At this time last year, it was farfetched if not impossible to think of the immense changes and challenges to people and the society in 2020. Social distancing has become the norm in many cities, in-person meetings are kept to a minimum, and large-scale conferences have morphed into multiple sessions of online meetings. All these changes and beyond –so many deaths and disabilities –are due to the coronavirus disease 2019 (COVID-19) pandemic. While the world awaits more effective ways to contain the virus from further spread and a safe and effective vaccine, appropriate treatment of the afflicted patients remains a key goal. Initially, much of the focus has been on antiviral therapy for early disease [1]; and anti-inflammatory treatment, especially with corticosteroids [2], for patients with systematic inflammatory response syndrome. However, it has transpired that COVID-19 is also an endothelial disease [3,4], and may lead to microthrombosis and large-vessel thrombosis for various reasons [5,6].
Several observational studies indicated the high incidence of venous (and occasionally arterial) thrombosis in patients with COVID-19 [[7], [8], [9]]. Results from some observational studies indicated lower mortality rates in patients receiving anticoagulant therapy, including therapeutic anticoagulation [[10], [11], [12]]. Such results were embraced by some clinicians and clinical institutions, leading to a change in their routine practice, including recommendations for escalated-dose prophylaxis in all, or some hospitalized patients with COVID-19 [[12], [13], [14]]. In turn, other consensus documents cautioned against indiscriminate use of higher than prophylactic-dose anticoagulation outside of a research study protocol [6,15]. More intense anticoagulation may hypothetically reduce micro and macro thrombosis with potential for improving patient outcomes. However, it may confer extra risk for excess bleeding, and thrombocytopenia. Multiple randomized trials have been registered and are at various stages of progress to identify the optimal antithrombotic therapy for outpatients, inpatients, and critically-ill patients with COVID-19 [16,17].
In this issue of Thrombosis Research, Bertoldi Lemos and colleagues report the results of the first of such randomized trials: the HESACOVID trial [18]. In a pilot single-center study, the authors randomly assigned 20 patients with severe COVID-19 and elevated D-dimer (>1000 μg/L) but no overt bleeding diathesis who required mechanical ventilation to prophylactic anticoagulation versus therapeutic anticoagulation with enoxaparin.
They noted a significant improvement in PaO2/FiO2 ratio, the study primary endpoint, over time in patients receiving therapeutic, but not those receiving prophylactic anticoagulation. In addition, use of therapeutic anticoagulation was associated with improved rate of weaning from mechanical ventilation (hazard ratio: 4.0 [95% CI 1.035–15.053], p = 0.031) and more ventilator-free days. Three patients in the therapeutic anticoagulation group and only 1 patient in the prophylactic group died by 28-day follow-up, although the study was not powered to detect a change in clinical outcomes. There were two diagnosed venous thromboembolic events in patients from each group (total of 4 events). No major bleeding was reported, however, 4 patients in the therapeutic arm and 2 patients in the prophylactic arm developed clinically-relevant bleeding [18].
The authors should be congratulated for completing the first randomized controlled trial of anticoagulant therapy in patients with COVID-19. Despite this important accomplishment, key limitations of the study should be kept in mind. Details about the study protocol are not available. The authors report using blocked randomization, with block size of 10. Does this mean that only 1 block was used for each arm? This may bring concerns for true randomization. With respect to intervention, some experts believe that use of therapeutic enoxaparin may have limitations in critically-ill patients who may require urgent or emergent procedures due to acute changes in clinical setting [6,19]. In addition, although the primary endpoint of this study (P/F ratio) is used in critical-care setting, it is a surrogate marker, rather than a patient-important endpoint [20]. The open-label design is another challenge, although it may seem impractical to conduct a double-blind double-dummy design in such a study. Blinded assessment of the primary endpoint, as reported by the authors, is a reassuring feature [18].
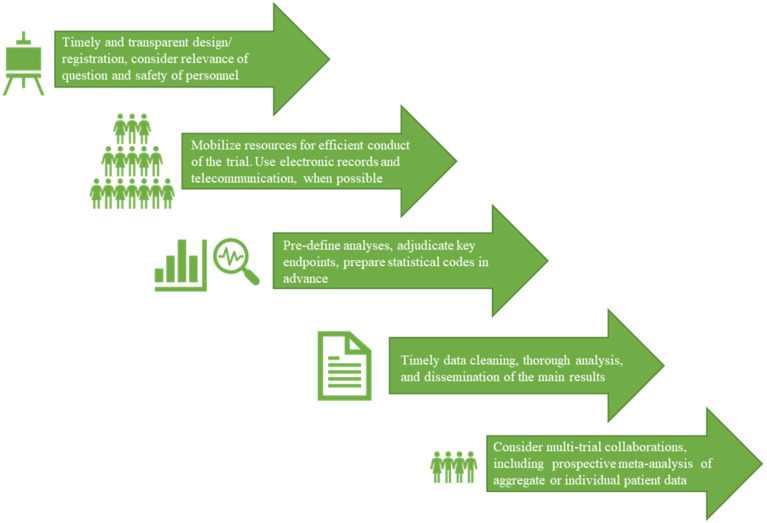
Designing randomized trials during the COVID-19 pandemic has brought unique challenges. Delay in the design phase –or working at the same pace as pre-pandemic periods –may simply fail since the disease wave has rapid turns. Securing sufficient resources in a short period of time is also a practical challenge. Ensuring the health of the research personnel is another design feature to consider, which was not typically a commonly encountered issue for pre-COVID-19 trials. Notwithstanding these challenges, the pandemic has brought opportunities to enhance the efficiency of clinical trial design and reporting, and to embrace additional collaborative efforts (Fig. 1 ).
Fig. 1.
Considerations for clinical trial design, conduct and reporting for COVID-19 therapeutics. Several concepts will be relevant outside of the pandemic, as well.
Where do we go from here? Are the results of HESACOVID practice changing? Probably not. However, this study represents an important step forward. Thinking of these exceedingly difficult months, I recalled of a poem from Hafez (Persian poet in the 14th century) says that “there is hope that the days of sorrow will not last… (Calm) days were not eternal and the opposite will not be everlasting either”. HESACOVID and other ongoing randomized trials will get us further closer to controlling this devastating pandemic.
Declaration of competing interest
Dr. Bikdeli reports that he is a consulting expert, on behalf of the plaintiff, for litigation related to two specific brand models of IVC filters.
References
- 1.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.JAC Sterne, Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., Annane D., LCP Azevedo, Berwanger O., Cavalcanti A.B., Dequin P.F., Du B., Emberson J., Fisher D., Giraudeau B., Gordon A.C., Granholm A., Green C., Haynes R., Heming N., JPT Higgins, Horby P., Jüni P., Landray M.J., Le Gouge A., Leclerc M., Lim W.S., Machado F.R., McArthur C., Meziani F., Møller M.H., Perner A., Petersen M.W., Savovic J., Tomazini B., Veiga V.C., Webb S., Marshall J.C. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1–13. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B., Madhavan M.V., Gupta A., Jimenez D., Burton J.R., Der Nigoghossian C., Chuich T., Nouri S.N., Dreyfus I., Driggin E., Sethi S., Sehgal K., Chatterjee S., Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Bertoletti L., Giri J., Cushman M., Quéré I., Dimakakos E.P., Gibson C.M., Lippi G., Favaloro E.J., Fareed J., Tafur A.J., Francese D.P., Batra J., Falanga A., Clerkin K.J., Uriel N., Kirtane A., McLintock C., Hunt B.J., Spyropoulos A.C., Barnes G.D., Eikelboom J.W., Weinberg I., Schulman S., Carrier M., Piazza G., Beckman J.A., Leon M.B., Stone G.W., Rosenkranz S., Goldhaber S.Z., Parikh S.A., Monreal M., Krumholz H.M., Konstantinides S.V., Weitz J.I., GYH Lip. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb Haemost. 2020;120:1004–1024. doi: 10.1055/s-0040-1713152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C., Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Giri J., Cushman M., Quere I., Dimakakos E.P., Gibson C.M., Lippi G., Favaloro E.J., Fareed J., Caprini J.A., Tafur A.J., Burton J.R., Francese D.P., Wang E.Y., Falanga A., McLintock C., Hunt B.J., Spyropoulos A.C., Barnes G.D., Eikelboom J.W., Weinberg I., Schulman S., Carrier M., Piazza G., Beckman J.A., Steg P.G., Stone G.W., Rosenkranz S., Goldhaber S.Z., Parikh S.A., Monreal M., Krumholz H.M., Konstantinides S.V., Weitz J.I., GYH Lip. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voicu S., Bonnin P., Stépanian A., Chousterman B.G., Le Gall A., Malissin I., Deye N., Siguret V., Mebazaa A., Mégarbane B. High prevalence of deep vein thrombosis in mechanically ventilated COVID-19 patients. J. Am. Coll. Cardiol. 2020;76:480–482. doi: 10.1016/j.jacc.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Angles-Cano E., Sattler L., Mertes P.M., Meziani F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paranjpe I., Fuster V., Lala A., Russak A.J., Glicksberg B.S., Levin M.A., Charney A.W., Narula J., Fayad Z.A., Bagiella E., Zhao S., Nadkarni G.N. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadkarni G.N., Lala A., Bagiella E., Chang H.L., Moreno P., Pujadas E., Arvind V., Bose S., Charney A.W., Chen M.D., Cordon-Cardo C., Dunn A.S., Farkouh M.E., Glicksberg B., Kia A., Kohli-Seth R., Levin M.A., Timsina P., Zhao S., Fayad Z.A., Fuster V. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: a single health system study. J. Am. Coll. Cardiol. 2020;S0735-1097(20):36408-1. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J., WEhmeyer G., Li H., Alshak M., Musarrat N., Mangala R. D-dimer cut-off points and risk of venous thromboembolism in adult hospitalized patients with COVID-19. Thromb Res. 2020;196:318–321. doi: 10.1016/j.thromres.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., Levi M., Samama C.M., Thachil J., Giannis D., Douketis J.D. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K., Holley A.B., Jimenez D., Le Gal G., Rali P., Wells P. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bikdeli B., Talasaz A.H., Rashidi F., Sharif-Kashani B., Farrokhpour M., Bakhshandeh H. Intermediate versus standard-dose prophylactic anticoagulation and statin therapy versus placebo in critically-ill patients with COVID-19: rationale and design of the INSPIRATION/INSPIRATION-S studies. Thromb Res. 2020;196:382–394. doi: 10.1016/j.thromres.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tritschler T., Mathieu M.E., Skeith L., Rodger M., Middeldorp S., Brighton T., Sandset P.M., Kahn S.R., Angus D.C., Blondon M., Bonten M.J., Cattaneo M., Cushman M., Derde L.P.G., MT DeSancho, Diehl J.L., Goligher E., Jilma B., Jüni P., Lawler P.R., Marietta M., Marshall J.C., McArthur C., Miranda C.H., Mirault T., Morici N., Perepu U., Schörgenhofer C., Sholzberg M., Spyropoulos A.C., Webb S.A., Zarychanski R., Zuily S., Le Gal G. Anticoagulant interventions in hospitalized patients with COVID-19: a scoping review of randomized controlled trials and call for international collaboration. J Thromb Haemost. 2020 doi: 10.1111/jth.15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertoldi Lemos A.C., do Espírito Santo D.A., Cabetti Salvetti M., Gilio R.N., Barbosa Agra L., Pazin-Filho A., Henrique Miranda C. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase II clinical trial (HESACOVID) Thromb Res. 2020;196:359–366. doi: 10.1016/j.thromres.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashemi A., Madhavan M.V., Bikdeli B. Pharmacotherapy for prevention and management of thrombosis in COVID-19. Semin. Thromb. Hemost. 2020 doi: 10.1055/s-0040-1714273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bikdeli B., Punnanithinont N., Akram Y., Lee I., Desai N.R., Ross J.S., Krumholz H.M. Two decades of cardiovascular trials with primary surrogate endpoints: 1990-2011. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005285. [DOI] [PMC free article] [PubMed] [Google Scholar]