Abstract
Background and Aims
Elective endoscopy resumed in our outpatient ambulatory center after instituting the preprocedure policy of a confirmed negative coronavirus disease 2019 (COVID-19) reverse transcriptase polymerase chain reaction (RT-PCR) status performed 72 hours before a scheduled procedure as mandated by the state of Illinois. In addition, all patients were required to contemporaneously complete the American Society for Gastrointestinal Endoscopy (ASGE) COVID-19 risk screening questionnaire published April 28, 2020 as outlined in the ASGE guidance document for reopening GI endoscopy during the COVID-19 pandemic. The aim of our study is to report the outcomes of 1000 patients who successfully completed the clinical aspects of the ASGE COVID-19 risk screening questionnaire and whose RT-PCR tests were valid for interpretation.
Methods
Data were retrospectively collected from patient medical records for demographics, symptom responses to the preprocedure ASGE COVID-19 risk screening questionnaire, and RT-PCR test results of patients scheduled to undergo an elective outpatient endoscopy at Rockford Gastroenterology Associates from May 22 through June 28, 2020. Descriptive statistics and standard calculation methods to determine both positive and negative predictive values were used for data analysis.
Results
Eight of 1000 patients included in the study tested positive for COVID-19. Three of 8 patients reported 1 or more symptoms on the ASGE COVID-19 risk screening questionnaire. One hundred nineteen additional patients reported symptoms on the ASGE COVID-19 risk screening questionnaire but tested negative for COVID-19. The positive and negative predictive values of the ASGE COVID-19 risk screening questionnaire were 2.46% and 99.43%, respectively.
Conclusions
The low incidence of COVID-19 infection in a community-based ambulatory surgery center is supported by a positive RT-PCR test rate of .80%. Absence of symptoms on the ASGE COVID-19 risk screening questionnaire was highly predictive of a negative RT-PCR test (99.43% negative predictive value), whereas the positive predictive value was low (2.46%) in symptomatic patients. A positive RT-PCR test was invaluable in preventing 5 asymptomatic patients from undergoing endoscopy. Similarly, 119 symptomatic patients underwent endoscopic evaluation who would have otherwise been excluded without RT-PCR testing. Symptom-based screening alone should not be the primary preprocedural assessment tool in selecting patients for undergoing endoscopy during the COVID-19 pandemic.
Abbreviations: ASGE, American Society for Gastrointestinal Endoscopy; COVID-19, coronavirus disease 2019; RGA, Rockford Gastroenterology Associates; RSQ, Risk Screening Questionnaire; RT-PCR, reverse transcriptase polymerase chain reaction
During the height of the coronavirus disease 2019 (COVID-19) pandemic, a joint statement by the U.S. gastroenterology professional societies recommended performing only those endoscopic procedures that were deemed urgent or emergent.1 This was done as part of the public health response to mitigate infection spread by diverting resources to unburden the supply chain for healthcare delivery systems.
In the state of Illinois, elective endoscopic procedures could begin on May 11, 2020 provided the facility was in compliance with the April 24, 2020 Illinois Department of Public Health’s guideline of self-quarantine and confirmed negative status of a COVID-19 reverse transcriptase polymerase chain reaction (RT-PCR) 72 hours before the scheduled procedure.2 On April 28, 2020, the American Society for Gastrointestinal Endoscopy (ASGE) recommended adopting a preprocedural COVID-19 risk screening questionnaire but did not endorse preprocedural COVID-19 testing until the assays were standardized, validated, and widely available.3 Rockford Gastroenterology Associates (RGA) developed stringent policies to meet the Illinois Department of Public Health’s mandate, including adoption of the ASGE preprocedure COVID-19 risk screening questionnaire (Appendix 1, available online at www.giejournal.org). Inadequate local resources for high-volume, rapid RT-PCR test results prompted our development of an onsite outdoor testing facility limited to RGA patients to meet the requirements set forth by the Illinois Department of Public Health.
Methods
The study protocol was designed as a retrospective review of existing records from patients within our practice who were ages 18 to 85 years and scheduled to undergo an endoscopic procedure from May 22 through June 28, 2020. To be included in this study, patients must have fulfilled the inclusion criteria of responses to the presence or absence of symptoms contained in the ASGE preprocedure COVID-19 Risk Screening Questionnaire (RSQ) as well as an RGA onsite 72-hour preprocedure real-time nasopharyngeal RT-PCR test result that was valid for interpretation. Patients with invalid RT-PCR test results, such as insufficient quantity of specimen, were removed from this study.
Patient demographics for age, race, gender, and pertinent history pertaining to procedural indication and risks were extracted from the medical record. The protocol was reviewed and approved by the Institutional Review Board of the University of Illinois College of Medicine (Rockford, Ill, USA).
The RSQ required yes or no responses to the following symptoms: fever of 100.4°F (38°C) or higher, difficulty breathing, cough, loss of sense of smell or taste, shortness of breath, chest pain, sore throat, new onset of fatigue or lack of energy, nausea with or without vomiting, and diarrhea. Answers to those questions on the RSQ were obtained by telephone or an in-person interview by a limited number of trained RGA medical personnel. Likewise, the nasopharyngeal specimens were collected by a select group of RGA registered nurses and physicians who had completed methodologic training in the handling and acquisition of the sample for subsequent analysis by the U.S. Food and Drug Administration–approved (for use under Emergency Use Authorization) Roche COBAS (Roche, Basel, Switzerland) 6800/8800 real-time RT-PCR COVID-19 test for the detection of severe acute respiratory syndrome coronavirus 2 RNA and pan-sarbecovirus including severe acute respiratory syndrome coronavirus 2.4 , 5 The performance characteristics of the test were verified by Poplar Healthcare, which is regulated under Clinical Laboratory Improvement Amendments as qualified to perform high-complexity testing.6 Percentage of positivity rates obtained from the Winnebago County Health Department served as a marker for disease prevalence within our community.
Results
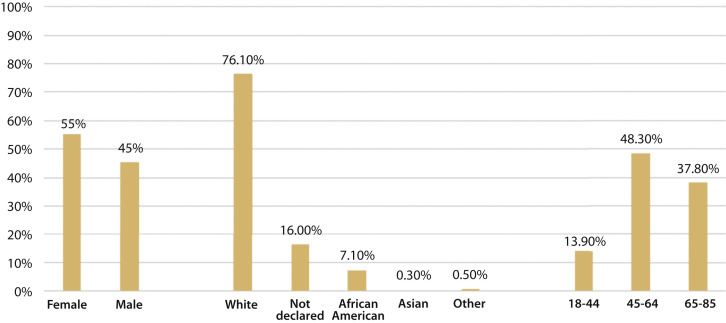
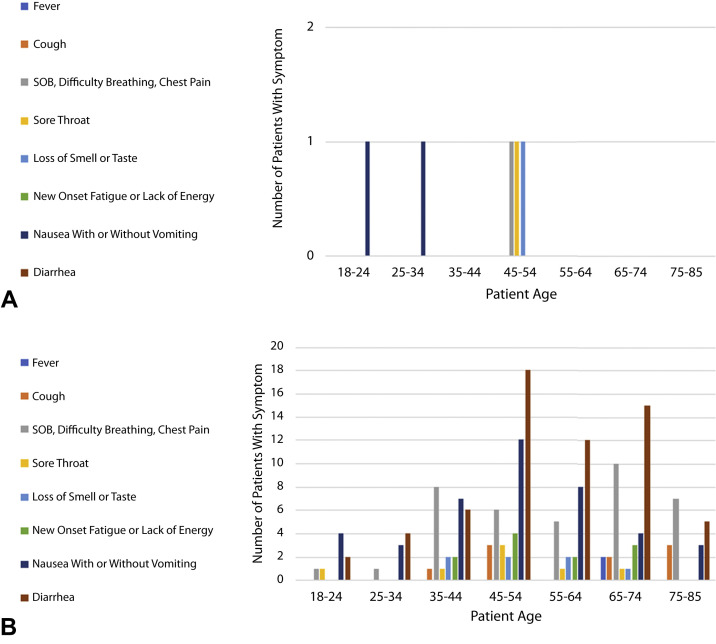
Of 1163 patients,1000 met inclusion criteria for evaluation. Patient demographics are summarized in Figure 1 . Of the 1000 patients included in this study, 8 tested positive for COVID-19, of whom 3 reported symptoms on the RSQ as shown in the 2 × 2 contingency tabulation (Table 1 ). Calculations from the 2 × 2 contingency table for negative and positive predictive values were 99.43% and 2.46%, respectively. Age and reported symptoms of the 8 RT-PCR–positive patients are summarized in Figure 2 . Two patients reported nausea. One patient reported chest pain, sore throat, and the loss of taste or smell.
Figure 1.
Cohort demographics: gender, race, and age.
Table 1.
Contingency table
| Positive reverse transcriptase polymerase chain reaction | Negative reverse transcriptase polymerase chain reaction | Total | |
|---|---|---|---|
| Positive questionnaire | 3 | 119 | 122 |
| Negative questionnaire | 5 | 873 | 878 |
| Column totals | 8 | 992 | 1000 |
Figure 2.
A, Symptoms by age of patients with a positive reverse transcriptase polymerase chain reaction (RT-PCR). B, Symptoms by age of patients with a negative RT-PCR. SOB, Shortness of breath.
Details of symptoms by age for the 8 PCR-positive patients and the 119 PCR-negative symptomatic patients (false positives) are shown Figure 2A and B, respectively. Three of the 5 asymptomatic RT-PCR–positive patients were younger than 45 years. Patients younger than 45 years represented 13.9% of all patients enrolled in the study.
In the false-positive group, diarrhea was the most commonly reported symptom (n = 62) followed by nausea and or vomiting (41); shortness of breath, chest pain, and difficulty breathing (38); cough (11); new-onset fatigue (11); sore throat (7); loss of taste or smell (7); and fever (2). Known nausea, chest pain, and cough accounted for 22.6% of the procedural indications for upper endoscopy, whereas known diarrhea accounted for 60.4% of the procedural indications for colonoscopy. Symptom frequency was independent of race and gender in both the PCR-positive and false-negative groups.
Discussion
This is the first outcomes study for preprocedure symptom screening followed by universal COVID-19 RT-PCR testing in patients undergoing endoscopic procedures within a community-based ambulatory surgery center. Eight of 1000 patients had a positive RT-PCR test result. Four of 5 asymptomatic RT-PCR–positive patients were 45 years old or less in age, suggesting the potential for a higher incidence of asymptomatic infection in younger patients. None of the 3 symptomatic RT-PCR patients reported symptoms highly suggestive of infection (fever, cough, shortness of breath, or difficulty breathing). Nausea, vomiting, and diarrhea accounted for the most frequent symptoms in all age categories as anticipated in a gastroenterology practice.
Based on the data collected from our cohort, the positive predictive value of the RSQ was 2.46% and negative predictive value was 99.43%. The RT-PCR positivity rate was .80%. In reference to published7 and unpublished data obtained from the Winnebago County Health Department (S. Martell, personal communication, September 1 and 9, 2020), the calculated average positivity rate for our service area of Winnebago County, Illinois during this study was 8.37%. The percent positivity rate in Winnebago County (8.37%) was notably higher than the infection rate in our patient cohort (.80%).
Our findings were comparable with infection rates observed in patients presenting for endoscopic procedures from academic centers in Stanford, California (.14%)8 and New York City, New York (.96%).9 Preprocedure RT-PCR testing in areas of higher viral prevalence would be expected to detect higher rates of infection. This was not supported by our findings (8.37%) in comparison with the infection rate of New York City, New York (6.27%) as reported by Dolinger et al.9 The marked similarity of low infection rates in patients presenting for endoscopic procedures from coastal academic centers and our Midwestern community-based ambulatory surgery center is not readily explained; however, we suspect that patients who have symptoms highly suggestive of COVID-19 are unlikely to schedule an elective endoscopy, precluding requisite RT-PCR testing. This is supported by the paucity of fever, cough, loss of sense of smell or taste, new-onset fatigue, and sore throat in our 119 patients with a false-positive result from the RSQ. In addition, health-conscious individuals desirous of surveillance or screening endoscopy may have practiced social distancing, hand hygiene, and worn face masks to a greater degree than their counterparts within the community, which in turn would have comparatively reduced their risk of infection.
The benefit of universal COVID-19 testing should not be underestimated. In our study, 5 asymptomatic patients were prevented from undergoing endoscopy as a result of positive RT-PCR testing, whereas procedures were performed on 119 patients with symptoms not highly suggestive of COVID-19 as a result of their negative RT-PCR tests. Nearly 12% of our patients scheduled to undergo endoscopy would have been unnecessarily excluded from indicated procedures if symptom screening alone was the primary tool in determining the likelihood of infection. Of course, the overall clinical suspicion of COVID-19 in this group was low because many presented with known GI symptoms. The reassurance of a negative RT-PCR test is important for those patients who were worried that exacerbation of their GI symptoms was related to COVID-19. Indeed, recent findings from the research of Podboy et al8 demonstrated a significant decrease in anxiety among patients and endoscopy unit staff after implementation of universal preprocedure testing. Furthermore, in a detailed economic analysis, Corral et al10 recently reported PCR testing to be an effective strategy for the resumption of endoscopy during the pandemic.
The limitations of universal PCR testing are nonetheless relevant. Although the analytic performance in a laboratory setting of currently available assays for detecting severe acute respiratory syndrome coronavirus 2 RNA can be determined, to our knowledge, there are no published data on the sensitivity of such assays in clinical practice. Recent publications from 2 research groups have emphasized the implications of false-negative and false-positive test results related to imperfect diagnostic performance of currently used assays.10 , 11 Although RT-PCR testing is the primary metric for determining the presence or absence of disease, the uncertainty of its accuracy should not be ignored. Disease prevalence and the pretest suspicion of infection based on symptoms and known risks factors for disease are major determinants in assessing test results in the absence of validated reference standards. Clinical discernment is critical when RT-PCR results conflict with the pretest assessment for risk of infection. Universal RT-PCR testing and prerequisite 72-hour preprocedure quarantine create barriers for timely elective and semiurgent procedures. Researchers from the United Kingdom reported an estimated 15.3% to 16.6% increase in colorectal cancer deaths related to delays in diagnosis during the pandemic.12 From a healthcare facility standpoint, universal PCR testing is disruptive and diverts provider resources from patient care. Outdoor testing facilities face significant challenges during inclement weather, which presents an additional barrier for procedural access.
In conclusion, our study is the first to report the outcomes of preprocedure symptom screening followed by universal COVID-19 RT-PCR testing in patients undergoing endoscopy within a community-based ambulatory surgery center. Although universal COVID-19 testing presents logistical obstacles for patients as well as healthcare facilities and the in vivo diagnostic accuracy of RT-PCR is unclear, we believe this remains the best strategy for minimizing exposure risk in endoscopy centers while avoiding delays in diagnosis for those RT-PCR–negative symptomatic patients. As practices resume scheduling of elective and semiurgent endoscopy, they must attempt to balance safety and optimal procedural access within the context of their disease prevalence and local testing capabilities. In our study, the absence of symptoms was predictive of a negative RT-PCR in 99.43% of patients, whereas the presence of symptoms predicted RT-PCR positivity in only 2.46%. Additional studies are needed to determine in vivo accuracy of RT-PCR tests and an acceptable performance threshold for symptom-based screening.
Footnotes
DISCLOSURE: All authors disclosed no financial relationships.
Appendix 1
COVID-19 Questionnaire (suggested; adapt as needed)
-
1.Have you had testing for COVID-19? Clarify if this was a direct viral test (eg, swab, saliva) or serologic (blood antibody) test.
-
a.Was your test positive or negative?
-
a.
-
2.Do you have any of the following? (yes or no)
-
a.Fever to 100.4°F (38°C) or higher
-
b.Cough c. Shortness of breath, difficulty breathing, chest pain
-
d.sore throat
-
e.Loss of sense of smell or taste
-
f.New onset of fatigue or lack of energy
-
a.
-
3.
Do you have nausea with or without vomiting?
-
4.
Do you have diarrhea?
-
5.
Have you recently traveled to any current COVID-19 hot spot? If so, where?
(The top impacted states in the United States and hot spots around the world can be found in the New York Times Coronavirus Map: Tracking the Global Outbreak, https://www.nytimes.com/interactive/2020/world/coronavirus-maps.html.)
-
6.
In the past 14 days, have you come into close contact (within 6 feet [2 m]) with someone who has a laboratory-confirmed COVID-19 diagnosis?
-
7.
Are you a first responder, healthcare worker, or do you work or volunteer at a hospital or healthcare facility?
-
8.
Are you an employee of a daycare facility, senior living location, adult day care, or extended care or rehabilitation care facility?
Answering “yes” to any of the above symptom questions (1-4) should result in referral to a primary care provider for assessment and possible testing. Answering “yes” to any other question should trigger COVID-19 testing performed less than 72 hours before the procedure. Questions 2, 3, and 4 required responses for study inclusion.
References
- 1.Joint Society message on endoscopy during COVID-19. https://gi.org/2020/04/01/joint-gi-society-message-on-endoscopy-during-covid-19 Available at: Accessed August 10, 2020.
- 2.https://www.dph.illinois.gov/sites/default/files/Elective_Surgery_04.24.20.pdf Available at: Accessed August 10, 2020.
- 3.https://.default-document-library/asge-guidance-for-reopening 4-28-2020.pdf Available at: Accessed August 10, 2020.
- 4.Cobas SARS-CoV-2 test. 2020. https://diagnostics.roche.com/global/en/products/params/cobas-sars-cov-2-2-test.html Available at: Accessed September 3, 2020.
- 5.Fact sheet for healthcare providers, coronavirus disease 2019 (COVID-19), Cobas SARS-CoV-2, Molecular Systems, Inc. U.S. Food and Drug Administration. 2020. https://www.fda.gov/media/136047/download Available at: Accessed September 3, 2020.
- 6.Poplar Healthcare, 3495 Hacks Road, Memphis, Tennessee 38125. https://www.poplarhealthcare.com Available at: Accessed September 7, 2020.
- 7.https://www.wchd.org/images/June_29_2020_WCHD_COVID19Report.pdf Available at: Accessed September 7, 2020.
- 8.Podboy A., Cholankeril G., Cianfichi L. Implementation and impact of universal pre-procedure testing of patients for COVID-19 prior to endoscopy. Gastroenterology. 2020;159:1586–1588. doi: 10.1053/j.gastro.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolinger M.T., Kumta N.A., Greenwald D.A. Outcomes of universal pre-procedure COVID-19 testing prior to endoscopy in a tertiary care center in New York City. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.07.015. ;159:1962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corral J.E., Hoogenboom S.A., Kroner P.T. COVID-19 polymerase chain reaction testing before endoscopy: an economic analysis. Gastrointest Endosc. 2020;92:524–534. doi: 10.1016/j.gie.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woloshin S., Patel N., Kesselheim A. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med. 2020;383:e38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 12.Maringe C., Spicer J., Morris M. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modeling study. Lancet Oncol. 2020:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]