Abstract.
Snakebite envenoming is a neglected, public health problem in tropical and subtropical regions. Local tissue necrosis, neurotoxic, and hemo-vasculotoxic effects are well-recognized features, whereas the endocrine and metabolic derangements are not as well known. In addition to contributing to morbidity, some of these manifestations can be potentially life-threatening if not recognized early. The most prominent endocrine manifestation is hypopituitarism (HP), which can manifest acutely or remain asymptomatic and present years later. Unexplained recurrent hypoglycemia and refractory hypotension are early clinical clues to suspect corticotroph axis involvement in acute settings. Chronic pituitary failure may present, like Sheehan’s syndrome, several years after the bite. The occurrence of acute kidney injury, capillary leak syndrome, and disseminated intravascular coagulation are predictors of HP. Adrenal hemorrhages are documented in autopsy series; however, primary adrenal insufficiency is very rare and confounded by the presence of HP. Hyponatremia, hypokalemia or hyperkalemia, and dysglycemia can occur, but the mechanisms involved are only partially understood. Awareness, a high index of suspicion, correct interpretation of hormonal parameters, and timely treatment of these abnormalities can be lifesaving.
INTRODUCTION
Snakebite envenoming is a common but neglected health problem in tropical countries.1,2 The annual worldwide mortality attributed to snakebite is as high as 138,000.3–6 This is likely to be an underestimation, as snakebites are not notifiable in many countries, and several bite-related deaths may remain unreported. The estimated mortality from snakebite in India alone is approximately 58,000 per year.7
There are nearly 200 species of medically relevant venomous snakes, most of which belong to Elapidae and Viperidae family (and occasionally family Lamprophiidae and subfamily Atractaspidinae, and family Colubridae [sensu lato]).8 They differ in the chemical composition of their venom and the structure of the venom-delivery apparatus. Snake venom is a complex mixture of proteins, peptides, carbohydrates, lipids, amines, and other small molecules, with effects depending on the type of bite, the amount delivered, and several other factors.9,10 Usually, elapid bites (e.g., cobras, kraits, mambas, coral snakes and certain sea snakes) cause neurotoxic effects, whereas viperid bites cause local tissue destruction and vascular toxicity. This dichotomy in clinical manifestations is not absolute, with an occasional overlap in features of envenoming.11 Clinical manifestations may be local (swelling, blebs, and tissue necrosis) or systemic, involving the neuromuscular system (paralysis and/or rhabdomyolysis), cardiovascular system (hypotension and collapse), and hemostatic system (disseminated intravascular coagulation [DIC]).12,13 Endocrine and metabolic derangements, which include pituitary gland (anterior and rarely posterior) dysfunction, adrenal involvement, dysglycemia, electrolyte abnormalities, and type 4 renal tubular acidosis (T4RTA), are lesser known systemic manifestations which contribute significantly to mortality and morbidity. This review focuses on endocrine and metabolic consequences of snakebite envenoming.
LITERATURE SEARCH STRATEGY
References for this review were identified through searches of PubMed for articles published till May 2020, by use of the terms “snakebite,” “snake bite,” “snakebite envenoming,” “snakebite envenomation,” “snake envenoming,” “snake envenomation,” “snakebite poisoning,” “Russell’s viper (RV)” and “Russell’s viper (RVE) envenoming.” in combination with the words “endocrine,” “hormone,” “hypopituitarism (HP),” “pituitary insufficiency,” “hypocortisolism,” “hypothyroidism,” “hypogonadism,” “diabetes insipidus (DI)” “hypoglycemia,” “hyperglycemia,” “electrolytes,” “dyselectrolytemia,” “hyponatremia,” “hypernatremia,” “hypokalemia,” “hyperkalemia,” “alkalosis,” and “acidosis.” Relevant articles were also identified through searches in the authors’ files and Google Scholar. Articles resulting from these searches and related references cited in those articles were reviewed. Articles published in the English language were included.
PITUITARY DISORDERS
Hypopituitarism is an uncommon but well-recognized complication of viperid envenomings, particularly of RV species (both Daboia russelii and Daboia siamensis).14–20 Hypopituitarism following snake envenoming was first described by Wolff in 1958 after bite of Bothrops jararacussu.14,15 Eapen et al.16 working in Angamaly, Kerala, provided the first reports from India, of both anterior and, later, posterior pituitary dysfunction following D. russelii bite. Tun-Pe et al.17 first reported RVE–related acute and chronic pituitary failure from Myanmar. There are reports of both acute and chronic forms of HP from India, Myanmar, and Sri Lanka, but not from other South Asian countries (e.g., Pakistan, Bangladesh, Nepal, Thailand, Cambodia, China, Taiwan, and Indonesia), which are also home to this deadly species. This peculiar geographical difference in clinical features parallels the variation in venom composition of the same snake species inhabiting different geographic locations. This observation is further supported by geographic clustering of other clinical manifestations like conjunctival edema and capillary leak syndrome (CLS) in Myanmar and India, and rhabdomyolysis and presynaptic neurotoxicity in Sri Lanka and India.11,21–23 Recent venomics and proteomics-based analysis have demonstrated considerable compositional, functional, and immunological differences among geographic variants of RV venoms across the Indian subcontinent.24
Pathophysiology of pituitary insufficiency.
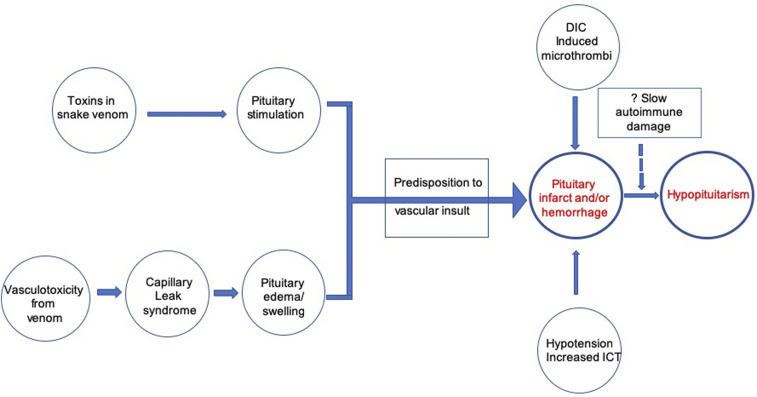
The exact pathophysiology of HP following RVE is unknown. It is postulated that mechanisms may be similar to Sheehan’s syndrome (SS), where pituitary apoplexy occurs in an enlarged and vulnerable gland with limited vascular supply (Figure 1).18,19,25 The heightened vulnerability of the pituitary gland to vascular insults following RVE presumably results from two mechanisms:
2. Direct stimulatory effects of RV toxin on pituitary cells—RV venom can stimulate a dose-dependent release of growth hormone (GH), adrenocorticotrophic hormone (ACTH), and thyroid-stimulating hormone (TSH) from rat pituitary cell cultures, without cell lysis.27,28
Figure 1.
Pathophysiology of hypopituitarism following Russell’s viper bite. DIC = disseminated intravascular coagulation; ICT = intracranial tension. This figure appears in color at www.ajtmh.org.
Vascular insult to this stimulated and engorged pituitary gland may ensue from the following changes:
1. Microthrombi deposition or overt bleeding due to DIC, impairing pituitary vascular supply.26,29
2. Changes in pituitary intravascular pressure triggered by CLS.18,19,30
3. Susceptibility of anterior pituitary vasculature to compressive effects of even minor intrasellar pressure increments, owing to its location in an enclosed, bony sella turcica.31
4. Hypotension from circulatory shock.26
5. Increase in intracranial pressure.26
In an autopsy series of 52 patients, DIC was found to be a significant predictor of pituitary hemorrhage or necrosis.26 In another large series, increased whole blood clotting time (a quantitative indicator of DIC) at presentation was predictive for the development of HP.32 Thus, whereas the vascular insult is multifactorial in etiology, DIC plays an important role, with contribution from CLS.
Lastly, it has been hypothesized that identical to SS, there may be an additional, autoantibody-mediated slow destruction of the pituitary gland over time after the initial envenoming, that presents as delayed HP.19 Pituitary autoimmunity, triggered by the disclosure of “sequestered” pituitary antigens by pituitary tissue necrosis during the acute insult, could play a role in the pathogenesis of RVE-associated delayed HP. In SS, anti-pituitary and anti-hypothalamic antibodies have been demonstrated, suggesting a probable role of pituitary autoimmunity in anterior pituitary dysfunction.33,34 Literature search did not reveal that the presence of antibodies to pituitary has been studied in RVE-induced HP to date and remains an area of potential research.
Clinical features of pituitary insufficiency.
Russell’s viper envenoming–related HP can occur acutely within 1 day to 2 weeks of the bite or be delayed as long as 24 years.17,19,35,36 Acute features are recurrent hypoglycemia and refractory hypotension, which respond to glucocorticoids.36,37 Chronic pituitary dysfunction may remain asymptomatic or present insidiously, weeks to years later, with fatigue, weight loss, and loss of appetite.17,32,38 Occasionally, HP is detected only by dynamic pituitary function testing.39,40 The mean time to diagnosis of delayed HP after viper bite was 8 years in a series from Southern India.38
Chronic HP may or may not be preceded by acute pituitary dysfunction.17,36,38 On the other hand, acute pituitary insufficiency (API) can be transient or persist indefinitely.20,32,39 It is unknown whether the pituitary function remains normal, in the interval between initial envenoming and diagnosis of delayed pituitary dysfunction. In all probability, chronic HP may be a continuum of clinically manifest or occult pituitary damage, sustained at initial envenoming.
Cortisol and GH axes have been reported to be most commonly affected; however, this pattern of pituitary dysfunction is not universal.17,28 Naik et al.39 reported that somatotrophs (83%) are most commonly affected, followed by gonadotrophs (50%). In the setting of vasculotoxic bite–induced AKI, corticotrophs were found to be commonly involved (39%) followed by gonadotrophs and lactotrophs.32 Gonadotroph axis involvement before puberty can lead to pubertal delay or arrest, resulting in primary amenorrhea in females and the absence of secondary sexual characters in both the genders. Affection, later on, can cause secondary amenorrhea in females, erectile dysfunction in males, and infertility, loss of libido, and loss of secondary sexual characters in both the genders. Delayed puberty and short stature have been reported in a boy who was bitten at the age of 12 years.40 Hypogonadism can also occur from primary involvement of gonads, as observed in the case report of an elderly man who presented with bilateral painful gynecomastia 2 months after a taipan (Oxyuranus scutellatus) envenomation.41
Potential risk factors of API in a study of nine patients of RVE were severe envenoming, AKI, mechanical ventilation, CLS, and DIC.36 In those developing AKI, HP was associated in cases requiring more hemodialysis sessions and having high 20-minute whole blood clotting time at presentation.32 Golay et al.37 found that in snake bite patients with AKI, the occurrence of chronic kidney disease on follow-up predicted the development of chronic HP. In the case series by Naik et al.,39 the occurrence of HP did not correlate with the existence of renal failure, coagulopathy, or clinical scoring of the severity of illness.
Diagnosis.
Several factors hinder pituitary function assessment in snakebite patients in emergency settings, especially in resource-limited conditions. These include critical illness–related pituitary dysfunction, prior glucocorticoid injection, and possible preexisting pituitary or adrenal dysfunction due to unrelated causes. Sometimes adrenal hemorrhage due to CLS or DIC can cause hypocortisolism seen in the acute phase of snakebite envenoming (discussed in the following text). Hypoglycemia and unexplained hypotension are critical indicators of HP. Hypotension is often multifactorial with the contribution of cardiogenic causes, vasodilation due to direct venom effect or systemic inflammatory response syndrome, CLS, sepsis, and endocrine reasons.26
Among the pituitary hormonal disturbances, identification of hypocortisolism takes precedence, given the therapeutic implication of glucocorticoid supplementation, which is lifesaving in the acute phase of envenoming. If secondary hypocortisolism (low ACTH) is documented, then the thyroid axis also requires assessment. A diagnosis of secondary hypothyroidism may be entertained if thyroxine (T4) and tri-iodothyronine (T3) levels are low along with low or inappropriately normal TSH levels. A similar pattern can also occur in sick euthyroid syndrome; thus, these results should be interpreted with caution. Evaluation of the gonadal axis and GH-insulin-like growth factor-1 axis should be deferred until the patient is stable.
Assessment of adrenal insufficiency (AI) in a critically ill patient is complex. Table 1 summarizes various criteria that have been used in various studies to define AI in RVE. A critical care task force on critical illness–related corticosteroid insufficiency (CIRCI) suggested that a rise in serum cortisol < 9 µg/dL after 60 minutes of injecting 250 µg of cosyntropin or a random plasma cortisol < 10 µg/dL may be considered diagnostic of CIRCI. The task force also suggested administering intravenous hydrocortisone up to a full dose of 400 mg/day for 3 days or more in patients with septic shock not responding to fluid and moderate- to high-dose vasopressors (conditional, low quality of evidence).42,43
Table 1.
Criteria used in various studies for the diagnosis of adrenal insufficiency after Russell’s viper envenoming
| Author (year) | Selection criteria | Diagnostic criteria | Number of HP cases | Follow-up |
|---|---|---|---|---|
| Golay et al. 37* | Clinical signs and symptoms which were relevant in the acute setting | A peak stimulated cortisol (after ACTH 250 μg) < 18 μg/dL | 2 | Both persistent HP at 1 year and 13 months |
| Rajagopala et al. 36 | 1. Hypotension [SBP < 90 mm Hg] and unexplained hypoglycemia (at least one episode of VBG level < 55 mg/dL) | 1. Random cortisol values < 4.3 μg/dL | 9 | Three of five survivors had HP at 1 year follow-up, two not completed 1 year, and four died |
| 2. Unexplained hypoglycemia or persistent hypotension and resolution on institution of parenteral hydrocortisone | ||||
| 2. Persistent hypotension (SBP < 90 mm Hg despite administration of at least 20 mL/kg crystalloids or a need for vasopressors to achieve SBP > 90 mm Hg) | ||||
| 3. Unexplained hypoglycemia alone | ||||
| Naik et al. 39 | Envenoming by venomous snake | Plasma cortisol of less than 350 nmol/L (established from institute’s in‐house assay) with inappropriately low ACTH | Five (three with secondary AI and two with primary AI) | At 6 months, secondary AI in 2 and primary AI in 1 (confirmed by provocation test) |
| Gopalakrishnan et al. 26 | Circulatory shock defined as SBP < 90 mm Hg despite intravenous fluid support of 20 mL/kg crystalloids and persistent oliguria (urine output < 0.5 mL/kg/hour for > 2 hours) or the need for vasopressor support to maintain SBP > 90 mm Hg | Random serum cortisol < 10 μg/dL | 12 (63%) of 19 patients with circulatory shock. HP also diagnosed in 4/5 patients who had hypoglycemia, hypotension, and hyperkalemia | Not available |
| Bhat et al. 32 | Patients with acute kidney injury following vasculotoxic envenoming | Serum cortisol < 3μg/dL with low or inappropriately normal ACTH (OR) cosyntropin stimulated cortisol < 18 μg/dL | 11 (21%) of 51 in acute phase. None were symptomatic. | 13 (two more) at 3 months |
ACTH = adrenocorticotrophic hormone; AI = adrenal insufficiency; HP = hypopituitarism; SBP = systolic blood pressure; VBG = venous blood glucose.
Of nine cases, two were diagnosed within first 2 weeks and considered as acute HP.
There is limited evidence regarding the applicability of these criteria, primarily proposed for use in septic shock, for RVE cases; however, in the absence of any other standards, the authors recommend the use of these definitions for the diagnosis of AI (Table 2). Thus, in resource-limited settings, which usually is the case in snakebite envenoming, a random plasma cortisol value of < 10 µg/dL in a critically ill patient of RVE can be considered to be diagnostic of hypocortisolism. If the immediate estimation of cortisol is not possible in a rural setup, then glucocorticoid supplementation can be started without waiting for the reports, in those with a high index of suspicion, after collecting a blood sample for random cortisol estimation.
Table 2.
Recommendations for management of acute pituitary insufficiency following snakebite envenoming
| A high index of suspicion for the development of acute HP following suspected or proven RVE must be maintained (especially in India, Sri Lanka, and Myanmar) |
| Presence of clinical features such as capillary leak syndrome, disseminated intravascular coagulation, or AKI are risk factors for developing acute HP |
| Laboratory tests for HP to be performed in acute settings in the following situations |
| Persistent or unexplained hypotension |
| Hypoglycemia |
| Persistent hyperkalemia |
| Evidence of pituitary hemorrhage on imaging (magnetic resonance imaging) |
| Random plasma cortisol of < 10 µg/dL or a delta cortisol (change in baseline cortisol at 60 minutes after cosyntropin 250 µg) of < 9 µg/dL is consistent with the diagnosis of hypocortisolism (results not interpretable if glucocorticoid has been administered before testing) |
| Intravenous hydrocortisone up to 400 mg/day in three to four divided doses can be administered. In case of strong clinical suspicion and pressing indications (e.g., unresponsive hypotension), hydrocortisone should be started after collecting appropriate samples and not delayed till the availability of hormone analysis |
| Consider shifting to oral glucocorticoid once clinically stable and oral intake is adequate |
| Diagnosis of secondary hypothyroidism should be strongly suspected if serum T4 and T3 levels are low along with a low or inappropriately normal serum thyroid-stimulating hormone level, and thyroxine should be supplemented (in conjugation with corticosteroid axis affection, and, in its absence, sick euthyroid syndrome is a possibility) |
| Rest of the pituitary and related hormones (LH, FSH, testosterone or estradiol, prolactin, and IGF-1) should be analyzed, and diagnosis of hypocortisolism and hypothyroidism should be reconfirmed during a follow-up visit after 6–8 weeks of discharge |
| Resource-limited settings |
| Random plasma cortisol value of < 10 µg/dL in a critically ill patient of RVE can be considered to be diagnostic of hypocortisolism |
| In those with high index of suspicion, if immediate estimation of cortisol is not possible, then glucocorticoid supplementation can be started without waiting for the reports after collecting a blood sample for random cortisol estimation |
FSH = follicle-stimulating hormone; HP = hypopituitarism; IGF-1 = insulin like growth factor 1; LH = luteinizing hormone; RVE = Russell’s viper envenoming; T3 = tri-iodothyronine; T4 = thyroxine.
Diabetes insipidus.
Diabetes insipidus is characterized by large quantities of hypotonic urine, caused by deficiency (central) or resistance to the (nephrogenic) action of arginine vasopressin (AVP). A rare disease in itself, its occurrence in snake envenoming is even more unusual.
In the series by Eapen et al.,16 of 600 cases of snakebite, only one had DI. In another report, a 20-year-old man was diagnosed with central diabetes insipidus (CDI), HP and growth retardation eight years after the bite.40 Another case of HP with CDI presented peculiarly with torsades de pointes.44 There is one reported case of isolated CDI, with normal anterior pituitary hormones in a 14-year-old boy.45 Diabetes insipidus was diagnosed in two of 13 patients with HP, in another cohort with AKI.32
Central diabetes insipidus occurs only when 80–90% of AVP-producing hypothalamic magnocellular neurons are lost because their capacity for AVP synthesis far exceeds the daily requirements.46 The posterior pituitary acts as a storage and secretory organ rather than a site of synthesis; thus, to cause CDI, the hypothalamus must be significantly affected. The anterior pituitary is supplied by a low-pressure hypothalamo-pituitary portal system from the superior hypophyseal artery. The posterior pituitary, however, receives direct arterial supply from the inferior hypophyseal artery. The intrasellar pressure changes that can accompany CLS and DIC may be insufficient to compromise posterior pituitary arterial circulation, as opposed to the low-pressure portal system in the anterior part. Thus, the posterior pituitary is resistant to vascular insults, and RVE-related CDI is rare.47 A crucial clinical correlate in envenoming is that polyuria, an important indicator of DI, may not manifest in the presence of coexistent hypocortisolism.
Pituitary imaging
Normal sellar appearance on magnetic resonance imaging (MRI), even in the presence of HP, is a common finding in snakebite patients. In the series by Golay et al.,37 seven of eight cases had no abnormality on pituitary imaging (five of whom were imaged within a year of envenoming). One of the cases diagnosed with HP and DI after eight years had findings of partial empty sella with a loss of posterior bright spot. In a series of delayed HP cases, MRI revealed normal findings in two and empty or partially empty sella in six.38 In a prospective study, Naik et al.39 documented empty sella in two patients and normal imaging in four patients at 6 months of follow-up. Rajagopala et al.36 have also described normal pituitary in the setting of acute HP following RVE. In the case of isolated CDI diagnosed 4 months after snake bite, the posterior pituitary bright spot was absent and lower infundibular stalk was thinned out, whereas anterior pituitary was normal.48 The current literature suggests that most HP cases have a normal sellar appearance in the first year, whereas those diagnosed later usually have partial or total empty sella.
Normal pituitary sellar imaging in RVE-induced pituitary dysfunction is contrary to findings in SS, where partial or complete empty sella is usually seen.49 In the early phase of SS, a non-hemorrhagic enlarged pituitary gland with a thin rim of enhancement is seen, following which the gland atrophies gradually, resulting in an empty sella typically by 1 year.50 The authors presume that a similar sequence of events occurs in HP induced by RVE, with a normal-appearing pituitary being observed in the initial year, with subsequent progression to partial or complete empty sella in later years. It is still unclear if this is indeed true and whether sellar imaging needs to be repeated after a year of initial envenoming to detect structural pituitary changes.
Autopsy findings
Unpublished reports of acute hemorrhagic necrosis affecting 25–35% of the anterior pituitary in patients surviving 8–72 hours after RVE have been discussed by Proby et al.35 In an autopsy series from South India, 46% of pituitary specimens demonstrated either ischemic or hemorrhagic necrosis.26 In another report, focal hemorrhages and small fibrin thrombi in the pituitary were observed along with microthrombi and histological evidence of acute tubular necrosis in the kidneys, suggesting that DIC may contribute to the pathogenesis of AKI and acute hemorrhagic necrosis of pituitary.29 Rajagopala et al.36 reported areas of ischemic necrosis with central hemorrhage in two patients. Another autopsy series from Myanmar showed pituitary hemorrhage and necrosis in 36 of 84 (43%) snakebite deaths.51 Thus, the most common pituitary findings in postmortem examination were ischemic necrosis and hemorrhage.
ADRENAL DISORDERS
Adrenal hemorrhage and primary AI are rare complications of snakebite envenoming. Two patients initially diagnosed with secondary AI in the acute phase were subsequently found to have primary AI as confirmed by recovery of ACTH levels along with the persistence of inadequate response to Synacthen stimulation test.39 A diagnosis of primary AI might be missed in the presence of HP.
Adrenal hemorrhages have been documented in several small autopsy series.17,29 Right-sided adrenal hematoma along with the right hemothorax, following the saw-scaled viper (Echis carinatus) envenoming, has been reported.52 In a large autopsy series, 25% had bilateral adrenal hemorrhage, and 6% showed ischemic necrosis.26 In a recent study, of 84 cases of lethal snakebite, the autopsy revealed AKI in 98%, pituitary hemorrhage/necrosis in 43%, and adrenal gland hemorrhage in 36%.51
Like the pituitary, the adrenal gland is a highly vascular organ, and the etiology of hemorrhage and necrosis is likened to that of Waterhouse–Friderichsen syndrome associated with severe bacterial sepsis with Neisseria meningitidis and Streptococcus pneumoniae infections.53 Snake venom has active constituents with both procoagulant and hemorrhagic effects that predispose to the development of DIC and, in turn, adrenal hemorrhage or necrosis.54,55 Bilateral adrenal hemorrhage may occur because of circulatory shock due to other causes such as bleeding or CLS in the setting of RVE.56,57
DYSGLYCEMIA
Hyperglycemia has been reported in pediatric and adult populations with snakebite envenoming. Plasma glucose levels as high as 486 mg/dL at 2 hours and 223 mg/dL at 4 hours have been documented in an infant, after a lethal bite from the nose-horned viper (Vipera ammodytes ammodytes) in Croatia.58
In a retrospective study of viper bites in a pediatric population, hyperglycemia was found to correlate with the risk of high-grade envenoming based on clinical severity criteria described by Audebert et al.59 Plasma glucose > 200 mg/dL has also been reported in seven of 44 cases of the many-banded krait (Bungarus multicinctus multicinctus) envenoming in Taiwan. One of the victims was later diagnosed to have diabetes mellitus.60
Transient hyperglycemia has been reported with viperid as well as elapid envenoming. Snakebite-related dysglycemia is believed to be pathophysiologically similar to autonomic dysfunction seen in severe scorpion sting poisoning or pheochromocytoma. Scorpion envenoming results in an autonomic storm with massive catecholamine release and increases in glucagon and cortisol levels.61 The counter-regulatory hormones oppose anabolic actions of insulin, leading to hyperglycemia. Catecholamine excess is known to have deleterious effects on glucose and insulin homeostasis.62 The transient nature of this dysglycemia further supports the possibility of autonomic dysfunction.
ELECTROLYTE DISORDERS
Electrolyte imbalance unrelated to pituitary or adrenal disorders has been reported in association with snakebite envenoming. Hyponatremia, hypokalemia, and hyperkalemia have been reported in the literature.
Hyponatremia.
Hyponatremia is a potentially life-threatening complication of elapid as well as viperid snakebite envenoming. There are isolated reports of hyponatremia following krait bite from Sri Lanka and the South American coral snake (Micrurus corallinus).63,64 Severe hyponatremia has been documented in 41% of 60 consecutive victims of Chinese krait bites (B. multicinctus) of the Elapidae family. Hyponatremia usually occurred on the second or third day following the bite and was associated with raised urine sodium levels.65 A similar observation was made in another series from Vietnam where 74% of cases of the Malayan krait (Bungarus candidus) envenoming developed hyponatremia with low serum osmolality inappropriately elevated urine osmolality with appropriately low ADH levels.66 Another series of 78 cases of krait bite from Thailand reported hyponatremia in 17.6%, with severe hyponatremia (< 120 mmol/L) in four pediatric patients, two of whom developed seizures.67
Hyponatremia has also been described after envenoming from viperids, with reported cases following bites by the berg adder (Bitis Atropos), endemic to South Africa, and the hump-nosed pit viper (Hypnale hypnale) from Sri Lanka.68,69 In a series of berg adder bite victims, hyponatremia was documented in eight of nine patients. The lowest documented sodium was 111 mmol/L, and hyponatremia occurred after 18–48 hours of envenoming, similar to the temporal profile of krait bite–induced hyponatremia. Detailed laboratory assessment of four cases revealed mean serum sodium of 123 mmol/L, mean urine sodium of 114.7 mmol/L, mean plasma osmolality of 262.2 mosm/kg, and mean urine osmolality of 663.2 mosm/kg.70 By contrast, another series describing 224 envenomings of the hump-nosed pit viper (Hypnale hypnale) from western Malabar coast of India did not describe hyponatremia in the clinical manifestations.71
Early reports suggested that hyponatremia occurred because of the syndrome of inappropriate antidiuretic hormone (SIADH). Interestingly, fluid restriction, the standard management of SIADH, resulted in severe dehydration in three children, which ruled out SIADH as a possible cause and subsequent management of all hyponatremic patients with normal saline infusions yielded favorable response.72
Hyponatremia can occur because of HP in vasculotoxic envenoming (e.g., RVE), whereas in neurotoxic envenoming, it is because of a different mechanism. It may be a direct effect of venom components per se, as natriuretic peptides (NPs) have been identified in the venom of several snake species. Dendroaspis NP has been isolated from venom glands of the green mamba (Dendroaspis angusticeps) and is found to have higher potency and stability than mammalian NP. It was investigated for therapeutic benefit in heart failure.73,74 Novel NPs have been isolated and characterized from venoms of other snakes such as the inland taipan (Oxyuranus microlepidotus), Iranian viper (Pseudocerastes persicus), Brazilian rattle snake (Crotalus durissus cascavella), blunt-nosed viper (Macrovipera lebetina), eastern brown snake (Pseudonaja textilis), and mulga snake (Pseudechis australis).75
Although most cases of hyponatremia observed after snakebite envenoming are acute in nature, but standard precautions related to slow normalization of serum sodium levels should be undertaken if there is any suspicion of chronic hyponatremia (> 48 hours), to avoid osmotic demyelination syndrome.76–78
Hypokalemia.
Hypokalemia can occur as a complication of neurotoxic envenoming and contribute to muscle weakness associated with these bites. Hypokalemia was previously presumed to be due to respiratory alkalosis from hyperventilation.79 A series of common krait (Bungarus caeruleus) bite from Sri Lanka found hypokalemia in 71% (n = 210), which was associated with metabolic acidosis and normal blood gases. The authors hypothesized that β-adrenergic stimulation from the autonomic dysfunction related to neurotoxic envenoming resulted in an intracellular shift of potassium, causing hypokalemia. This study, however, did not assess for external potassium losses and the possible role of other hormonal factors like insulin and aldosterone.80 This association between the potential pathogenic role of autonomic dysfunction in hypokalemia is supported by another report from India of hypokalemia following a Sind krait (Bungarus sindanus) bite where the patient had severe autonomic disturbances and cardiac complications.81
Another report documented two victims of common krait bite, who developed deep coma and hypokalemia, with low renal potassium excretion and no evidence of gastrointestinal potassium loss (no diarrhea or ileus).82 The authors proposed that hypokalemia occurred due to an intracellular shift in a mechanism similar to barium poisoning. Barium ions increase the activity of Na+-K+-ATPase enzyme and block potassium channels to interfere with its passive diffusion, leading to a drop in extracellular potassium.83,84
Hypokalemia has also been reported following Malayan krait bites in Thailand while being conspicuously absent in envenoming by banded kraits. However, the authors acknowledge the low numbers of banded krait victims (n = 9) as compared with Malayan krait bites (n = 68) in the series.67
Although most reported cases of hypokalemia are from envenoming by elapids, it has also been reported from Hungary and Croatia following bites by Balkan adders (Vipera berus bosniensis). Despite belonging to the viperid family, this species predominantly causes neurotoxic manifestations.85 It has also been reported in RVE associated with API, which persisted for 5 days, despite high-dose intravenous potassium replacement. Because renal potassium loss was ruled out, a direct effect of components of RV venom per se, with or without contribution from stress-induced catecholamine release, was postulated to be responsible for the hypokalemia. The patient was treated with dexamethasone for API, which has no mineralocorticoid action (ruling out iatrogenic hypokalemia).20 Snake envenoming–induced redistributive hypokalemia should be managed similarly to hypokalemic periodic paralysis, and there lies a potential risk for rebound hyperkalemia later.86
Type 4 renal tubular acidosis.
Type 4 renal tubular acidosis is characterized by hyperkalemia, normal anion gap metabolic acidosis, and inappropriately low urine transtubular potassium gradient (TTKG).87 There are three reports from SriLanka of T4RTA, following bites from the hump-nosed viper (Hypnale hypnale).88,89 The first patient developed AKI, required dialysis initially, and renal function gradually recovered over 10 weeks. Acute onset lower limb paraparesis occurred at 18 weeks, accompanied by hyperkalemia and mild renal dysfunction (eGFR-76 mL/minute), normal anion gap acidosis, urine pH of 5, and TTKG of 1.8. The findings suggested T4RTA, which was further corroborated by a response to fludrocortisone. Interestingly, this was transient, with a decrease in fludrocortisone requirement and complete recovery in the next 3 weeks.88 Two other cases were documented during the polyuric phase of recovery from AKI, with transient but severe intractable hyperkalemia (requiring dialysis) with normal anion gap metabolic acidosis and low TTKG.89
It is unclear whether the mechanism of type 4 RTA in snake bite survivors is due to specific factors present in the venom or due to renal damage sustained at initial envenoming.
FUTURE DIRECTIONS
Many questions remain regarding endocrine and metabolic manifestations of snakebite envenoming. Early antivenom administration in RVE can prevent the progression of both coagulopathy and CLS, which are implicated in the pathogenesis of API. It is unclear whether this measure can prevent the occurrence of acute or chronic HP. Mortality benefits of early glucocorticoid administration in RVE, particularly in cases of refractory shock, merit exploration. Pathogenesis of electrolyte disorders and the role of pituitary autoimmunity in pituitary insufficiency are largely speculative. Specific interventions for these are possible only after a systematic investigation in larger populations. Advances in venom sequencing and next-generation proteomics may help identify specific venom components that cause particular clinical features.
CONCLUSION
Snakebite envenoming can cause major endocrine complications, which, if unrecognized, can be lethal. Acute pituitary insufficiency, hyponatremia resulting from natriuresis, and hypokalemia by the intracellular shift of potassium are emergencies that can occur after snakebite. Sometimes, the pituitary insult may go unrecognized in the acute phase and manifest years later with chronic HP. Lacunae exist in our understanding of the mechanism of these abnormalities and remain to be explored. Awareness and knowledge of these conditions will decrease morbidity and mortality resulting from snakebite.
Acknowledgments:
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1.Gutiérrez JM, Theakston RDG, Warrell DA, 2006. Confronting the neglected problem of snake bite envenoming: the need for a global partnership. PLoS Med 3: e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habib AG, Kuznik A, Hamza M, Abdullahi MI, Chedi BA, Chippaux J-P, Warrell DA, 2015. Snakebite is under appreciated: appraisal of burden from West Africa. PLoS Negl Trop Dis 9: e0004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasturiratne A, Wickremasinghe AR, de Silva N, Kithsiri Gunawardena N, Pathmeswaran A, Premaratna R, Savioli L, Lalloo DG, de Silva HJ, 2008. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med 5: e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison RA, Hargreaves A, Wagstaff SC, Faragher B, Lalloo DG, 2009. Snake envenoming: a disease of poverty. PLoS Negl Trop Dis 3: e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization , 2018. Factsheet-“Snakebite Envenoming”. Available at: http://www.who.int/en/news-room/fact-sheets/detail/snakebite-envenoming. Accessed May 20, 2018. [Google Scholar]
- 6.Babo Martins S, et al. 2019. Snakebite and its impact in rural communities: the need for a one health approach. PLoS Negl Trop Dis 13: e0007608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suraweera W, et al. 2020. Trends in snakebite mortality in India from 2000 to 2019 in a nationally representative mortality study. medRxiv, 10.1101/2020.05.15.20103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization , 2018. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins. Available at: https://www.who.int/bloodproducts/AntivenomGLrevWHO_TRS_1004_web_Annex_5.pdf?ua=1. Accessed February 3, 2020. [Google Scholar]
- 9.Warrell DA, 2009. Researching nature’s venoms and poisons. Trans R Soc Trop Med Hyg 103: 860–866. [DOI] [PubMed] [Google Scholar]
- 10.Warrell DA, 2010. Snake bite. Lancet 375: 77–88. [DOI] [PubMed] [Google Scholar]
- 11.Silva A, Maduwage K, Sedgwick M, Pilapitiya S, Weerawansa P, Dahanayaka NJ, Buckley NA, Siribaddana S, Isbister GK, 2016. Neurotoxicity in Russell’s viper (Daboia russelii) envenoming in Sri Lanka: a clinical and neurophysiological study. Clin Toxicol (Phila) 54: 411–419. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization regional Office for South-East Asia , 2016. Guidelines for the Management of Snakebites, 2nd edition New Delhi, India: WHO. [Google Scholar]
- 13.Ariaratnam CA, Sheriff MHR, Arambepola C, Theakston RDG, Warrell DA, 2009. Syndromic approach to treatment of snake bite in Sri Lanka based on results of a prospective national hospital-based survey of patients envenomed by identified snakes. Am J Trop Med Hyg 81: 725–731. [DOI] [PubMed] [Google Scholar]
- 14.Wolff H, 2013. Is it time to denominate hypopituitarism after snake bite? QJM 106: 390. [DOI] [PubMed] [Google Scholar]
- 15.Wolff H, 1958. Insuficiência hipofisária anterior por picada de ofídio. Arq Bras Endocrinol Metab 7: 25–47. [Google Scholar]
- 16.Eapen CK, Chandy N, Kochuvarkey KL, Zacharia PK, Thomas PJ, Ipe TI, 1976. Unusual complication of snake bite: hypopituitarism after viper bites. Ohsaka A, Hayashi K, Sawai Y, Murata R, Funatsu M, Tamiya N, eds. Animal, Plant, and Microbial Toxins. New York, NY: Springer, 467–473. [Google Scholar]
- 17.Tun-Pe , Phillips RE, Warrell DA, Moore RA, Swe TN, Lwin M, Burke CW, 1987. Acute and chronic pituitary failure resembling Sheehan’s syndrome following bites by Russell’s viper in Burma. Lancet 2: 763–767. [DOI] [PubMed] [Google Scholar]
- 18.Burke CW, 1990. The anterior pituitary, snakebite and Sheehan’s syndrome. Q J Med 75: 331–333. [PubMed] [Google Scholar]
- 19.Antonypillai CN, Wass JaH, Warrell DA, Rajaratnam HN, 2011. Hypopituitarism following envenoming by Russell’s vipers (Daboia siamensis and D. russelii) resembling Sheehan’s syndrome: first case report from Sri Lanka, a review of the literature and recommendations for endocrine management. QJM 104: 97–108. [DOI] [PubMed] [Google Scholar]
- 20.Jeevagan V, Katulanda P, Gnanathasan CA, Warrell DA, 2013. Acute pituitary insufficiency and hypokalaemia following envenoming by Russell’s viper (Daboia russelii) in Sri Lanka: exploring the pathophysiological mechanisms. Toxicon 63: 78–82. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan R, Kaliaperumal S, Dutta TK, 2005. Bilateral angle closure glaucoma following snake bite. J Assoc Physicians India 53: 46–48. [PubMed] [Google Scholar]
- 22.Phillips RE, Theakston RD, Warrell DA, Galigedara Y, Abeysekera DT, Dissanayaka P, Hutton RA, Aloysius DJ, 1988. Paralysis, rhabdomyolysis and haemolysis caused by bites of Russell’s viper (Vipera russelli pulchella) in Sri Lanka: failure of Indian (Haffkine) antivenom. Q J Med 68: 691–715. [PubMed] [Google Scholar]
- 23.Udayabhaskaran V, Arun Thomas ET, Shaji B, 2017. Capillary leak syndrome following snakebite envenomation. Indian J Crit Care Med 21: 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pla D, Sanz L, Quesada-Bernat S, Villalta M, Baal J, Wahed Chowdhury MA, León G, Gutiérrez JM, Kuch U, Calvete JJ, 2019. Phylovenomics of Daboia russelii across the Indian subcontinent. Bioactivities and comparative in vivo neutralization and in vitro third-generation antivenomics of antivenoms against venoms from India, Bangladesh and Sri Lanka. J Proteomics 207: 103443. [DOI] [PubMed] [Google Scholar]
- 25.Sheehan HL, 1937. Post-partum necrosis of the anterior pituitary. J Pathol Bacteriol 45: 189–214. [Google Scholar]
- 26.Gopalakrishnan M, Vinod KV, Dutta TK, Shaha KK, Sridhar MG, Saurabh S, 2018. Exploring circulatory shock and mortality in viper envenomation: a prospective observational study from India. QJM 111: 799–806. [DOI] [PubMed] [Google Scholar]
- 27.Hart GR, Caldwell G, Burrin JM, 1990. Intracellular mechanisms involved in the stimulation of growth hormone release from rat anterior pituitary cells by Russell’s viper venom. J Endocrinol 127: 111–117. [DOI] [PubMed] [Google Scholar]
- 28.Hart GR, Proby C, Dedhia G, Yeo TH, Joplin GF, Burrin JM, 1989. Burmese Russell’s viper venom causes hormone release from rat pituitary cells in vitro. J Endocrinol 122: 489–494. [DOI] [PubMed] [Google Scholar]
- 29.Than T, Francis N, Swe TN, Lwin M, Pe T, Soe S, Oo MM, Phillips RE, Warrell DA, 1989. Contribution of focal haemorrhage and microvascular fibrin deposition to fatal envenoming by Russell’s viper (Vipera russelli siamensis) in Burma. Acta Trop 46: 23–38. [DOI] [PubMed] [Google Scholar]
- 30.Lwinl M, Warrell DA, Phillips RE, Swe TN, Pe T, Lay MM, 1985. Bites by Russell’s viper (Vipera russelli siamensis) in Burma: haemostatic, vascular, and renal disturbances and response to treatment. Lancet 2: 1259–1264. [DOI] [PubMed] [Google Scholar]
- 31.Sherif IH, Vanderley CM, Beshyah S, Bosairi S, 1989. Sella size and contents in Sheehan’s syndrome. Clin Endocrinol (Oxf) 30: 613–618. [DOI] [PubMed] [Google Scholar]
- 32.Bhat S, Mukhopadhyay P, Raychaudhury A, Chowdhury S, Ghosh S, 2019. Predictors of hypopituitarism due to vasculotoxic snake bite with acute kidney injury. Pituitary 22: 594–600. [DOI] [PubMed] [Google Scholar]
- 33.Goswami R, Kochupillai N, Crock PA, Jaleel A, Gupta N, 2002. Pituitary autoimmunity in patients with Sheehan’s syndrome. J Clin Endocrinol Metab 87: 4137–4141. [DOI] [PubMed] [Google Scholar]
- 34.De Bellis A, et al. 2008. Anti-hypothalamus and anti-pituitary antibodies may contribute to perpetuate the hypopituitarism in patients with Sheehan’s syndrome. Eur J Endocrinol 158: 147–152. [DOI] [PubMed] [Google Scholar]
- 35.Proby C, Aung T, Win T, Mon H, Burrin JM, Joplin GF, 1990. Immediate and long-term effects on hormone levels following bites by the Burmese Russell’s viper. Q J Med 75: 399–411. [PubMed] [Google Scholar]
- 36.Rajagopala S, Thabah MM, Ariga KK, Gopalakrishnan M, 2015. Acute hypopituitarism complicating Russell’s viper envenomation: case series and systematic review. QJM 108: 719–728. [DOI] [PubMed] [Google Scholar]
- 37.Golay V, Roychowdhary A, Dasgupta S, Pandey R, 2014. Hypopituitarism in patients with vasculotoxic snake bite envenomation related acute kidney injury: a prospective study on the prevalence and outcomes of this complication. Pituitary 17: 125–131. [DOI] [PubMed] [Google Scholar]
- 38.Shivaprasad C, Aiswarya Y, Sridevi A, Anupam B, Amit G, Rakesh B, Annie PA, Anish K, 2019. Delayed hypopituitarism following Russell’s viper envenomation: a case series and literature review. Pituitary 22: 4–12. [DOI] [PubMed] [Google Scholar]
- 39.Naik BN, Bhalla A, Sharma N, Mokta J, Singh S, Gupta P, Rai A, Subbiah S, Bhansali A, Dutta P, 2018. Pituitary dysfunction in survivors of Russell’s viper snake bite envenomation: a prospective study. Neurol India 66: 1351–1358. [DOI] [PubMed] [Google Scholar]
- 40.Golay V, Roychowdhary A, Pandey R, Pasari A, Praveen M, Arora P, Sarkar D, 2013. Growth retardation due to panhypopituitarism and central diabetes insipidus following Russell’s viper bite. Southeast Asian J Trop Med Public Health 44: 697–702. [PubMed] [Google Scholar]
- 41.Van Der Meer E, Conway L, Little M, Hanson J, 2020. A case of acute hypogonadism following taipan (Oxyuranus scutellatus) envenomation. Toxicon 180: 28–30. [DOI] [PubMed] [Google Scholar]
- 42.Annane D, et al. 2017. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med 43: 1751–1763. [DOI] [PubMed] [Google Scholar]
- 43.Pastores SM, Annane D, Rochwerg B; Corticosteroid Guideline Task Force of SCCM and ESICM , 2018. Guidelines for the diagnosis and management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in critically ill patients (Part II): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med 46: 146–148. [DOI] [PubMed] [Google Scholar]
- 44.Krishnan MN, Kumar S, Ramamoorthy KP, 2001. Severe panhypopituitarism and central diabetes insipidus following snake bite: unusual presentation as torsades de pointes. J Assoc Physicians India 49: 923–924. [PubMed] [Google Scholar]
- 45.Gupta UC, Garg OP, Kataria ML, 1992. Cranial diabetes insipidus due to viper bite. J Assoc Physicians India 40: 686–687. [PubMed] [Google Scholar]
- 46.Christ-Crain M, Bichet DG, Fenske WK, Goldman MB, Rittig S, Verbalis JG, Verkman AS, 2019. Diabetes insipidus. Nat Rev Dis Primer 5: 54. [DOI] [PubMed] [Google Scholar]
- 47.Anderson JR, Antoun N, Burnet N, Chatterjee K, Edwards O, Pickard JD, Sarkies N, 1999. Neurology of the pituitary gland. J Neurol Neurosurg Psychiatry 66: 703–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grace M, Shanoj KC, 2014. An unusual complication of snake bite. Egypt J Intern Med 26: 91. [Google Scholar]
- 49.Lavallée G, Morcos R, Palardy J, Aubé M, Gilbert D, 1995. MR of nonhemorrhagic postpartum pituitary apoplexy. AJNR Am J Neuroradiol;16: 1939–1941. [PMC free article] [PubMed] [Google Scholar]
- 50.Diri H, Karaca Z, Tanriverdi F, Unluhizarci K, Kelestimur F, 2016. Sheehan’s syndrome: new insights into an old disease. Endocrine 51: 22–31. [DOI] [PubMed] [Google Scholar]
- 51.Thein CM, Byard RW, 2019. Characteristics and relative numbers of lethal snake bite cases in medicolegal practice in central Myanmar - a five year study. J Forensic Leg Med 63: 52–55. [DOI] [PubMed] [Google Scholar]
- 52.Lakhotia M, Pahadiya HR, Singh J, Gandhi R, Bhansali S, 2014. Adrenal hematoma and right hemothorax after Echis carinatus bite: an unusual manifestation. Toxicol Int 21: 325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bjorklund SI, 1953. The Waterhouse-Friderichsen syndrome. Acta Paediatr 42: 77–83. [DOI] [PubMed] [Google Scholar]
- 54.Waheed H, Moin SF, Choudhary MI, 2017. Snake venom: from deadly toxins to life-saving therapeutics. Curr Med Chem 24: 1874–1891. [DOI] [PubMed] [Google Scholar]
- 55.Warrell DA, 1989. Snake venoms in science and clinical medicine. 1. Russell’s viper: biology, venom and treatment of bites. Trans R Soc Trop Med Hyg 83: 732–740. [DOI] [PubMed] [Google Scholar]
- 56.Szabo S, Hüttner I, Kovacs K, Horvath E, Szabo D, Horner HC, 1980. Pathogenesis of experimental adrenal hemorrhagic necrosis (“apoplexy”): ultrastructural, biochemical, neuropharmacologic, and blood coagulation studies with acrylonitrile in the rat. Lab Investig J Tech Methods Pathol 42: 533–546. [PubMed] [Google Scholar]
- 57.Vongphoumy I, Chanthilat P, Vilayvong P, Blessmann J, 2016. Prospective, consecutive case series of 158 snakebite patients treated at Savannakhet provincial hospital, Lao People’s Democratic Republic with high incidence of anaphylactic shock to horse derived F(ab’)2 antivenom. Toxicon 117: 13–21. [DOI] [PubMed] [Google Scholar]
- 58.Lukšić B, Culić V, Stričević L, Brizić I, Poljak NK, Tadić Z, 2010. Infant death after nose-horned viper (Vipera ammodytes ammodytes) bite in Croatia: a case report. Toxicon 56: 1506–1509. [DOI] [PubMed] [Google Scholar]
- 59.Audebert F, Sorkine M, Bon C, 1992. Envenoming by viper bites in France: clinical gradation and biological quantification by ELISA. Toxicon 30: 599–609. [DOI] [PubMed] [Google Scholar]
- 60.Mao Y-C, Liu P-Y, Chiang L-C, Liao S-C, Su H-Y, Hsieh S-Z, Yang C-C, 2017. Bungarus multicinctus multicinctus snakebite in Taiwan. Am J Trop Med Hyg 96: 1497–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bahloul M, Turki O, Chaari A, Bouaziz M, 2018. Incidence, mechanisms and impact outcome of hyperglycaemia in severe scorpion-envenomed patients. Ther Adv Endocrinol Metab 9: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ronen JA, Gavin M, Ruppert MD, Peiris AN, 2019. Glycemic disturbances in pheochromocytoma and paraganglioma. Cureus 11: e4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kankananarachchi I, Fernando MP, Denipitiya T, Navabalasooriyar P, Kitulwatte NC, 2018. Persistent hyponatraemia following suspected krait envenomation in a 5 year old Sri Lankan child: a case report. Ceylon Med J 63: 24–25. [DOI] [PubMed] [Google Scholar]
- 64.Ho PL, Soares MB, Maack T, Gimenez I, Puorto G, Furtado MF, Raw I, 1997. Cloning of an unusual natriuretic peptide from the South American coral snake Micrurus corallinus. Eur J Biochem 250: 144–149. [DOI] [PubMed] [Google Scholar]
- 65.Hung HT, Höjer J, Du NT, 2009. Clinical features of 60 consecutive ICU-treated patients envenomed by Bungarus multicinctus. Southeast Asian J Trop Med Public Health 40: 518–524. [PubMed] [Google Scholar]
- 66.Trinh KX, Khac QL, Trinh LX, Warrell DA, 2010. Hyponatraemia, rhabdomyolysis, alterations in blood pressure and persistent mydriasis in patients envenomed by Malayan kraits (Bungarus candidus) in southern Viet Nam. Toxicon 56: 1070–1075. [DOI] [PubMed] [Google Scholar]
- 67.Tongpoo A, Sriapha C, Pradoo A, Udomsubpayakul U, Srisuma S, Wananukul W, Trakulsrichai S, 2018. Krait envenomation in Thailand. Ther Clin Risk Manag 14: 1711–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wium CA, Marks CJ, Du Plessis CE, Müller GJ, 2017. Berg adder (Bitis atropos): an unusual case of acute poisoning. South Afr Med J 107: 1075–1077. [DOI] [PubMed] [Google Scholar]
- 69.de Silva U, Sarathchandra C, Senanayake H, Pilapitiya S, Siribaddana S, Silva A, 2018. Hyponatraemia and seizures in Merrem’s hump-nosed pit viper (Hypnale hypnale) envenoming: a case report. J Med Case Rep 12: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van der Walt AJ, Muller GJ, 2019. Berg adder (Bitis atropos) envenoming: an analysis of 14 cases. Clin Toxicol (Phila) 57: 131–136. [DOI] [PubMed] [Google Scholar]
- 71.Kumar KS, Narayanan S, Udayabhaskaran V, Thulaseedharan NK, 2018. Clinical and epidemiologic profile and predictors of outcome of poisonous snake bites - an analysis of 1,500 cases from a tertiary care center in Malabar, North Kerala, India. Int J Gen Med 11: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cotton MF, Shahak E, Muller GJ, Heyns L, Kalis NN, Aalbers C, 1991. Syndrome of inappropriate antidiuretic hormone secretion, an unusual complication of an elapid snakebite. South Afr Med J 79: 735–736. [PubMed] [Google Scholar]
- 73.Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M, 1992. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps). J Biol Chem 267: 13928–13932. [PubMed] [Google Scholar]
- 74.Chen HH, Lainchbury JG, Burnett JC, 2002. Natriuretic peptide receptors and neutral endopeptidase in mediating the renal actions of a new therapeutic synthetic natriuretic peptide dendroaspis natriuretic peptide. J Am Coll Cardiol 40: 1186–1191. [DOI] [PubMed] [Google Scholar]
- 75.Vink S, Jin AH, Poth KJ, Head GA, Alewood PF, 2012. Natriuretic peptide drug leads from snake venom. Toxicon 59: 434–445. [DOI] [PubMed] [Google Scholar]
- 76.Hoorn EJ, Zietse R, 2017. Diagnosis and treatment of hyponatremia: compilation of the guidelines. J Am Soc Nephrol 28: 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buffington MA, Abreo K, 2016. Hyponatremia: a review. J Intensive Care Med 31: 223–236. [DOI] [PubMed] [Google Scholar]
- 78.Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, Thompson CJ, 2013. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med 126: S1–S42. [DOI] [PubMed] [Google Scholar]
- 79.Sellahewa K, 1997. Lessons from four studies on the management of snake bite in Sri Lanka. Ceylon Med J 42: 8–15. [PubMed] [Google Scholar]
- 80.Kularatne S, 2002. Common krait (Bungarus caeruleus) bite in Anuradhapura, Sri Lanka: a prospective clinical study, 1996–98. Postgrad Med J 78: 276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pillai LV, Ambike D, Husainy S, Khaire A, Captain A, Kuch U, 2012. Severe neurotoxic envenoming and cardiac complications after the bite of a “Sind krait” (Bungarus cf. sindanus) in Maharashtra, India. Trop Med Health 40: 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gawarammana IB, Mudiyanselage Kularatne SA, Kularatne K, Waduge R, Weerasinghe VS, Bowatta S, Senanayake N, 2010. Deep coma and hypokalaemia of unknown aetiology following Bungarus caeruleus bites: exploration of pathophysiological mechanisms with two case studies. J Venom Res 1: 71–75. [PMC free article] [PubMed] [Google Scholar]
- 83.Struyk AF, Cannon SC, 2008. Paradoxical depolarization of BA2+- treated muscle exposed to low extracellular K+: insights into resting potential abnormalities in hypokalemic paralysis. Muscle Nerve 37: 326–337. [DOI] [PubMed] [Google Scholar]
- 84.Bahlmann H, Lindwall R, Persson H, 2005. Acute barium nitrate intoxication treated by hemodialysis. Acta Anaesthesiol Scand 49: 110–112. [DOI] [PubMed] [Google Scholar]
- 85.Malina T, Krecsák L, Jelić D, Maretić T, Tóth T, Siško M, Pandak N, 2011. First clinical experiences about the neurotoxic envenomings inflicted by lowland populations of the Balkan adder, Vipera berus bosniensis. Neurotoxicology 32: 68–74. [DOI] [PubMed] [Google Scholar]
- 86.Weiner ID, Wingo CS, 1997. Hypokalemia–consequences, causes, and correction. J Am Soc Nephrol 8: 1179–1188. [DOI] [PubMed] [Google Scholar]
- 87.Batlle D, Arruda J, 2018. Hyperkalemic forms of renal tubular acidosis: clinical and pathophysiological aspects. Adv Chronic Kidney Dis 25: 321–333. [DOI] [PubMed] [Google Scholar]
- 88.Karunarathne S, Udayakumara Y, Govindapala D, Fernando H, 2013. Type IV renal tubular acidosis following resolution of acute kidney injury and disseminated intravascular coagulation due to hump-nosed viper bite. Indian J Nephrol 23: 294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weerakkody RM, Lokuliyana PN, Lanerolle RD, 2016. Transient distal renal tubular acidosis following hump nosed viper bite: two cases from Sri Lanka. Saudi J Kidney Dis Transpl 27: 1018–1020. [DOI] [PubMed] [Google Scholar]