Abstract
The renin-angiotensin system (RAS) is one of the oldest hormone systems in vertebrate phylogeny. RAS was initially related to regulation of blood pressure and sodium and water homeostasis. However, local or paracrine RAS were later identified in many tissues, including brain, and play a major role in their physiology and pathophysiology. In addition, a major component, ACE2, is the entry receptor for SARS-CoV-2. Overactivation of tissue RAS leads several oxidative stress and inflammatory processes involved in aging-related degenerative changes. In addition, a third level of RAS, the intracellular or intracrine RAS (iRAS), with still unclear functions, has been observed. The possible interaction between the intracellular and extracellular RAS, and particularly the possible deleterious or beneficial effects of the iRAS activation are controversial. The dopaminergic system is particularly interesting to investigate the RAS as important functional interactions between dopamine and RAS have been observed in the brain and several peripheral tissues. Our recent observations in mitochondria and nucleus of dopaminergic neurons may clarify the role of the iRAS. This may be important for the developing of new therapeutic strategies, since the effects on both extracellular and intracellular RAS must be taken into account, and perhaps better understanding of COVID-19 cell mechanisms.
Abbreviations: ACE, angiotensin converting enzyme; Ang II, angiotensin II; AREs, antioxidant response elements; AT1, ang II type 1 receptor; AT2, ang II type 2 receptor; CNS, Central Nervous System; ER, endoplasmic reticulum; GCPR, G-protein coupled receptor; IGF-1, insulin-like growth factor 1; mitoKATP, mitochondrial ATP-sensitive potassium channels; NAC, N-acetyl cysteine; NFE2, L2, nuclear factor- erythroid 2 related factor 2; NO, mitochondrial nitric oxide; NOS, nitric oxide synthase; Nox, NADPH-oxidase complex; OS, oxidative stress; PGC-1α, peroxisome proliferator-activated receptor γ co-activator 1 α; PRR, prorenin receptors; RAS, renin-angiotensin system; ROS, reactive oxygen species; SHRs, spontaneously hypertensive rats; SIRT1, sirtuin 1
Subject codes: ACE/angiotensin receptors/renin angiotensin system, Cell biology/structural biology, Cell signaling/signal transduction, Oxidant stress
Keywords: Angiotensin, COVID-19, Intracrine, Mitochondria, Nucleus, Oxidative stress
1. Introduction
The renin-angiotensin system (RAS) was initially described more than a century ago by Tigerstedt and Bergman (1898) using extracts of rabbit’s kidney. The RAS is one of the oldest hormone systems in vertebrate phylogeny, being already observed in vertebrates such as lamprey, and it may have played a key role in the process of adaptation from aquatic to terrestrial ecosystems (Nishimura, 2017; Wong and Takei, 2018). Consistent with this, the RAS was initially considered a circulating hormonal system that plays a major role in regulation of blood pressure and sodium and water homeostasis. However, local or paracrine RAS were later identified in many tissues, including brain tissue, and they play a major role in the physiology and pathophysiology of those tissues. In peripheral tissues, both the hormonal RAS and tissue paracrine RAS may act together. However, several studies suggest that the circulating RAS is notably less important than the paracrine RAS for the activity of the tissue (Ganong, 1994). Consistent with this, heart local or tissue angiotensin may be 75 % of the total heart tissue angiotensin (Danser et al., 1994; De Mello and Frohlich, 2014). However, RAS components from the circulation can participate in local angiotensin synthesis together local synthesis of RAS components that generate angiotensin peptides (Danser et al., 1994)
Angiotensin II (Ang II) is the principal effector peptide of the system and is generated by the sequential action of two enzymes, renin and angiotensin converting enzyme (ACE), on the precursor glycoprotein angiotensinogen. Ang II acts on two major G-protein coupled receptors (GCPR): Ang II type 1 and 2 (AT1 and AT2) receptors. AT2 receptor actions are usually opposed to those exerted by AT1 receptors (McCarthy et al., 2013). Overactivation of tissue RAS, via AT1 receptors, leads to several oxidative stress and inflammatory processes, which appear to be involved in aging-related degenerative changes in a number of tissues (Benigni et al., 2009; de Cavanagh et al., 2015d). Activation of the plasma membrane NADPH-oxidase complex 2 (Nox2), which leads to intracellular generation of superoxide and superoxide-derived ROS (reactive oxygen species), plays a major role in the pro-oxidative and pro-inflammatory effects of AT1 activity (Grammatopoulos et al., 2007; Labandeira-Garcia et al., 2013; Rodriguez-Pallares et al., 2008).
More recently, several new peptides and receptors have been involved in the RAS function. Additional angiotensin peptides such as Ang (1–7), Ang III and Ang IV induce functional effects. Ang IV acts via a transmembrane enzyme, insulin-regulated membrane aminopeptidase (IRAP) (Albiston et al., 2001; Chai et al., 2004). Ang (1–7) and alamandine counteract the effects of activation of the pro-oxidative Ang II/AT1 axis by acting via G-protein coupled receptor Mas (Kostenis et al., 2005; Santos et al., 2003, 2018) and the Mas-related GPCR members (Mrg) such as MrgD (Hrenak et al., 2016). Alamandine is formed by decarboxylation of the Asp residue of angiotensin-(1–7), leading to the formation of Ala as the N-terminal amino acid, and can be generated both from the “deleterious” Ang A as well as from the “protective” angiotensin 1–7 (Hrenak et al., 2016). New receptors for renin and its precursor prorenin were also observed, which bind prorenin giving to this precursor catalytic properties similar to those of renin. Furthermore, prorenin receptors (PRR) trigger their own signaling pathway that leads to pro-oxidative effects similar to those induced by activation of AT1 receptors (Nguyen and Contrepas, 2008).
Altogether the data suggest the presence in the RAS of two major axes: a pro-oxidative and pro-inflammatory arm constituted by the Ang II/AT1 axis and a protective antioxidative and antiinflammatory arm that includes the Ang II/AT2, Ang (1–7)/Mas, and alamandine/Mas-related receptors; the role of other RAS components such as prorenin/PRR, Ang A, Ang III, Ang IV has been more controversial and possibly tissue-dependent (see for review Chappell, 2016; Hrenak et al., 2016; Li et al., 2017; Paz Ocaranza et al., 2020). In this field, as in most research areas, there are controversial results that should be considered with caution when analyzing the literature. Most controversies are possibly related to methodological differences. A first methodological problem is the use of different experimental doses that may lead to different conclusions. Concentrations of RAS components vary depending on different tissues, cells and subcellular compartments (see Chappell, 2016 for a detailed review). A second confusing factor may be the use of commercial non-selective antibodies, particularly in the case of GPCRs (Benicky et al., 2012; Chappell, 2016) without confirmation of the specificity of the antibody and/or without a simultaneous confirmation of the results with different methodological approaches such as RT-PCR, binding experiments or functional assays with receptor agonists and antagonists. We normally use several parallel methods, as well as confirmation of specificity of antibodies using methods such as western blot analysis of lysates from cells transfected with the corresponding GPCR tagged to fusion tail DDK (i.e. a C-terminal DDK epitope tag DYKDDDDK) or GFP (green fluorescent protein), or preadsortion with the corresponding synthetic peptide antigen (Valenzuela et al., 2016)
In addition to the circulating and the paracrine or tissular RAS, a number of recent studies suggest the presence of an intracellular RAS that further increases the complexity of the RAS. An intracellular synthesis of Ang II and other RAS components, as well as different RAS receptors, were observed in a number of cells including, fibroblasts, vascular smooth muscle cells, cardiac cells, kidney cells, and neurons (Escobales et al., 2019; Li et al., 2018; Re, 2018; Re and Cook, 2015). Renin is classically known as a secretory glycoprotein produced, stored and released by the kidney. However, whereas the kidney expresses transcripts encoding secretory renin, other tissues and cells additionally or exclusively express transcripts encoding cytosolic renin protein that cannot be secreted, and can act on intracellular angiotensinogen. Several studies have shown that cytosolic renin exerts effects opposite to those of circulating renin, as cytosolic renin appears to be cell protective (Nakagawa et al., 2020; Wanka et al., 2018, 2020). This is consistent with our observations of cell protective effects of the intracellular RAS (see below). However, the functional role of the intracellular RAS is still unclear and controversial.
2. The brain RAS. Dopamine and RAS
In the brain, the actions of RAS were initially related to neurons regulating blood pressure and sodium and water homeostasis (Phillips and de Oliveira, 2008) as a result of the activity of the circulating RAS via the circumventricular organs, because Ang II does not cross the blood-brain barrier in normal physiological conditions (Harding et al., 1988). However, a paracrine and independent brain RAS has now been shown. Brain levels of Ang II are higher than circulating levels (Hermann et al., 1984), and other RAS components were shown in several brain regions. The precursor protein of the brain paracrine system (angiotensinogen) is mainly produced by astrocytes (Hermann et al., 1984; Milsted et al., 1990; Stornetta et al., 1988), and other cells such as neurons may make small contributions (Kumar et al., 1988; Thomas et al., 1992). As renin is located at low levels in the brain, some authors were unable to detect it, suggesting that brain Ang II may be taken up from the blood and questioning a brain RAS independent from the circulating RAS (van Thiel et al., 2017v). However, low levels of renin were observed in many other studies (Bader and Ganten, 2002; Lavoie et al., 2004) and, more importantly, the brain has high levels of prorenin and prorenin receptors, and prorenin bound to prorenin receptors provides catalytic properties similar to those of renin (Nguyen and Contrepas, 2008; Valenzuela et al., 2010). This point has been discussed in detail by Sigmund et al., 2017.
Over the last decade, we have shown a local or paracrine RAS in the substantia nigra and striatum of rodents and primates (Joglar et al., 2009; Rodriguez-Pallares et al., 2008; Valenzuela et al., 2010), including humans (Garrido-Gil et al., 2017, 2013). In addition to its physiological functions, overactivation of the nigrostriatal RAS, via AT1 receptors, enhances neuroinflammation, oxidative stress and dopaminergic degeneration (Grammatopoulos et al., 2007; Labandeira-Garcia et al., 2013; Rodriguez-Pallares et al., 2008). Consistent with this, we have observed overactivity of the Ang II/AT1 pro-oxidative/proinflammatory axis in animal models with increased dopaminergic neuron vulnerability such as aged animals and menopausal animals (Rodriguez-Perez et al., 2012; Villar-Cheda et al., 2012). Modulation of the neuroinflammatory/microglial response is a major mechanism by which brain RAS is involved in the progression of a number of brain diseases, including neurodegenerative diseases, stroke and traumatic brain injuries. However, this has already been reviewed in detail in previous articles (Hammer et al., 2017; Labandeira-Garcia et al., 2017; Rodriguez-Perez et al., 2020; Saavedra, 2012).
The dopaminergic system is particularly interesting to investigate the RAS as important functional interactions between dopamine and RAS have been observed in several tissues. In the CNS, dopamine is a neurotransmitter that regulates movement and behavior. However, dopamine is also involved in cardiovascular, renal, endocrine, gastrointestinal and immune functions (Mackie et al., 2018; Missale et al., 1998; Vidal and Pacheco, 2019). Dopamine D1-like and D2-like receptor subtypes are expressed in the brain, but also in peripheral organs such as the kidney, heart, blood vessels, adrenal gland, postganglionic sympathetic nerve terminals, gastrointestinal tract and almost all immune cell subpopulations (Mackie et al., 2018; Missale et al., 1998; Vidal and Pacheco, 2019). In most of these organs, an important functional interaction between dopamine and the local RAS has been demonstrated. This interaction has been particularly investigated in the regulation of kidney sodium excretion and cardiovascular function, where dopamine and angiotensin systems directly counterregulate each other (Gildea, 2009; Gildea et al., 2019). In addition, dimerization of Ang II and dopamine receptors has been observed in renal cells (Durdagi et al., 2019). Consistent with this, several studies have shown that dysregulation of interactions between DA and RAS, such as changes in dopamine and angiotensin receptor expression (Chugh et al., 2012) or dopamine or angiotensin levels (Yang et al., 2012), play a major role in renal degenerative diseases and hypertension.
In the brain, Ang II-dopamine interaction was initially suggested by microdialysis studies, which observed that acute striatal perfusion of Ang II led to striatal dopamine release that was inhibited by AT1 antagonists (Brown et al., 1996; Mendelsohn et al., 1993). More recently, Ang II was observed to regulate the axonal synthesis of norepinephrine and dopamine by modulating trafficking and expression of tyrosine hydroxylase and dopamine β-hydroxylase, two key enzymes for catecholamine biosynthesis (Aschrafi et al., 2019). We have shown counterregulation between dopamine and angiotensin receptors in the nigrostriatal system (Villar-Cheda et al., 2014, 2010), and dimerization between AT1 and D2 receptors in striatal neurons (Martinez-Pinilla et al., 2015). Similar to that observed in the renal and cardiovascular systems, we have shown that dysregulation of RAS/dopamine interactions leads to exacerbation of neuroinflammation and dopaminergic neuron degeneration (Dominguez-Meijide et al., 2017; Villar-Cheda et al., 2014).
3. The intracellular RAS
In the circulating/hormonal RAS and the paracrine/tissue RAS, functional effects are induced by angiotensin-related peptides that act on different angiotensin receptor types located on the cell membrane, which leads to intracellular changes. However, in a variety of cells such as cardiomyocytes, kidney cells, fibroblasts, vascular smooth muscle, and neurons, among others, a number of studies have shown the presence of different RAS components at intracellular organelles such as nucleus, mitochondria, endoplasmic reticulum and others. In addition, introduction of angiotensin at an intracellular level using intracellular injections or viral vectors to produce intracellular Ang II led to a series of functional effects, including changes in gene expression, independent of those derived from activation of plasma membrane receptors (Baker et al., 2004; Deliu and Tica, 2011). On this basis, the existence of a third level of RAS (i.e. the intracellular or intracrine RAS; iRAS) has been suggested (Abadir et al., 2011; Alzayadneh and Chappell, 2015; Cook et al., 2001; da Silva Novaes et al., 2018d; Eggena et al., 1993; Gwathmey et al., 2012; Kumar et al., 2012a; Re and Cook, 2010). In addition, several effects of the intracellular RAS have been shown to be independent of administration of extracellular angiotensin receptor antagonists, further confirming that changes induced by intracellular angiotensin are independent of plasma membrane receptors (De Mello and Monterrubio, 2004; Tadevosyan et al., 2017). This is consistent with recent studies showing the important role of other intracellular GPCRs, such as the purine receptors, 5-hydroxytrptamine (5-HT4) receptors, melatonin MT1 receptors or cannabinoid CB1 receptors, in cell function (see for review Jong et al., 2018).
Several studies have shown that the iRAS is involved in important cellular processes including ion-channel activity, regulation of Ca2+ homeostasis, or secretion of extracellular matrix components (Deliu et al., 2014; Jong et al., 2018; Kamal et al., 2017; Zhuo et al., 2006). In addition to the effects of iRAS in mitochondria and nucleus (see below), AT1 and AT2 receptors have been observed in the endoplasmic reticulum (ER). ER expresses different proteins regulating Ca2+ storage, and Ang II may stimulate the sarco(endo)plasmic reticulum calcium ATPase (SERCA) activity, which plays a major role in Ca2+ mobilization (Ferrao et al., 2017, 2012). However, the possible interaction between the intracellular and extracellular RAS, and particularly the possible deleterious or beneficial effects of iRAS activation have been particularly controversial (Baker et al., 2004; Cook et al., 2001; Re, 2018; Tadevosyan et al., 2017). This is of crucial importance for the design of new therapies based on manipulation of RAS components for major cardiovascular, renal and possibly brain diseases. A number of studies in peripheral cells such as cardiomyocytes, fibroblasts and kidney cells have shown that high levels of intracellular Ang II, such as those induced by diabetic hyperglycemia, lead to oxidative stress and cell and tissue damage (Kumar et al., 2012b; Redding et al., 2010; Singh et al., 2008). Consistent with this, it has usually been assumed that the iRAS may be amplifying the effects of the pro-oxidative Ang II/AT1 axis of the extracellular RAS, and that inhibition of the intracellular RAS may induce clinical benefits against RAS-induced pro-oxidative pro-inflammatory effects (Carey, 2012; Cook and Re, 2012). However, the results of our recent studies on effects of iRAS on brain mitochondria and nuclei from dopaminergic neurons (Costa-Besada et al., 2018; Valenzuela et al., 2016; Villar-Cheda et al., 2017), show that this issue may be more complicated. The intracellular RAS may be physiologically buffering the pro-oxidative effects of the AngII/AT1/NADPH-oxidase activation. However, the buffering capacity of the iRAS may be overwhelmed by an excess of the Ang II/AT1 activity and/or an excess of intracellular Ang II related to excessive internalization of the Ang II/AT1 complex. Differences in distribution or balance between intracellular receptor types in different cell types or circumstances such as aging or pathology may modify the effects of iRAS activity. Consistent with this, an increase in mitochondrial AT1/AT2 ratio was observed in renal cells in diabetic nephropathy, and rats overexpressing mitochondrial AT2 receptors showed a decrease in diabetes-induced kidney alterations (Friederich-Persson and Persson, 2020; Micakovic et al., 2018). Our recent observations on the effects of iRAS on mitochondria and nucleus of dopaminergic neurons appear particularly interesting to clarify this issue.
4. The mitochondrial RAS
4.1. The presence of RAS components in the mitochondria
The possible presence of a mitochondrial RAS was suggested after observing different RAS components such as renin, ACE, and Ang II in cells from adrenal gland, kidney or cerebellar cortex, particularly using electron microscopy or uptake of labelled Ang II studies (Erdmann et al., 1996; Peters et al., 1996; van Kats et al., 2001). More recently the presence of a number of RAS components such as Ang I, Ang II, and Ang 1–7 was confirmed in mitochondria isolated from kidney cortex (Wilson et al., 2016, 2017). Mitochondrial angiotensins may be imported from the cytoplasm. It is known that the mitochondrial outer membrane contains a multisubunit complex responsible for the recognition and translocation of proteins within mitochondria (Model et al., 2002). However, the possible uptake of different RAS components has not been at present clarified. Interestingly, it has been shown that mitochondrial angiotensinogen can be imported from the cytoplasm in kidney cells (Wilson et al., 2017).Cytosolic renin has also been localized within mitochondria (Clausmeyer et al., 1999; Wanka et al., 2018, 2020). Altogether suggests that angiotensins, including Ang 1–7 may also be produced within the mitochondria. Consistent with this we have observed high levels of ACE2 in the mitochondria, which may mediate degradation of mitochondrial Ang II and generation of protective Ang 1–7 (see below).
AT1 and AT2 receptors were observed in mitochondria of several types of cells (Abadir et al., 2011, 2012), and the presence of G-proteins in the mitochondria has been also shown (Lyssand and Bajjalieh, 2007; Suofu et al., 2017). As current data indicate that these receptors are not encoded by mitochondrial DNA (Calvo et al., 2016), it appears that they reach the mitochondria after a translocation process. In the cytoplasm, AT1 receptors are available both from internalization of the Ang II/AT1 complex or de novo synthesized AT1 receptors (Hunyady, 1999; Thekkumkara and Linas, 2002). It is usually considered that AT2 receptors are not internalized after binding Ang II and the cytoplasmatic pool is derived from de novo synthesized AT2 receptors (Gwathmey et al., 2011; Tadevosyan et al., 2010). Posttranscriptional changes including incorporation of signal peptides or glycosylations may lead to trafficking of AT1 and AT2 receptors to different cell organelles including mitochondria (Helenius and Aebi, 2004; Singh et al., 2008). It has also been suggested the possibility that extracellular Ang II induces heterodimerization of AT1 and AT2 receptors so that AT2 receptors may be internalized as heterodimers (Ferrao et al., 2012, 2017).
4.2. Functional effects of the mitochondrial angiotensin receptors
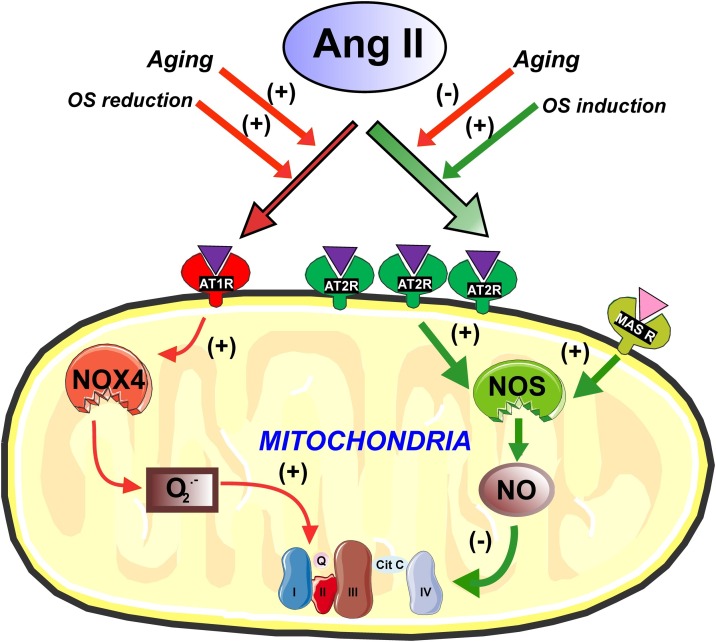
We have shown the location of AT1, AT2 and Mas receptors in mitochondria of cultured dopaminergic neurons and mitochondria of neurons of the rodent substantia nigra, as well as mitochondria isolated from these sources (Costa-Besada et al., 2018; Garrido-Gil et al., 2017; Valenzuela et al., 2016). In addition, we performed functional studies to clarify the possible role of angiotensin mitochondrial receptors, including functional studies in isolated mitochondria to prevent possible effects derived from non-mitochondrial angiotensin receptors, and particularly from plasma membrane receptors. We observed that AT2 receptors were located at the cell membrane level, but were particularly abundant at mitochondrial level, while AT1 receptors were more abundant at the cell membrane and nucleus but less abundant than AT2 in the mitochondria, where Mas receptors were also identified (Costa-Besada et al., 2018; Valenzuela et al., 2016; Villar-Cheda et al., 2017). In isolated mitochondria from dopaminergic neurons, we showed that AT1 and AT2 receptors play a role in the control of the respiratory function. As previously observed for plasma membrane AT1 and AT2 receptor function (McCarthy et al., 2013; Rodriguez-Perez et al., 2020), mitochondrial AT1 and AT2 receptors induce opposite effects on mitochondrial respiratory rates. AT2 receptors, which are normally much more abundant than AT1 in mitochondria, induce a moderate but significant decrease in respiration. However, AT1 activation increases mitochondrial respiration (Abadir et al., 2011; Valenzuela et al., 2016). Consistent with this, mitochondria isolated from mice deficient in AT2 or AT1 receptors showed an increase or decrease in respiratory rates, respectively (Valenzuela et al., 2016). In isolated mitochondria, we observed that AT2 receptor activation promotes nitric oxide synthase (NOS) activation leading to an increase in mitochondrial nitric oxide (NO) levels, which modulate mitochondrial respiration without significant changes in mitochondrial membrane potential, meaning that the bioenergetic properties of the mitochondria are not altered. Mitochondrial AT1 activation led to superoxide production, via mitochondrial Nox4, and increased mitochondrial respiration (Valenzuela et al., 2016) (Fig. 1 ).
Fig. 1.
Modulation of oxidative phosphorylation by mitochondrial angiotensin receptors. Mitochondrial AT1 receptors, via Nox4, induce superoxide production and increase respiration. AT2 receptors are more numerous than AT1 in mitochondria. AT2 and Mas receptors induce, via nitric oxide, a reduction in mitochondrial respiration and regulate oxidative phosphorylation without significant change in mitochondrial membrane potential, which suggests that mitochondrial bioenergetic properties are not altered. Treatment of cells with oxidative stress (OS) inducers leads to a compensatory increase in levels of mitochondrial AT2 receptors, while treatment of cells with antioxidants increases AT1 receptor levels. Mitochondrial AT2 and Mas receptors may modulate respiration and offset the effects of low levels of OS during the normal cell functioning. Aging leads to a decrease in AT2 and Mas receptors and an increase in AT1 mitochondrial receptors, which may contribute to mitochondrial dysfunction and cell death. Images were produced using Servier Medical Art (http://www.servier.com).
In dopaminergic cells, Ang 1–7 was abundantly located at mitochondrial level. Ang 1–7 (see below), increased mitochondrial level of nitric oxide and inhibited the Ang II-induced increase in levels of superoxide and changes in mitochondrial respiration (Costa-Besada et al., 2018). This is consistent with previous studies showing that the pro-oxidative proinflammatory effects of the Ang II/AT1/Nox2/superoxide axis are counteracted by the Ang 1–7/MasR and the Ang II/AT2 and protective axes (Paz Ocaranza et al., 2020; Santos et al., 2018). Our observations in dopaminergic cells suggest the mitochondria as a major site for modulation of ROS levels by the Ang II/AT2 and Ang 1–7 protective axes.
Our data in dopaminergic neurons suggest that mitochondrial angiotensin receptors may buffer the effects of other factors affecting mitochondrial respiration. We observed that an increase in neuronal oxidative stress induced by administration of very low doses of the dopaminergic neurotoxin MPP+ induces an increase in the levels of mitochondrial AT2 receptors. On the contrary, treatment of neurons with antioxidants such as N-acetyl cysteine (NAC) led to an increase in the levels of mitochondrial AT1 receptors. An increase in cell oxidative stress may also be the result of activation of the plasma membrane AT1 receptors by the paracrine Ang II, which induces activation of the plasma membrane NADPH-oxidase complex (Nox2), the second major intracellular source of superoxide after the mitochondria (Babior, 2004). In different cell types, a ROS-mediated interaction (i.e. cross-talk signaling) between the plasma membrane Nox2 and mitochondria was observed, and ROS derived from the Nox2-released superoxide leads to opening of mitochondrial ATP-sensitive potassium channels (mitoKATP), which enhances generation of mitochondrial ROS (Daiber, 2010; Ou et al., 2016; Wosniak et al., 2009; Zhang and Gutterman, 2007). We have confirmed this in cultures of dopaminergic neurons, and that blockers of mitoKATP channels inhibit the Ang II-induced increase in superoxide production in dopaminergic neurons (Rodriguez-Pallares et al., 2009, 2012). Enhanced ROS production may lead to mitochondrial DNA damage and oxidation of mitochondrial proteins, leading to mitochondrial dysfunction. Increased ROS production also affects cytoplasm and leads to cell dysfunction. It is known that intracellular superoxide is primarily produced by the oxidation of NADPH by NAPH-oxidases or by electron leak from aerobic respiration in mitochondria. Superoxide is rapidly converted into hydrogen peroxide (H2O2) by compartment-specific superoxide dismutases (SODs). H2O2 is capable of oxidizing cysteine residues on proteins to initiate redox processes. Alternatively, H2O2 may be converted to H2O by cellular antioxidant proteins. When cell antioxidant mechanisms are insufficient and H2O2 levels increase uncontrollably, hydroxyl radicals (OH·) are produced via reactions with metal cations (Fe2+), and irreversibly damage cellular macromolecules (see for review Schieber and Chandel, 2014). As indicated above, oxidative stress induced by activation of the cell membrane AT1/NADPH-oxidase axis by paracrine Ang II or other sources of ROS acting on mitochondria may be counteracted by mitochondrial RAS antioxidative components such as AT2 receptors and Ang 1–7 receptors, possibly within moderate or physiological levels of oxidative stress.
4.3. Mitochondrial RAS in aging and disease
It is possible that the buffering capacity of the mitochondrial Ang II AT2 and Ang 1–7 receptors change in different types of cells or different conditions. Interestingly, it was found that aging affects mitochondrial AT1/AT2 receptor ratio and the above-mentioned mitochondrial receptor response to cell oxidative stress (Abadir et al., 2011; Valenzuela et al., 2016). Overactivation of the plasma membrane Ang II/AT1/NADPH-oxidase axis and an increase in levels of oxidative stress were observed in dopaminergic neurons from aged animals (Villar-Cheda et al., 2014, 2012). Mitochondria isolated from aged rats showed an increase in mitochondrial AT1 receptor levels and a decrease in mitochondrial AT2 and Ang 1–7/Mas protective axis relative to young rats (Costa-Besada et al., 2018; Valenzuela et al., 2016). This may contribute to the mitochondrial dysfunction observed during normal aging and lead to higher vulnerability of aged dopaminergic neurons and aging-related neurodegenerative disorders such as Parkinson’s disease (Hauser and Hastings, 2013; Rodriguez et al., 2015). Similar changes may occur under disease conditions. A recent study in kidney cortex mitochondria has found that Ang II, via mitochondrial AT1 receptors, increased mitochondria leak respiration in diabetic animals, and AT2 mitochondrial activation decreased mitochondrial respiration (Friederich-Persson and Persson, 2020).
4.4. Possible role of mitochondrial ACE2 in the cellular effects of SARS-CoV-2 and COVID-19 disease
As indicated above, ACE2 is a key component of the anti-inflammatory antioxidative RAS axis because it mediates degradation of deleterious Ang II and generation of protective Ang 1–7. However, ACE2 is also the entry receptor for SARS-CoV viruses to invade cells (Kuba et al., 2005; Yan et al., 2020). These two opposite effects have led to consider ACE2 as a double-edged sword for COVID-19 disease. Several organs are affected by the virus, being lung lesions particularly dangerous. The CNS is also affected (Baig et al., 2020; Saavedra, 2020), and about 36 % of COVID-19 patients (45 % of severe cases) showed neurological manifestations (Mao et al., 2020). It is normally considered that the virus-induced decrease in tissue ACE2 and the subsequent dysregulation tissue RAS function towards the Ang II/AT1 proinflammatory and prooxidative axis plays a major role in the inflammatory processes observed in COVID-19 disease in the different organs. However, the exact mechanisms connecting virus-related ACE2 downregulation with the cellular pro-oxidative and pro-inflammatory responses are unclear. It is usually assumed that the effects of the ACE2-derived Ang 1–7 on cell membrane Mas receptors are responsible for the intracellular beneficial antioxidative effects, which would be downregulated by the effects of the virus on cell membrane ACE2.
In isolated mitochondria from rat brain (Costa-Besada et al., 2018), which was recently confirmed in mitochondria isolated from the brain of non-human primates (unpublished), we observed that both ACE2 and Ang 1–7 are much highly concentrated in the mitochondrial fraction than in the whole cell homogenate, and that levels of Ang 1–7 in the mitochondrial fraction are around 3 times higher than those of Ang II. This suggests an important role for mitochondrial ACE2 and its product Ang 1–7 in the mitochondrial function. Several previous studies have shown that viruses, including SARS-CoV viruses, modulate cell function by modifying mitochondrial processes (Boya et al., 2004; Yuan et al., 2006), and dysregulation of the mitochondrial ACE2/Ang 1–7 axis as a result of the effects of SARS-CoV-2 internalization and replication may play a major role in cell changes in COVID-19 disease. Several proteins generated from the SARS-CoV viral genome have mitochondrial targeting sequence (Singh et al., 2020; Yuan et al., 2006). Coronavirus spike proteins contain endoplasmic reticulum retrieval signals that can retrieve spike proteins to the endoplasmic reticulum (Lontok et al., 2004; Sadasivan et al., 2017). Although direct interaction between viral spike protein and mitochondria has not been demonstrated at the present time, the interaction with mitochondrial ACE2 may be via MAMs (mitochondrial associated membrane compartment) (Williamson and Colberg-Poley, 2009), or mechanisms that remain to be clarified.
5. The nuclear RAS
5.1. The presence of RAS components in the nucleus
Angiotensin receptors have been observed in nuclei from different types of renal and cardiovascular cells (Alzayadneh and Chappell, 2015; Eggena et al., 1993; Gwathmey et al., 2012; Tadevosyan et al., 2017). Ang II binding sites on chromatin have also been suggested (Re et al., 1984), although the nature of chromatin binding is unclear. The mechanisms of transportation of the receptors to the nucleus have not been totally clarified. Several studies have shown the translocation of AT1 receptors from the cell membrane to the nucleus after binding Ang II (i.e. internalization of the Ang II-AT1 receptor complex via receptor-mediated endocytosis), although de novo synthesized AT1 receptors may be also incorporated to the nuclear membranes (Bkaily et al., 2003; Cook et al., 2006; Hunyady, 1999; Thekkumkara and Linas, 2002; Villar-Cheda et al., 2017). Transportation of AT2 receptors to the nucleus is more controversial because they do not have common nuclear transportation domains (i.e. AT2 lack a canonical nuclear localization sequence as AT1 receptors do) (da Silva Novaes et al., 2018d; Zhou et al., 2014). Translocation of AT2 receptors from the cell membrane to the nucleus after binding Ang II has not been observed (Gwathmey et al., 2012; Tadevosyan et al., 2017). It has been suggested that AT2 receptors may be transported to the nucleus by active transport using an importin/exportin system (Matsushima-Otsuka et al., 2018). A recent in vitro study of brain stem neurons has shown that after Ang 1–7 stimulation Mas receptors are internalized through clathrin-coated pits and caveolae into early endosomes and slowly recycled back to the plasma membrane, and that that in neurons from spontaneously hypertensive rats (SHRs) Ang 1–7 induced Mas receptor translocation to the nucleus together with its ligand Ang 1–7 (Cerniello et al., 2019).
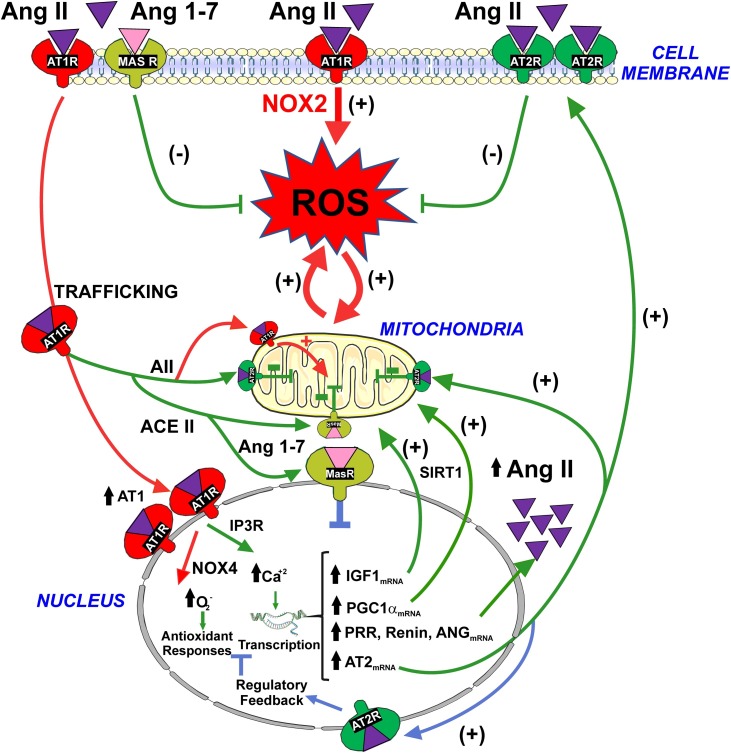
We have shown nuclear location of Ang II, Ang 1–7, AT1, AT2, MAS receptors and translocation of AT1/Ang II to the nucleus of dopaminergic neurons (Costa-Besada et al., 2018; Villar-Cheda et al., 2017). The function of nuclear angiotensin receptors has not been clarified. Several studies have suggested that the iRAS may lead to amplification of the effects of the paracrine RAS that acts on the plasma membrane receptors (Carey, 2012; Cook and Re, 2012). However, our recent studies on the iRAS of dopaminergic neurons suggest that, at least in physiological conditions, the nuclear RAS is a cell protective system that may contribute to counteract collateral deleterious effects of activation of the plasma membrane Ang II/AT1 pro-oxidative axis during the normal cell function. It is known that extracellular Ang II activates the AT1/Nox2 axis leading to the increase in levels of intracellular superoxide. However, a simultaneous translocation of the Ang II/AT1 complex to the nucleus activates a number of protective mechanisms against oxidative stress as detailed below (Fig. 2 ).
Fig. 2.
Modulation by nuclear angiotensin receptors of the pro-oxidative effects of activation of the plasma membrane AT1-Nox2 axis . Activation of surface AT1 leads to generation of intracellular superoxide/H2O2 and oxidative stress (red arrows). However, activation of AT1 also induces internalization of the Ang II-AT1 receptor complex to the nucleus (red arrows). Nuclear AT1 receptor activation leads to an increase in NOX4/superoxide/H2O2 and IP3/Ca2+ levels that, via regulation of gene expression, trigger several mechanisms that may protect cells against oxidative stress (green arrows): (i) an increase in the levels of protective AT2 receptors that traffic to mitochondria and cell membrane leading to a compensatory upregulation of the RAS protective arm (i.e. AII/AT2); (ii) an increase in the synthesis of intracellular angiotensinogen/AngII to act on intracellular AT2 receptors and, via Ang 1-7, intracellular Mas receptors; (iii) upregulation of mRNA expression for PGC-1α and IGF-1, which, possibly interacting with SIRT1, enhance mitochondrial protection and reduce oxidative damage. Nuclear AT2 and Mas receptors modulate the effects of nuclear AT1 receptors by increasing nuclear levels of NO (blue arrows). Abbreviations: ANG, angiotensinogen; Ang II, angiotensin II; Ang 1-7, angiotensin 1-7; AT1, angiotensin type 1; AT2, angiotensin type 2; IGF-1, insulin-like growth factor 1; IP3R, inositol 1,4,5-trisphosphate receptor; MAS, Mas receptors; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PRR, prorenin/renin receptors; ROS, reactive oxygen species; SIRT1, sirtuin 1. Images were produced using Servier Medical Art (http://www.servier.com).
A number of recent data support this protective view (Nunez et al., 2014, 2017, 2018; Wanka et al., 2020; Wilson et al., 2016). Several studies have also shown that high levels of intracellular Ang II led to cell and tissue damage (Redding et al., 2010; Singh et al., 2008). Under pathological conditions, an excess of Ang II/AT1 stimulation at the cell membrane level, leading to excessive Nox-derived superoxide and intracellular AT1 and Ang II levels, may overwhelm the below described iRAS buffer capacity and eventually amplify the cell damage. Interestingly, this may also be the case of aged brains, where we observed that the iRAS protective responses were altered (see below). However, physiological intracellular levels of angiotensins are more difficult to estimate than tissue or circulating levels, as they have been usually estimated in cultured cells, which are affected by the culture conditions and the absence of the regulatory effect of the extracellular RAS and other possible physiological regulatory factors. The levels of intracellular angiotensins varied depending on different cell types and experimental conditions. However, they were around of 150–200 fmol/mg protein for Ang II and 250–400 fmol/mg protein for Ang 1–7, which may increase and decrease 3–5 times, respectively, under pathological conditions such as high glucose conditions (Alzayadneh and Chappell, 2014; Lavrentyev et al., 2007; see Chappell, 2016 for a detailed review)
5.2. Activation of nuclear RAS components after nuclear translocation of the Ang II/AT1 complex
In isolated nuclei from dopaminergic neurons, activation of nuclear AT1 receptors by Ang II induced an increase in the expression of AT2 receptor mRNA. The increase in traffic of AT2 receptors to different cell structures may counteract the effects of cell membrane AT1 activation. In different types of cells, plasma membrane AT2 receptors have been shown to counteract the effects of AT1 activation (Padia and Carey, 2013; Rodriguez-Perez et al., 2020; Wang et al., 2012). The responsible mechanisms include production of nitric oxide that modulates Nox2-derived free radical availability (Wang et al., 2012), the downregulation of AT1 mRNA expression (Padia and Carey, 2013; Rodriguez-Perez et al., 2020; Steckelings et al., 2005) or by forming AT2-AT1 heteromers at the plasma membrane level (AbdAlla et al., 2001; Patel and Hussain, 2018). In addition, AT2 receptors may be transported to the mitochondria, where, via NO generation, downregulate mitochondrial respiration and cell oxidative stress (Valenzuela et al., 2016) (see above).
Interestingly, activation of nuclear AT1 receptors in isolated nuclei also increased the expression of mRNA for angiotensinogen, renin and renin-prorenin receptors. This suggests a parallel increase of iRAS components, particularly intracellular Ang II and Ang 1–7, that may act on upregulated anti-oxidative AT2 and Mas receptors. However, it is possible that excessive production of intracellular and/or internalized Ang II or changes in the rate of intracellular AT1 receptors as observed in aging or disease may result, directly or interacting with other factors, in deleterious effects.
In isolated neuronal nuclei, activation of AT2 receptors with AT2 agonists, or activation of Mas receptors with Ang 1–7, leads to an increase nuclear NO levels, which counteracts the effect of nuclear AT1 stimulation. Similar results were observed using isolated nuclei from AT2 and AT1 KO mice (Costa-Besada et al., 2018; Villar-Cheda et al., 2017), and suggest that nuclear Mas and AT2 receptors may regulate the protective response induced by activation of nuclear AT1 receptors, in order to preserve an adequate equilibrium between the cell deleterious and protective RAS arms. In addition, treatment of isolated nuclei with Ang 1–7 significantly decreased the expression of AT2 mRNA and did not lead to changes in the expression of Mas and AT1 receptor mRNA. This suggests a compensatory regulation between nuclear AT2 and Mas receptor effects.
5.3. Other possible protective mechanisms induced by nuclear Ang II/AT1 activation
Activation of AT1 receptors in isolated dopaminergic neuronal nuclei led to an increase in mRNA expression for PGC-1α (peroxisome proliferator-activated receptor γ co-activator 1 α). The PGC-1 family members are master transcriptional regulators of the mitochondrial function and trigger several mechanisms against mitochondrial dysfunction to promote cell survival (Finley and Haigis, 2009; Scarpulla, 2008). Consistent with this and our observations in isolated dopaminergic neuron nuclei, conditional PGC-1α KO mice showed significant loss of dopaminergic neurons (Ciron et al., 2015; Jiang et al., 2016), while overexpression of PGC-1α was protective for dopaminergic neurons (Borniquel et al., 2006; Mudo et al., 2012). Therefore, the increase in the expression of PGC-1α, induced by activation of nuclear AT1 receptors, may also compensate the pro-oxidative effects of activation of the plasma membrane AT1 receptors.
In the substantia nigra, a series of previous studies have shown that Ang II modulates other compounds that regulate mitochondrial function, such as IGF-1 (insulin-like growth factor 1) or SIRT1 (sirtuin 1) (Diaz-Ruiz et al., 2015; Rodriguez-Perez et al., 2016). IGF-1 has been found to increase survival of dopaminergic neurons (Kao, 2009; Offen et al., 2001), which has been associated to protective effects on mitochondrial function leading to a decrease in free radical production, oxidative damage and apoptosis (Puche et al., 2008; Sadaba et al., 2016; Tang, 2016). SIRT1 increases cell resistance to oxidative stress by deacetylation of several proteins involved in cell survival, metabolism and stress response (Tanno et al., 2010; Yuan et al., 2016). In fact, IGF-1 increases the expression of SIRT1 to inhibit oxidative stress-induced cell death (Vinciguerra et al., 2009), and it has been shown that SIRT1 controls mitochondrial function at least in part by modulating PGC-1α activity (Finley and Haigis, 2009; Tang, 2016; Yuan et al., 2016). On this basis, we investigated possible effects of activation of nuclear AT1 receptors on these compounds. In neuronal isolated nuclei, activation of AT1 receptors increased the expression of IGF-1 mRNA and PGC-1α mRNA. However, activation of AT1 receptors did not produce any significant increase in SIRT1 mRNA in isolated nuclei from dopaminergic neurons (Villar-Cheda et al., 2017). As we observed an increase in expression of SIRT1 induced by activation of plasma membrane AT1 receptors in cultures of dopaminergic neurons (Diaz-Ruiz et al., 2015), components located in the cytoplasm are probably necessary. The observed increase in expression of IGF-1 may be one of these components, since IGF-1 increased SIRT1 expression in other cell types (Vinciguerra et al., 2009). Consistent with this, we observed that nuclei isolated from the brain of transgenic mice overexpressing SIRT1 showed a decrease in expression of nuclear AT1 receptors, which supports a possible feedback regulation of the above-mentioned mechanism (i.e. indirect upregulation of SIRT1 by activation of nuclear AT1 receptors). This is also supported by observations in muscle cells showing that overexpression of SIRT1 downregulates PGC-1α (Gurd et al., 2009). In conclusion, activation of nuclear AT1 receptors by intracellular Ang II or nuclear translocation of the plasma membrane Ang II/AT1 complex may trigger a compensatory mechanism by increasing IGF-1, PGC-1α and SIRT1 levels.
5.4. Nuclear AT1 receptor activation induces an increase in nuclear Ca2+ and superoxide/H2O2 levels
Nuclear Ca2+ signaling is an important regulator of gene transcription (Bezin et al., 2008), and IP3 receptors have been shown to be involved in Ca2+ signaling (Kusnier et al., 2006). Calcium directly binds transcription factors such as DREAM (Carrion et al., 1999) and activates the nuclear CaM kinase pathways that modulate gene expression. (Chawla, 2002). We have observed that activation of nuclear AT1 receptors increases nuclear Ca2+ levels, and that inhibition of IP3 receptors inhibits the above-mentioned Ang II-induced increase in AT2 and PGC-1α mRNAs, which indicates that Ca2+ signaling is responsible for the above described transcriptional changes (Villar-Cheda et al., 2017).
Activation of nuclear AT1 receptors also induces an increase in nuclear superoxide/H2O2 levels via Nox4 activation (Pendergrass et al., 2009; Villar-Cheda et al., 2017). This may have been responsible of the above mentioned protective mechanisms, as Nox4 can regulate gene expression in a manner dependent on regulatory DNA sequence Maf-recognition element (MARE), constituting part of the antioxidant response (Hoshino et al., 2000). However, treatment of isolated nuclei with Ang II and the antioxidant NAC or the Nox inhibitor DPI, did not block the Ang II-induced AT2 or PGC-1α mRNA expression. Superoxide derived from nuclear AT1/Nox4 activation may be responsible for the transcription of other components of the antioxidant response. Low levels of superoxide/H2O2 derived from nuclear AT1 activation may lead to beneficial hormetic adaptations to oxidative stress, similar to those described at mitochondrial level (Yun and Finkel, 2014). However, an excessive production of superoxide may lead to oxidative damage of nuclear components and cell and tissue damage, as reported in studies that used high levels of intracellular Ang II (Kumar et al., 2012a; Micakovic et al., 2018; Singh et al., 2008) (Fig. 3 ).
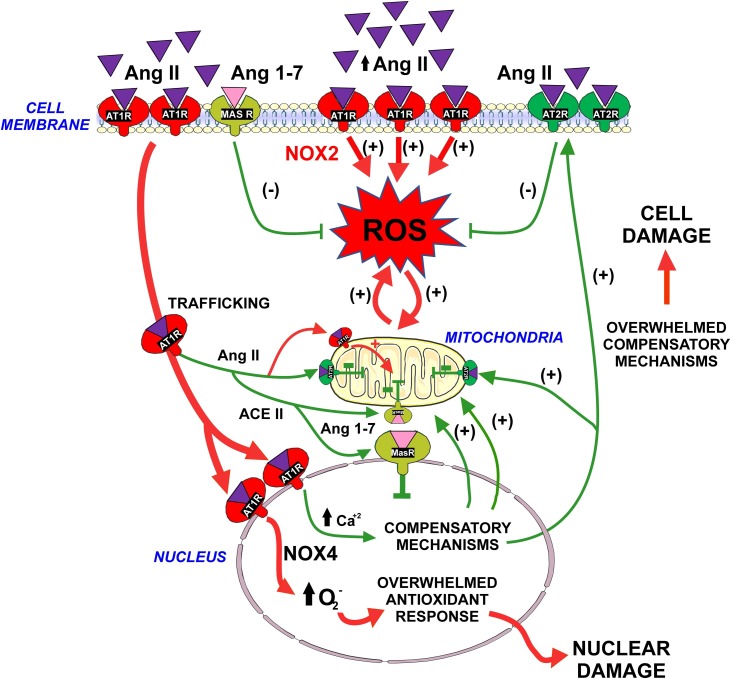
Fig. 3.
Physiologic compensatory mechanisms may be overwhelmed. Intracellular RAS may buffer the pro-oxidative effects of activation of plasma membrane AT1 receptors by extracellular Ang II. Internalization of the Ang II-AT1 complex to the nucleus, and activation of nuclear AT1 receptors by intracellular Ang II triggers a number of mechanisms that may protect cells against oxidative stress during the normal cell function (see Fig. 1, Fig. 2). However, excessive Ang II /AT1 activation or an increase in the AT1/AT2 ratio, as a result of disease-related mechanisms or aging, may overwhelm the compensatory mechanisms, leading to cell damage and progression of disease. Abbreviations: Ang II, angiotensin II; Ang 1-7, angiotensin 1-7; AT1, angiotensin type 1; AT2, angiotensin type 2; MAS, Mas receptors; ROS, reactive oxygen species. Images were produced using Servier Medical Art (http://www.servier.com).
5.5. Nuclear RAS in aging and disease
Aging is a major factor involved in degenerative changes in peripheral tissues such as cardiovascular and renal tissues, and the main risk factor for neurodegenerative diseases such as Alzheimer’s disease and PD. In several peripheral tissues, overactivity of the Ang II/AT1/Nox2 pro-oxidative proinflammatory axis has been associated to aging (Benigni et al., 2009, 2013; Elkahloun and Saavedra, 2019). In tissue homogenates from the nigral region of aged rats and mice, we also observed overactivity of the Ang II/AT1/Nox2 axis and a marked decrease in the expression of AT2 receptors (Rodriguez-Pallares et al., 2012; Villar-Cheda et al., 2014, 2012), together with an increase in markers of oxidative stress and neuroinflammation. The Ang 1–7/Mas receptor axis is also downregulated in the aged nigra (Costa-Besada et al., 2018). As commented above, isolated mitochondria from aged rats also showed an increase in AT1 and a decrease in AT2 receptors, which may affect mitochondrial protection against oxidative stress. Interestingly, aged rats also showed a decrease in the levels of IGF-1 and SIRT1 in the nigral region (Diaz-Ruiz et al., 2015; Rodriguez-Perez et al., 2016).
In isolated nuclei from aged rats we observed a significant decrease in both nuclear AT1 and AT2 receptor levels (Villar-Cheda et al., 2017). In contrast with that observed in the case of nuclei isolated from young rats, nuclei from aged animals treated with similar doses of Ang II did not produce a significant increase in the expression of AT2, angiotensinogen, IGF-1 or PGC-1α mRNA. The lack of a significant nuclear compensatory response to the increase in intracellular levels of Ang II may explain the decrease in AT2, IGF-1 and SIRT1 expression observed in the nigra of aged animals (Diaz-Ruiz et al., 2015; Rodriguez-Perez et al., 2016; Villar-Cheda et al., 2014, 2012), and the increase in cell vulnerability to the increase in paracrine Ang II/AT1 levels that takes place in aged animals.
As indicated above, different components of the intracellular RAS may be deregulated under pathological conditions such as an excess of cell oxidative stress or high glucose conditions (Kumar et al., 2012b; Micakovic et al., 2018; Singh et al., 2008; Valenzuela et al., 2016; Villar-Cheda et al., 2017).
6. Additional intracellular mechanisms counteract the pro-oxidative effects of the paracrine Ang II/AT1 axis
In addition to the above-mentioned mechanisms, other cell protective mechanisms compensate the possible deleterious effects of the activation of the pro-oxidative Ang II/ AT1 arm. In dopaminergic neurons, we have recently observed that Ang II, alone or combined with other pro-oxidative factors, activates the transcription factor NRF2 (i.e., NFE2L2, nuclear factor- erythroid 2 related factor 2) pathway (Parga et al., 2018). NRF2 is a key regulator of cell antioxidant mechanisms and redox homeostasis. NRF2 and other transcription factors promote phase II antioxidant enzymes after binding to the promoter regions of the antioxidant response elements (AREs) (Zhang et al., 2013). Consistent with this, activation of the NRF2 pathway has shown neuroprotective properties in dopaminergic neurons by regulation of the expression of antioxidant enzymes and other transcription factors, such as KLF9, which reduce oxidative stress both in vitro and in vivo. The release of superoxide from the AT1/Nox2 pathway induces NRF2 activation (Parga et al., 2018). However, AT1 receptor activation may promote NRF2 pathway via alternative mechanisms such as ERK1/2 (Huang et al., 1996) and PKC (Huang et al., 2000), as phosphorylation of NRF2 by these kinases promote the dissociation from the inhibitor KEAP1 and nuclear localization of NRF2 (Xu et al., 2006).
7. Conclusions and perspectives for the future
Previous studies have suggested that the intracellular RAS may lead to amplification of the effects of the paracrine or circulating RAS that act on the plasma membrane receptors. On this basis, it was assumed that intracellular Ang II may contribute to progression of diseases and tissue and cell dysfunctions induced by overactivation of the RAS pro-oxidative arm. However, the intracellular RAS may buffer the pro-oxidative effects of activation of plasma membrane AT1 receptors by extracellular (paracrine) Ang II. Internalization of the Ang II-AT1 complex to the nucleus, and activation of nuclear AT1 receptors by intracellular Ang II triggers a number of mechanisms that protect cells against oxidative stress. Data from several recent studies suggest that the iRAS may play important roles for the normal cell function. Particularly, the iRAS may be buffering the potential deleterious effects of activation of the pro-oxidative arm of the extracellular RAS and other pro-oxidative stimuli. However, the iRAS may be overwhelmed and even enhance the cell damage after excessive Ang II /AT1 activation or after changes in pro-oxidative/antioxidative receptor (AT1/AT2-MasR) ratios induced by disease-related conditions or aging. This may be important for the developing of new therapeutic strategies since the effects on both extracellular and iRAS must be taken into account. For instance, differences in the efficacy of different AT1 receptor blockers to inhibit extracellular and intracellular receptors have been observed (Cook et al., 2001; Filipeanu et al., 2001). The presence of high levels of mitochondrial ACE2 open new avenues for research on intracellular effects of SARS-CoV viruses.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
Grant sponsor: Spanish Ministry of Science and Innovation (RTI2018-098830-B-I00). Grant sponsor: Spanish Ministry of Health (PI17/00828, RD16/0011/0016 and CIBERNED). Grant sponsor: Galician Government (XUGA, ED431C 2018/10, ED431G/05). Grant sponsor: FEDER (Regional European Development Fund).
Footnotes
The Peer Review Overview and Supplementary data associated with this article can be found in the online version, at doi: https://doi.org/10.1016/j.pneurobio.2020.101919.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Abadir P.M., Foster D.B., Crow M., Cooke C.A., Rucker J.J., Jain A., Smith B.J., Burks T.N., Cohn R.D., Fedarko N.S., Carey R.M., O’Rourke B., Walston J.D. Identification and characterization of a functional mitochondrial angiotensin system. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14849–14854. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abadir P.M., Walston J.D., Carey R.M. Subcellular characteristics of functional intracellular renin-angiotensin systems. Peptides. 2012;38:437–445. doi: 10.1016/j.peptides.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AbdAlla S., Lother H., Abdel-tawab A.M., Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J. Biol. Chem. 2001;276:39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- Albiston A.L., McDowall S.G., Matsacos D., Sim P., Clune E., Mustafa T., Lee J., Mendelsohn F.A., Simpson R.J., Connolly L.M., Chai S.Y. Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated aminopeptidase. J. Biol. Chem. 2001;276:48623–48626. doi: 10.1074/jbc.C100512200. [DOI] [PubMed] [Google Scholar]
- Alzayadneh E.M., Chappell M.C. Angiotensin-(1-7) abolishes AGE-induced cellular hypertrophy and myofibroblast transformation via inhibition of ERK1/2. Cell. Signal. 2014;26:3027–3035. doi: 10.1016/j.cellsig.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzayadneh E.M., Chappell M.C. Nuclear expression of renin-angiotensin system components in NRK-52E renal epithelial cells. J. Renin Angiotensin Aldosterone Syst. 2015;16:1135–1148. doi: 10.1177/1470320313515039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschrafi A., Berndt A., Kowalak J.A., Gale J.R., Gioio A.E., Kaplan B.B. Angiotensin II mediates the axonal trafficking of tyrosine hydroxylase and dopamine beta-hydroxylase mRNAs and enhances norepinephrine synthesis in primary sympathetic neurons. J. Neurochem. 2019;150:666–677. doi: 10.1111/jnc.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B.M. NADPH oxidase. Curr. Opin. Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Bader M., Ganten D. It’s renin in the brain: transgenic animals elucidate the brain renin angiotensin system. Circ. Res. 2002;90:8–10. [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Baker K.M., Chernin M.I., Schreiber T., Sanghi S., Haiderzaidi S., Booz G.W., Dostal D.E., Kumar R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul. Pept. 2004;120:5–13. doi: 10.1016/j.regpep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Benicky J., Hafko R., Sanchez-Lemus E., Aguilera G., Saavedra J.M. Six commercially available angiotensin II AT1 receptor antibodies are non-specific. Cell. Mol. Neurobiol. 2012;32:1353–1365. doi: 10.1007/s10571-012-9862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni A., Corna D., Zoja C., Sonzogni A., Latini R., Salio M., Conti S., Rottoli D., Longaretti L., Cassis P., Morigi M., Coffman T.M., Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J. Clin. Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni A., Orisio S., Noris M., Iatropoulos P., Castaldi D., Kamide K., Rakugi H., Arai Y., Todeschini M., Ogliari G., Imai E., Gondo Y., Hirose N., Mari D., Remuzzi G. Variations of the angiotensin II type 1 receptor gene are associated with extreme human longevity. Age (Dordr.) 2013;35:993–1005. doi: 10.1007/s11357-012-9408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezin S., Charpentier G., Lee H.C., Baux G., Fossier P., Cancela J.M. Regulation of nuclear Ca2+ signaling by translocation of the Ca2+ messenger synthesizing enzyme ADP-ribosyl cyclase during neuronal depolarization. J. Biol. Chem. 2008;283:27859–27870. doi: 10.1074/jbc.M804701200. [DOI] [PubMed] [Google Scholar]
- Bkaily G., Sleiman S., Stephan J., Asselin C., Choufani S., Kamal M., Jacques D., Gobeil F., Jr, D’Orleans-Juste P. Angiotensin II AT1 receptor internalization, translocation and de novo synthesis modulate cytosolic and nuclear calcium in human vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 2003;81:274–287. doi: 10.1139/y03-007. [DOI] [PubMed] [Google Scholar]
- Borniquel S., Valle I., Cadenas S., Lamas S., Monsalve M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1alpha. FASEB J. 2006;20:1889–1891. doi: 10.1096/fj.05-5189fje. [DOI] [PubMed] [Google Scholar]
- Boya P., Pauleau A.L., Poncet D., Gonzalez-Polo R.A., Zamzami N., Kroemer G. Viral proteins targeting mitochondria: controlling cell death. Biochim. Biophys. Acta. 2004;1659:178–189. doi: 10.1016/j.bbabio.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Brown D.C., Steward L.J., Ge J., Barnes N.M. Ability of angiotensin II to modulate striatal dopamine release via the AT1 receptor in vitro and in vivo. Br. J. Pharmacol. 1996;118:414–420. doi: 10.1111/j.1476-5381.1996.tb15418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo S.E., Clauser K.R., Mootha V.K. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016;44:D1251–1257. doi: 10.1093/nar/gkv1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey R.M. Functional intracellular renin-angiotensin systems: potential for pathophysiology of disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R479–481. doi: 10.1152/ajpregu.00656.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion A.M., Link W.A., Ledo F., Mellstrom B., Naranjo J.R. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398:80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- Cerniello F.M., Silva M.G., Carretero O.A., Gironacci M.M. Mas receptor is translocated to the nucleus upon agonist stimulation in brainstem neurons from spontaneously hypertensive rats but not normotensive rats. Cardiovasc. Res. 2019 doi: 10.1093/cvr/cvz332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai S.Y., Fernando R., Peck G., Ye S.Y., Mendelsohn F.A., Jenkins T.A., Albiston A.L. The angiotensin IV/AT4 receptor. Cell. Mol. Life Sci. 2004;61:2728–2737. doi: 10.1007/s00018-004-4246-1. [DOI] [PubMed] [Google Scholar]
- Chappell M.C. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am. J. Physiol. Heart Circ. Physiol. 2016;310:H137–52. doi: 10.1152/ajpheart.00618.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla S. Regulation of gene expression by Ca2+ signals in neuronal cells. Eur. J. Pharmacol. 2002;447:131–140. doi: 10.1016/s0014-2999(02)01837-x. [DOI] [PubMed] [Google Scholar]
- Chugh G., Lokhandwala M.F., Asghar M. Altered functioning of both renal dopamine D1 and angiotensin II type 1 receptors causes hypertension in old rats. Hypertension. 2012;59:1029–1036. doi: 10.1161/HYPERTENSIONAHA.112.192302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciron C., Zheng L., Bobela W., Knott G.W., Leone T.C., Kelly D.P., Schneider B.L. PGC-1alpha activity in nigral dopamine neurons determines vulnerability to alpha-synuclein. Acta Neuropathol. Commun. 2015;3:16. doi: 10.1186/s40478-015-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausmeyer S., Sturzebecher R., Peters J. An alternative transcript of the rat renin gene can result in a truncated prorenin that is transported into adrenal mitochondria. Circ. Res. 1999;84:337–344. doi: 10.1161/01.res.84.3.337. [DOI] [PubMed] [Google Scholar]
- Cook J.L., Re R.N. Lessons from in vitro studies and a related intracellular angiotensin II transgenic mouse model. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R482–493. doi: 10.1152/ajpregu.00493.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.L., Zhang Z., Re R.N. In vitro evidence for an intracellular site of angiotensin action. Circ. Res. 2001;89:1138–1146. doi: 10.1161/hh2401.101270. [DOI] [PubMed] [Google Scholar]
- Cook J.L., Mills S.J., Naquin R., Alam J., Re R.N. Nuclear accumulation of the AT1 receptor in a rat vascular smooth muscle cell line: effects upon signal transduction and cellular proliferation. J. Mol. Cell. Cardiol. 2006;40:696–707. doi: 10.1016/j.yjmcc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Costa-Besada M.A., Valenzuela R., Garrido-Gil P., Villar-Cheda B., Parga J.A., Lanciego J.L., Labandeira-Garcia J.L. Paracrine and intracrine angiotensin 1-7/Mas receptor axis in the substantia nigra of rodents, monkeys, and humans. Mol. Neurobiol. 2018;55:5847–5867. doi: 10.1007/s12035-017-0805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Novaes A., Ribeiro R.S., Pereira L.G., Borges F.T., Boim M.A. Intracrine action of angiotensin II in mesangial cells: subcellular distribution of angiotensin II receptor subtypes AT1 and AT2. Mol. Cell. Biochem. 2018;448:265–274. doi: 10.1007/s11010-018-3331-y. [DOI] [PubMed] [Google Scholar]
- Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Danser A.H., van Kats J.P., Admiraal P.J., Derkx F.H., Lamers J.M., Verdouw P.D., Saxena P.R., Schalekamp M.A. Cardiac renin and angiotensins. Uptake from plasma versus in situ synthesis. Hypertension. 1994;24:37–48. doi: 10.1161/01.hyp.24.1.37. [DOI] [PubMed] [Google Scholar]
- de Cavanagh E.M., Inserra F., Ferder L. Angiotensin II blockade: how its molecular targets may signal to mitochondria and slow aging. Coincidences with calorie restriction and mTOR inhibition. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H15–44. doi: 10.1152/ajpheart.00459.2014. [DOI] [PubMed] [Google Scholar]
- De Mello W.C., Frohlich E.D. Clinical perspectives and fundamental aspects of local cardiovascular and renal Renin-Angiotensin systems. Front. Endocrinol. (Lausanne) 2014;5:16. doi: 10.3389/fendo.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello W.C., Monterrubio J. Intracellular and extracellular angiotensin II enhance the L-type calcium current in the failing heart. Hypertension. 2004;44:360–364. doi: 10.1161/01.HYP.0000139914.52686.74. [DOI] [PubMed] [Google Scholar]
- Deliu E., Tica A.A., Motoc D., Brailoiu G.C., Brailoiu E. Intracellular angiotensin II activates rat myometrium. Am. J. Physiol., Cell Physiol. 2011;301:C559–65. doi: 10.1152/ajpcell.00123.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliu E., Brailoiu G.C., Eguchi S., Hoffman N.E., Rabinowitz J.E., Tilley D.G., Madesh M., Koch W.J., Brailoiu E. Direct evidence of intracrine angiotensin II signaling in neurons. Am. J. Physiol., Cell Physiol. 2014;306:C736–744. doi: 10.1152/ajpcell.00131.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ruiz C., Rodriguez-Perez A.I., Beiroa D., Rodriguez-Pallares J., Labandeira-Garcia J.L. Reciprocal regulation between sirtuin-1 and angiotensin-II in the substantia nigra: implications for aging and neurodegeneration. Oncotarget. 2015;6:26675–26689. doi: 10.18632/oncotarget.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Meijide A., Rodriguez-Perez A.I., Diaz-Ruiz C., Guerra M.J., Labandeira-Garcia J.L. Dopamine modulates astroglial and microglial activity via glial renin-angiotensin system in cultures. Brain Behav. Immun. 2017;62:277–290. doi: 10.1016/j.bbi.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Durdagi S., Erol I., Salmas R.E., Aksoydan B., Kantarcioglu I. Oligomerization and cooperativity in GPCRs from the perspective of the angiotensin AT1 and dopamine D2 receptors. Neurosci. Lett. 2019;700:30–37. doi: 10.1016/j.neulet.2018.04.028. [DOI] [PubMed] [Google Scholar]
- Eggena P., Zhu J.H., Clegg K., Barrett J.D. Nuclear angiotensin receptors induce transcription of renin and angiotensinogen mRNA. Hypertension. 1993;22:496–501. doi: 10.1161/01.hyp.22.4.496. [DOI] [PubMed] [Google Scholar]
- Elkahloun A.G., Saavedra J.M. Candesartan neuroprotection in rat primary neurons negatively correlates with aging and senescence: a transcriptomic analysis. Mol. Neurobiol. 2019 doi: 10.1007/s12035-019-01800-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann B., Fuxe K., Ganten D. Subcellular localization of angiotensin II immunoreactivity in the rat cerebellar cortex. Hypertension. 1996;28:818–824. doi: 10.1161/01.hyp.28.5.818. [DOI] [PubMed] [Google Scholar]
- Escobales N., Nunez R.E., Javadov S. Mitochondrial angiotensin receptors and cardioprotective pathways. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H1426–H1438. doi: 10.1152/ajpheart.00772.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrao F.M., Lara L.S., Axelband F., Dias J., Carmona A.K., Reis R.I., Costa-Neto C.M., Vieyra A., Lowe J. Exposure of luminal membranes of LLC-PK1 cells to ANG II induces dimerization of AT1/AT2 receptors to activate SERCA and to promote Ca2+ mobilization. Am. J. Physiol. Renal Physiol. 2012;302:F875–883. doi: 10.1152/ajprenal.00381.2011. [DOI] [PubMed] [Google Scholar]
- Ferrao F.M., Cardoso L.H.D., Drummond H.A., Li X.C., Zhuo J.L., Gomes D.S., Lara L.S., Vieyra A., Lowe J. Luminal ANG II is internalized as a complex with AT1R/AT2R heterodimers to target endoplasmic reticulum in LLC-PK1 cells. Am. J. Physiol. Renal Physiol. 2017;313:F440–F449. doi: 10.1152/ajprenal.00261.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipeanu C.M., Henning R.H., Nelemans S.A., de Zeeuw D. Intracellular angiotensin II: from myth to reality? J. Renin. Syst. 2001;2:219–226. doi: 10.3317/jraas.2001.035. [DOI] [PubMed] [Google Scholar]
- Finley L.W., Haigis M.C. The coordination of nuclear and mitochondrial communication during aging and calorie restriction. Ageing Res. Rev. 2009;8:173–188. doi: 10.1016/j.arr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich-Persson M., Persson P. Mitochondrial angiotensin II receptors regulate oxygen consumption in kidney mitochondria from healthy and type 1 diabetic rats. Am. J. Physiol. Renal Physiol. 2020 doi: 10.1152/ajprenal.00417.2019. [DOI] [PubMed] [Google Scholar]
- Ganong W.F. Origin of the angiotensin II secreted by cells. Proc. Soc. Exp. Biol. Med. 1994;205:213–219. doi: 10.3181/00379727-205-43699a. [DOI] [PubMed] [Google Scholar]
- Garrido-Gil P., Valenzuela R., Villar-Cheda B., Lanciego J.L., Labandeira-Garcia J.L. Expression of angiotensinogen and receptors for angiotensin and prorenin in the monkey and human substantia nigra: an intracellular renin-angiotensin system in the nigra. Brain Struct. Funct. 2013;218:373–388. doi: 10.1007/s00429-012-0402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Gil P., Rodriguez-Perez A.I., Fernandez-Rodriguez P., Lanciego J.L., Labandeira-Garcia J.L. Expression of angiotensinogen and receptors for angiotensin and prorenin in the rat and monkey striatal neurons and glial cells. Brain Struct. Funct. 2017;222:2559–2571. doi: 10.1007/s00429-016-1357-z. [DOI] [PubMed] [Google Scholar]
- Gildea J.J. Dopamine and angiotensin as renal counterregulatory systems controlling sodium balance. Curr. Opin. Nephrol. Hypertens. 2009;18:28–32. doi: 10.1097/MNH.0b013e32831a9e0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildea J.J., Xu P., Kemp B.A., Carey R.M., Jose P.A., Felder R.A. The dopamine D1 receptor and angiotensin II Type-2 receptor are required for inhibition of sodium transport through a protein phosphatase 2A pathway. Hypertension. 2019;73:1258–1265. doi: 10.1161/HYPERTENSIONAHA.119.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos T.N., Jones S.M., Ahmadi F.A., Hoover B.R., Snell L.D., Skoch J., Jhaveri V.V., Poczobutt A.M., Weyhenmeyer J.A., Zawada W.M. Angiotensin type 1 receptor antagonist losartan, reduces MPTP-induced degeneration of dopaminergic neurons in substantia nigra. Mol. Neurodegener. 2007;2:1. doi: 10.1186/1750-1326-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd B.J., Yoshida Y., Lally J., Holloway G.P., Bonen A. The deacetylase enzyme SIRT1 is not associated with oxidative capacity in rat heart and skeletal muscle and its overexpression reduces mitochondrial biogenesis. J. Physiol. 2009;587:1817–1828. doi: 10.1113/jphysiol.2008.168096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwathmey T.M., Shaltout H.A., Rose J.C., Diz D.I., Chappell M.C. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension. 2011;57:620–626. doi: 10.1161/HYPERTENSIONAHA.110.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwathmey T.M., Alzayadneh E.M., Pendergrass K.D., Chappell M.C. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R518–530. doi: 10.1152/ajpregu.00525.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer A., Stegbauer J., Linker R.A. Macrophages in neuroinflammation: role of the renin-angiotensin-system. Pflugers Arch. 2017;469:431–444. doi: 10.1007/s00424-017-1942-x. [DOI] [PubMed] [Google Scholar]
- Harding J.W., Sullivan M.J., Hanesworth J.M., Cushing L.L., Wright J.W. Inability of [125I]Sar1, Ile8-angiotensin II to move between the blood and cerebrospinal fluid compartments. J. Neurochem. 1988;50:554–557. doi: 10.1111/j.1471-4159.1988.tb02946.x. [DOI] [PubMed] [Google Scholar]
- Hauser D.N., Hastings T.G. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiol. Dis. 2013;51:35–42. doi: 10.1016/j.nbd.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- Hermann K., McDonald W., Unger T., Lang R.E., Ganten D. Angiotensin biosynthesis and concentrations in brain of normotensive and hypertensive rats. J. Physiol. (Paris) 1984;79:471–480. [PubMed] [Google Scholar]
- Hoshino H., Kobayashi A., Yoshida M., Kudo N., Oyake T., Motohashi H., Hayashi N., Yamamoto M., Igarashi K. Oxidative stress abolishes leptomycin B-sensitive nuclear export of transcription repressor Bach2 that counteracts activation of Maf recognition element. J. Biol. Chem. 2000;275:15370–15376. doi: 10.1074/jbc.275.20.15370. [DOI] [PubMed] [Google Scholar]
- Hrenak J., Paulis L., Simko F. Angiotensin A/Alamandine/MrgD Axis: another clue to understanding cardiovascular pathophysiology. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.C., Richards E.M., Sumners C. Mitogen-activated protein kinases in rat brain neuronal cultures are activated by angiotensin II type 1 receptors and inhibited by angiotensin II type 2 receptors. J. Biol. Chem. 1996;271:15635–15641. doi: 10.1074/jbc.271.26.15635. [DOI] [PubMed] [Google Scholar]
- Huang H.C., Nguyen T., Pickett C.B. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12475–12480. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunyady L. Molecular mechanisms of angiotensin II receptor internalization. J. Am. Soc. Nephrol. 1999;10(Suppl 11):S47–56. [PubMed] [Google Scholar]
- Jiang H., Kang S.U., Zhang S., Karuppagounder S., Xu J., Lee Y.K., Kang B.G., Lee Y., Zhang J., Pletnikova O., Troncoso J.C., Pirooznia S., Andrabi S.A., Dawson V.L., Dawson T.M. Adult conditional knockout of PGC-1alpha leads to loss of dopamine neurons. eNeuro. 2016;3 doi: 10.1523/ENEURO.0183-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglar B., Rodriguez-Pallares J., Rodriguez-Perez A.I., Rey P., Guerra M.J., Labandeira-Garcia J.L. The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: relevance to progression of the disease. J. Neurochem. 2009;109:656–669. doi: 10.1111/j.1471-4159.2009.05999.x. [DOI] [PubMed] [Google Scholar]
- Jong Y.I., Harmon S.K., O’Malley K.L. Intracellular GPCRs play key roles in synaptic plasticity. ACS Chem. Neurosci. 2018;9:2162–2172. doi: 10.1021/acschemneuro.7b00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal M., Jacques D., Bkaily G. Angiotensin II receptors’ modulation of calcium homeostasis in human vascular endothelial cells. Can. J. Physiol. Pharmacol. 2017;95:1289–1297. doi: 10.1139/cjpp-2017-0416. [DOI] [PubMed] [Google Scholar]
- Kao S.Y. Rescue of alpha-synuclein cytotoxicity by insulin-like growth factors. Biochem. Biophys. Res. Commun. 2009;385:434–438. doi: 10.1016/j.bbrc.2009.05.089. [DOI] [PubMed] [Google Scholar]
- Kostenis E., Milligan G., Christopoulos A., Sanchez-Ferrer C.F., Heringer-Walther S., Sexton P.M., Gembardt F., Kellett E., Martini L., Vanderheyden P., Schultheiss H.P., Walther T. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111:1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Rassoli A., Raizada M.K. Angiotensinogen gene expression in neuronal and glial cells in primary cultures of rat brain. J. Neurosci. Res. 1988;19:287–290. doi: 10.1002/jnr.490190302. [DOI] [PubMed] [Google Scholar]
- Kumar R., Thomas C.M., Yong Q.C., Chen W., Baker K.M. The intracrine renin-angiotensin system. Clin. Sci. 2012;123:273–284. doi: 10.1042/CS20120089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Yong Q.C., Thomas C.M., Baker K.M. Intracardiac intracellular angiotensin system in diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R510–517. doi: 10.1152/ajpregu.00512.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusnier C., Cardenas C., Hidalgo J., Jaimovich E. Single-channel recording of inositol trisphosphate receptor in the isolated nucleus of a muscle cell line. Biol. Res. 2006;39:541–553. doi: 10.4067/s0716-97602006000300015. [DOI] [PubMed] [Google Scholar]
- Labandeira-Garcia J.L., Rodriguez-Pallares J., Dominguez-Meijide A., Valenzuela R., Villar-Cheda B., Rodriguez-Perez A.I. Dopamine-angiotensin interactions in the basal ganglia and their relevance for Parkinson’s disease. Mov. Disord. 2013;28:1337–1342. doi: 10.1002/mds.25614. [DOI] [PubMed] [Google Scholar]
- Labandeira-Garcia J.L., Rodriguez-Perez A.I., Garrido-Gil P., Rodriguez-Pallares J., Lanciego J.L., Guerra M.J. Brain renin-angiotensin system and microglial polarization: implications for aging and neurodegeneration. Front. Aging Neurosci. 2017;9:129. doi: 10.3389/fnagi.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie J.L., Cassell M.D., Gross K.W., Sigmund C.D. Localization of renin expressing cells in the brain, by use of a REN-eGFP transgenic model. Physiol. Genomics. 2004;16:240–246. doi: 10.1152/physiolgenomics.00131.2003. [DOI] [PubMed] [Google Scholar]
- Lavrentyev E.N., Estes A.M., Malik K.U. Mechanism of high glucose induced angiotensin II production in rat vascular smooth muscle cells. Circ. Res. 2007;101:455–464. doi: 10.1161/CIRCRESAHA.107.151852. [DOI] [PubMed] [Google Scholar]
- Li X.C., Zhang J., Zhuo J.L. The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol. Res. 2017;125:21–38. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.C., Zhu D., Zheng X., Zhang J., Zhuo J.L. Intratubular and intracellular renin-angiotensin system in the kidney: a unifying perspective in blood pressure control. Clin. Sci. 2018;132:1383–1401. doi: 10.1042/CS20180121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lontok E., Corse E., Machamer C.E. Intracellular targeting signals contribute to localization of coronavirus spike proteins near the virus assembly site. J. Virol. 2004;78:5913–5922. doi: 10.1128/JVI.78.11.5913-5922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssand J.S., Bajjalieh S.M. The heterotrimeric [corrected] G protein subunit G alpha i is present on mitochondria. FEBS Lett. 2007;581(December (30)):5765–5768. doi: 10.1016/j.febslet.2007.11.044. [DOI] [PubMed] [Google Scholar]
- Mackie P., Lebowitz J., Saadatpour L., Nickoloff E., Gaskill P., Khoshbouei H. The dopamine transporter: an unrecognized nexus for dysfunctional peripheral immunity and signaling in Parkinson’s disease. Brain Behav. Immun. 2018;70:21–35. doi: 10.1016/j.bbi.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pinilla E., Rodriguez-Perez A.I., Navarro G., Aguinaga D., Moreno E., Lanciego J.L., Labandeira-Garcia J.L., Franco R. Dopamine D2 and angiotensin II type 1 receptors form functional heteromers in rat striatum. Biochem. Pharmacol. 2015;96:131–142. doi: 10.1016/j.bcp.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Matsushima-Otsuka S., Fujiwara-Tani R., Sasaki T., Ohmori H., Nakashima C., Kishi S., Nishiguchi Y., Fujii K., Luo Y., Kuniyasu H. Significance of intranuclear angiotensin-II type 2 receptor in oral squamous cell carcinoma. Oncotarget. 2018;9:36561–36574. doi: 10.18632/oncotarget.26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C.A., Widdop R.E., Denton K.M., Jones E.S. Update on the angiotensin AT(2) receptor. Curr. Hypertens. Rep. 2013;15:25–30. doi: 10.1007/s11906-012-0321-4. [DOI] [PubMed] [Google Scholar]
- Mendelsohn F.A., Jenkins T.A., Berkovic S.F. Effects of angiotensin II on dopamine and serotonin turnover in the striatum of conscious rats. Brain Res. 1993;613:221–229. doi: 10.1016/0006-8993(93)90902-y. [DOI] [PubMed] [Google Scholar]
- Micakovic T., Papagiannarou S., Clark E., Kuzay Y., Abramovic K., Peters J., Sticht C., Volk N., Fleming T., Nawroth P., Hammes H.P., Alenina N., Grone H.J., Hoffmann S.C. The angiotensin II type 2 receptors protect renal tubule mitochondria in early stages of diabetes mellitus. Kidney Int. 2018;94:937–950. doi: 10.1016/j.kint.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Milsted A., Barna B.P., Ransohoff R.M., Brosnihan K.B., Ferrario C.M. Astrocyte cultures derived from human brain tissue express angiotensinogen mRNA. Proc. Natl. Acad. Sci. U. S. A. 1990;87:5720–5723. doi: 10.1073/pnas.87.15.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C., Nash S.R., Robinson S.W., Jaber M., Caron M.G. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Model K., Prinz T., Ruiz T., Radermacher M., Krimmer T., Kühlbrandt W., Pfanner N., Meisinger C. Protein translocase of the outer mitochondrial membrane: role of import receptors in the structural organization of the TOM complex. J. Mol. Biol. 2002;316:657–666. doi: 10.1006/jmbi.2001.5365. [DOI] [PubMed] [Google Scholar]
- Mudo G., Makela J., Di Liberto V., Tselykh T.V., Olivieri M., Piepponen P., Eriksson O., Malkia A., Bonomo A., Kairisalo M., Aguirre J.A., Korhonen L., Belluardo N., Lindholm D. Transgenic expression and activation of PGC-1alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell. Mol. Life Sci. 2012;69:1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa P., Nair A.R., Agbor L.N., Gomez J., Wu J., Zhang S.Y., Lu K.T., Morgan D.A., Rahmouni K., Grobe J.L., Sigmund C.D. Increased susceptibility of mice lacking Renin-b to angiotensin II-Induced organ damage. Hypertension. 2020;76:468–477. doi: 10.1161/HYPERTENSIONAHA.120.14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen G., Contrepas A. The (pro)renin receptors. J. Mol. Med. 2008;86:643–646. doi: 10.1007/s00109-008-0319-1. [DOI] [PubMed] [Google Scholar]
- Nishimura H. Renin-angiotensin system in vertebrates: phylogenetic view of structure and function. Anat. Sci. Int. 2017;92:215–247. doi: 10.1007/s12565-016-0372-8. [DOI] [PubMed] [Google Scholar]
- Nunez R.E., Castro M., Javadov S., Escobales N. Angiotensin II and ischemic preconditioning synergize to improve mitochondrial function while showing additive effects on ventricular postischemic recovery. J. Cardiovasc. Pharmacol. 2014;64:172–179. doi: 10.1097/FJC.0000000000000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez R.E., Javadov S., Escobales N. Angiotensin II-preconditioning is associated with increased PKCepsilon/PKCdelta ratio and prosurvival kinases in mitochondria. Clin. Exp. Pharmacol. Physiol. 2017;44:1201–1212. doi: 10.1111/1440-1681.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez R.E., Javadov S., Escobales N. Critical role of angiotensin II type 2 receptors in the control of mitochondrial and cardiac function in angiotensin II-preconditioned rat hearts. Pflugers Arch. 2018;470:1391–1403. doi: 10.1007/s00424-018-2153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offen D., Shtaif B., Hadad D., Weizman A., Melamed E., Gil-Ad I. Protective effect of insulin-like-growth-factor-1 against dopamine-induced neurotoxicity in human and rodent neuronal cultures: possible implications for Parkinson’s disease. Neurosci. Lett. 2001;316:129–132. doi: 10.1016/s0304-3940(01)02344-8. [DOI] [PubMed] [Google Scholar]
- Ou Z., Jiang T., Gao Q., Tian Y.Y., Zhou J.S., Wu L., Shi J.Q., Zhang Y.D. Mitochondrial-dependent mechanisms are involved in angiotensin II-induced apoptosis in dopaminergic neurons. J. Renin. Syst. 2016;17 doi: 10.1177/1470320316672349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padia S.H., Carey R.M. AT2 receptors: beneficial counter-regulatory role in cardiovascular and renal function. Pflugers Arch. 2013;465:99–110. doi: 10.1007/s00424-012-1146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parga J.A., Rodriguez-Perez A.I., Garcia-Garrote M., Rodriguez-Pallares J., Labandeira-Garcia J.L. Angiotensin II induces oxidative stress and upregulates neuroprotective signaling from the NRF2 and KLF9 pathway in dopaminergic cells. Free Radic. Biol. Med. 2018;129:394–406. doi: 10.1016/j.freeradbiomed.2018.10.409. [DOI] [PubMed] [Google Scholar]
- Patel S., Hussain T. Dimerization of AT2 and mas receptors in control of blood pressure. Curr. Hypertens. Rep. 2018;20:41. doi: 10.1007/s11906-018-0845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz Ocaranza M., Riquelme J.A., Garcia L., Jalil J.E., Chiong M., Santos R.A.S., Lavandero S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020;17:116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergrass K.D., Gwathmey T.M., Michalek R.D., Grayson J.M., Chappell M.C. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem. Biophys. Res.Commun. 2009;384:149–154. doi: 10.1016/j.bbrc.2009.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Kranzlin B., Schaeffer S., Zimmer J., Resch S., Bachmann S., Gretz N., Hackenthal E. Presence of renin within intramitochondrial dense bodies of the rat adrenal cortex. Am. J. Physiol. 1996;271:E439–450. doi: 10.1152/ajpendo.1996.271.3.E439. [DOI] [PubMed] [Google Scholar]
- Phillips M.I., de Oliveira E.M. Brain renin angiotensin in disease. J. Mol. Med. 2008;86:715–722. doi: 10.1007/s00109-008-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puche J.E., Garcia-Fernandez M., Muntane J., Rioja J., Gonzalez-Baron S., Castilla Cortazar I. Low doses of insulin-like growth factor-I induce mitochondrial protection in aging rats. Endocrinology. 2008;149:2620–2627. doi: 10.1210/en.2007-1563. [DOI] [PubMed] [Google Scholar]
- Re R.N. Role of intracellular angiotensin II. Am. J. Physiol. Heart Circ. Physiol. 2018;314:H766–H771. doi: 10.1152/ajpheart.00632.2017. [DOI] [PubMed] [Google Scholar]
- Re R.N., Cook J.L. The mitochondrial component of intracrine action. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H577–583. doi: 10.1152/ajpheart.00421.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R.N., Cook J.L. Studies of intracellular angiotensin II. Methods Mol. Biol. 2015;1234:1–8. doi: 10.1007/978-1-4939-1755-6_1. [DOI] [PubMed] [Google Scholar]
- Re R.N., Vizard D.L., Brown J., LeGros L., Bryan S.E. Angiotensin II receptors in chromatin. J. Hypertens. Suppl. 1984;2:S271–273. [PubMed] [Google Scholar]
- Redding K.M., Chen B.L., Singh A., Re R.N., Navar L.G., Seth D.M., Sigmund C.D., Tang W.W., Cook J.L. Transgenic mice expressing an intracellular fluorescent fusion of angiotensin II demonstrate renal thrombotic microangiopathy and elevated blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H1807–1818. doi: 10.1152/ajpheart.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., Rodriguez-Sabate C., Morales I., Sanchez A., Sabate M. Parkinson’s disease as a result of aging. Aging Cell. 2015;14:293–308. doi: 10.1111/acel.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pallares J., Rey P., Parga J.A., Munoz A., Guerra M.J., Labandeira-Garcia J.L. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol. Dis. 2008;31:58–73. doi: 10.1016/j.nbd.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pallares J., Parga J.A., Joglar B., Guerra M.J., Labandeira-Garcia J.L. The mitochondrial ATP-sensitive potassium channel blocker 5-hydroxydecanoate inhibits toxicity of 6-hydroxydopamine on dopaminergic neurons. Neurotox. Res. 2009;15:82–95. doi: 10.1007/s12640-009-9010-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pallares J., Parga J.A., Joglar B., Guerra M.J., Labandeira-Garcia J.L. Mitochondrial ATP-sensitive potassium channels enhance angiotensin-induced oxidative damage and dopaminergic neuron degeneration. Relevance for aging-associated susceptibility to Parkinson’s disease. Age (Dordr.) 2012;34:863–880. doi: 10.1007/s11357-011-9284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Perez A.I., Valenzuela R., Villar-Cheda B., Guerra M.J., Labandeira-Garcia J.L. Dopaminergic neuroprotection of hormonal replacement therapy in young and aged menopausal rats: role of the brain angiotensin system. Brain. 2012;135:124–138. doi: 10.1093/brain/awr320. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Perez A.I., Borrajo A., Diaz-Ruiz C., Garrido-Gil P., Labandeira-Garcia J.L. Crosstalk between insulin-like growth factor-1 and angiotensin-II in dopaminergic neurons and glial cells: role in neuroinflammation and aging. Oncotarget. 2016;7:30049–30067. doi: 10.18632/oncotarget.9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Perez A.I., Garrido-Gil P., Pedrosa M.A., Garcia-Garrote M., Valenzuela R., Navarro G., Franco R., Labandeira-Garcia J.L. Angiotensin type 2 receptors: role in aging and neuroinflammation in the substantia nigra. Brain Behav. Immun. 2020;87:256–271. doi: 10.1016/j.bbi.2019.12.011. [DOI] [PubMed] [Google Scholar]
- Saavedra J.M. Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin. Sci. 2012;123:567–590. doi: 10.1042/CS20120078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra J.M. COVID-19, angiotensin receptor blockers, and the brain. Cell. Mol. Neurobiol. 2020;40:667–674. doi: 10.1007/s10571-020-00861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaba M.C., Martin-Estal I., Puche J.E., Castilla-Cortazar I. Insulin-like growth factor 1 (IGF-1) therapy: mitochondrial dysfunction and diseases. Biochim. Biophys. Acta. 2016;1862:1267–1278. doi: 10.1016/j.bbadis.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Sadasivan J., Singh M., Sarma J.D. Cytoplasmic tail of coronavirus spike protein has intracellular targeting signals. J. Biosci. 2017;42:231–244. doi: 10.1007/s12038-017-9676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R.A., Simoes e Silva A.C., Maric C., Silva D.M., Machado R.P., de Buhr I., Heringer-Walther S., Pinheiro S.V., Lopes M.T., Bader M., Mendes E.P., Lemos V.S., Campagnole-Santos M.J., Schultheiss H.P., Speth R., Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R.A.S., Sampaio W.O., Alzamora A.C., Motta-Santos D., Alenina N., Bader M., Campagnole-Santos M.J. The ACE2/Angiotensin-(1-7)/MAS Axis of the renin-angiotensin system: focus on angiotensin-(1-7) Physiol. Rev. 2018;98:505–553. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla R.C. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann. N. Y. Acad. Sci. 2008;1147:321–334. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–62. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund C.D., Diz D.I., Chappell M.C. No brain renin-angiotensin system: deja vu all over again? Hypertension. 2017;69:1007–1010. doi: 10.1161/HYPERTENSIONAHA.117.09167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V.P., Le B., Khode R., Baker K.M., Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes. 2008;57:3297–3306. doi: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.K., Chaubey G., Chen J.Y., Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am. J. Physiol. Cell Physiol. 2020;319:C258–C267. doi: 10.1152/ajpcell.00224.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckelings U.M., Kaschina E., Unger T. The AT2 receptor--a matter of love and hate. Peptides. 2005;26:1401–1409. doi: 10.1016/j.peptides.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Stornetta R.L., Hawelu-Johnson C.L., Guyenet P.G., Lynch K.R. Astrocytes synthesize angiotensinogen in brain. Science. 1988;242:1444–1446. doi: 10.1126/science.3201232. [DOI] [PubMed] [Google Scholar]
- Suofu Y., Li W., Jean-Alphonse F.G. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E7997–E8006. doi: 10.1073/pnas.1705768114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadevosyan A., Maguy A., Villeneuve L.R., Babin J., Bonnefoy A., Allen B.G., Nattel S. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J. Biol. Chem. 2010;285:22338–22349. doi: 10.1074/jbc.M110.121749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadevosyan A., Xiao J., Surinkaew S., Naud P., Merlen C., Harada M., Qi X., Chatenet D., Fournier A., Allen B.G., Nattel S. Intracellular Angiotensin-II interacts with nuclear angiotensin receptors in cardiac fibroblasts and regulates RNA synthesis, cell proliferation, and collagen secretion. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B.L. Sirt1 and the mitochondria. Mol. Cells. 2016;39:87–95. doi: 10.14348/molcells.2016.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno M., Kuno A., Yano T., Miura T., Hisahara S., Ishikawa S., Shimamoto K., Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J. Biol. Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thekkumkara T., Linas S.L. Role of internalization in AT(1A) receptor function in proximal tubule epithelium. Am. J. Physiol. Renal Physiol. 2002;282:F623–629. doi: 10.1152/ajprenal.00118.2001. [DOI] [PubMed] [Google Scholar]
- Thomas W.G., Greenland K.J., Shinkel T.A., Sernia C. Angiotensinogen is secreted by pure rat neuronal cell cultures. Brain Res. 1992;588:191–200. doi: 10.1016/0006-8993(92)91575-y. [DOI] [PubMed] [Google Scholar]
- Tigerstedt R., Bergman P.G. Niere und kreislauf. Skand. Arch. Physiol. 1898;8:223–271. [Google Scholar]
- Valenzuela R., Barroso-Chinea P., Villar-Cheda B., Joglar B., Munoz A., Lanciego J.L., Labandeira-Garcia J.L. Location of prorenin receptors in primate substantia nigra: effects on dopaminergic cell death. J. Neuropathol. Exp. Neurol. 2010;69:1130–1142. doi: 10.1097/NEN.0b013e3181fa0308. [DOI] [PubMed] [Google Scholar]
- Valenzuela R., Costa-Besada M.A., Iglesias-Gonzalez J., Perez-Costas E., Villar-Cheda B., Garrido-Gil P., Melendez-Ferro M., Soto-Otero R., Lanciego J.L., Henrion D., Franco R., Labandeira-Garcia J.L. Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell Death Dis. 2016;7:e2427. doi: 10.1038/cddis.2016.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kats J.P., van Meegen J.R., Verdouw P.D., Duncker D.J., Schalekamp M.A., Danser A.H. Subcellular localization of angiotensin II in kidney and adrenal. J. Hypertens. 2001;19:583–589. doi: 10.1097/00004872-200103001-00010. [DOI] [PubMed] [Google Scholar]
- van Thiel B.S., Goes Martini A., Te Riet L., Severs D., Uijl E., Garrelds I.M., Leijten F.P.J., van der Pluijm I., Essers J., Qadri F., Alenina N., Bader M., Paulis L., Rajkovicova R., Domenig O., Poglitsch M., Danser A.H.J. Brain renin-angiotensin system: does it exist? Hypertension. 2017;69:1136–1144. doi: 10.1161/HYPERTENSIONAHA.116.08922. [DOI] [PubMed] [Google Scholar]
- Vidal P.M., Pacheco R. Targeting the dopaminergic system in autoimmunity. J. Neuroimmune Pharmacol. 2019 doi: 10.1007/s11481-019-09834-5. [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B., Rodriguez-Pallares J., Valenzuela R., Munoz A., Guerra M.J., Baltatu O.C., Labandeira-Garcia J.L. Nigral and striatal regulation of angiotensin receptor expression by dopamine and angiotensin in rodents: implications for progression of Parkinson’s disease. Eur. J. Neurosci. 2010;32:1695–1706. doi: 10.1111/j.1460-9568.2010.07448.x. [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B., Valenzuela R., Rodriguez-Perez A.I., Guerra M.J., Labandeira-Garcia J.L. Aging-related changes in the nigral angiotensin system enhances proinflammatory and pro-oxidative markers and 6-OHDA-induced dopaminergic degeneration. Neurobiol. Aging. 2012;33(204):e201–211. doi: 10.1016/j.neurobiolaging.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B., Dominguez-Meijide A., Valenzuela R., Granado N., Moratalla R., Labandeira-Garcia J.L. Aging-related dysregulation of dopamine and angiotensin receptor interaction. Neurobiol. Aging. 2014;35:1726–1738. doi: 10.1016/j.neurobiolaging.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B., Costa-Besada M.A., Valenzuela R., Perez-Costas E., Melendez-Ferro M., Labandeira-Garcia J.L. The intracellular angiotensin system buffers deleterious effects of the extracellular paracrine system. Cell Death Dis. 2017;8:e3044. doi: 10.1038/cddis.2017.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinciguerra M., Santini M.P., Claycomb W.C., Ladurner A.G., Rosenthal N. Local IGF-1 isoform protects cardiomyocytes from hypertrophic and oxidative stresses via SirT1 activity. Aging (Albany NY) 2009;2:43–62. doi: 10.18632/aging.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Coleman C.G., Glass M.J., Zhou P., Yu Q., Park L., Anrather J., Pickel V.M., Iadecola C. Angiotensin II type 2 receptor-coupled nitric oxide production modulates free radical availability and voltage-gated Ca2+ currents in NTS neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R1076–1083. doi: 10.1152/ajpregu.00571.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanka H., Lutze P., Staar D., Grunow B., Peters B.S., Peters J. An alternative renin isoform is cardioprotective by modulating mitochondrial metabolism. J. Cell. Mol. Med. 2018;22:5991–6001. doi: 10.1111/jcmm.13872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanka H., Lutze P., Staar D., Albers A., Bäumgen I., Grunow B., Peters J. Non-secretory renin reduces oxidative stress and increases cardiomyoblast survival during glucose and oxygen deprivation. Sci. Rep. 2020;10:2329. doi: 10.1038/s41598-020-59216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson C.D., Colberg-Poley A.M. Access of viral proteins to mitochondria via mitochondria-associated membranes. Rev. Med. Virol. 2009;19:147–164. doi: 10.1002/rmv.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B.A., Nautiyal M., Gwathmey T.M., Rose J.C., Chappell M.C. Evidence for a mitochondrial angiotensin-(1-7) system in the kidney. Am. J. Physiol. Renal Physiol. 2016;310:F637–F645. doi: 10.1152/ajprenal.00479.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B.A., Cruz-Diaz N., Su Y., Rose J.C., Gwathmey T.M., Chappell M.C. Angiotensinogen import in isolated proximal tubules: evidence for mitochondrial trafficking and uptake. Am. J. Physiol. Renal Physiol. 2017;312:F879–F886. doi: 10.1152/ajprenal.00246.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M.K.S., Takei Y. Molecular and evolutionary perspectives of the renin-angiotensin system from lamprey. Gen. Comp. Endocrinol. 2018;257:137–142. doi: 10.1016/j.ygcen.2017.01.031. [DOI] [PubMed] [Google Scholar]
- Wosniak J., Jr., Santos C.X., Kowaltowski A.J., Laurindo F.R. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid. Redox Signal. 2009;11:1265–1278. doi: 10.1089/ars.2009.2392. [DOI] [PubMed] [Google Scholar]
- Xu C., Yuan X., Pan Z., Shen G., Kim J.H., Yu S., Khor T.O., Li W., Ma J., Kong A.N. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol. Cancer Ther. 2006;5:1918–1926. doi: 10.1158/1535-7163.MCT-05-0497. [DOI] [PubMed] [Google Scholar]
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Yao B., Zhou Y., Yin H., Zhang M.Z., Harris R.C. Intrarenal dopamine modulates progressive angiotensin II-mediated renal injury. Am. J. Physiol. Renal Physiol. 2012;302:F742–749. doi: 10.1152/ajprenal.00583.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Shan Y., Yao Z., Li J., Zhao Z., Chen J., Cong Y. Mitochondrial location of severe acute respiratory syndrome coronavirus 3b protein. Mol. Cells. 2006;21:186–191. [PubMed] [Google Scholar]
- Yuan Y., Cruzat V.F., Newsholme P., Cheng J., Chen Y., Lu Y. Regulation of SIRT1 in aging: roles in mitochondrial function and biogenesis. Mech. Ageing Dev. 2016;155:10–21. doi: 10.1016/j.mad.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Yun J., Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.X., Gutterman D.D. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2023–2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- Zhang M., An C., Gao Y., Leak R.K., Chen J., Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Luo Y., Sato S., Tanabe E., Kitayoshi M., Fujiwara R., Sasaki T., Fujii K., Ohmori H., Kuniyasu H. Role of two types of angiotensin II receptors in colorectal carcinoma progression. Pathobiology. 2014;81:169–175. doi: 10.1159/000362092. [DOI] [PubMed] [Google Scholar]
- Zhuo J.L., Li X.C., Garvin J.L., Navar L.G., Carretero O.A. Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am. J. Physiol. Renal Physiol. 2006;290:F1382–1390. doi: 10.1152/ajprenal.00269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.