandez S., Pascual N.M., Gomez C. Rapid recovery of a SARS-CoV-2-infected X-linked agammaglobulinemia patient after infusion of COVID-19 convalescent plasma. J Allergy Clin Immunol Pract. 2020;8:2793–2795. doi: 10.1016/j.jaip.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soresina A., Moratto D., Chiarini M., Paolillo C., Baresi G., Foca E. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol. 2020;31:565–569. doi: 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.Q. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roschewski M., Lionakis M.S., Sharman J.P., Roswarski J., Goy A., Monticelli M.A. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treon S.P., Castillo J.J., Skarbnik A.P., Soumerai J.D., Ghobrial I.M., Guerrera M.L. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135:1912–1915. doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akalin E., Azzi Y., Bartash R., Seethamraju H., Parides M., Hemmige V. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]