Abstract.
Phaeohyphomycosis causes a wide spectrum of systemic manifestations and can affect even the immunocompetent hosts. Involvement of the central nervous system is rare. A 48-year-old farmer presented with chronic headache, fever, and impaired vision and hearing. Serial MRIs of the brain showed enhancing exudates in the basal cisterns, and lesions in the sella and perichiasmatic and cerebellopontine angle regions along with enhancement of the cranial nerves and leptomeninges. Cerebrospinal fluid (CSF) showed lymphocytic pleocytosis with elevated protein and decreased glucose on multiple occasions. Clinical, imaging, and CSF abnormalities persisted despite treatment with antitubercular drugs and steroids for 2 years. Biopsy of the dura mater at the cervicomedullary junction revealed necrotizing granulomatous lesions, neutrophilic abscesses, and giant cells containing slender, pauci-septate, pigmented fungal hyphae. Fungal culture showed growth of Fonsecaea pedrosoi, which is classically known to cause brain abscesses. Here, we report the diagnostic odyssey in a patient with chronic meningitis from a region endemic for tuberculosis and describe the challenges in establishing the accurate diagnosis. Lack of therapeutic response to an adequate trial of empirical antitubercular therapy warrants search for alternative causes, including fungal meningitis. We highlight the uncommon manifestation of F. pedrosoi with chronic meningitis as well as the protracted clinical course despite not receiving antifungal therapy.
INTRODUCTION
Fungal meningitis is a relatively uncommon infection, among others, affecting the central nervous system. It usually occurs in immunocompromised individuals. Several fungi can cause meningitis, including the commonly recognized cryptococcal meningitis. Phaeohyphomycosis is a term used to describe infections caused by fungi, which contain melanin in their cell walls giving a characteristic dark color to their conidia and hyphae. These fungi are termed as dematiaceous, darkly pigmented, or phaeoid and can cause a wide spectrum of diseases that range from innocuous subcutaneous nodules to life-threatening cerebral abscess and disseminated disease.1 A unique feature of these fungi is that they can affect even the immunocompetent hosts and those without any underlying predisposing systemic conditions.2 Here, we report the clinical course in a patient with fungal meningitis and the challenges in establishing the accurate diagnosis.
CASE REPORT
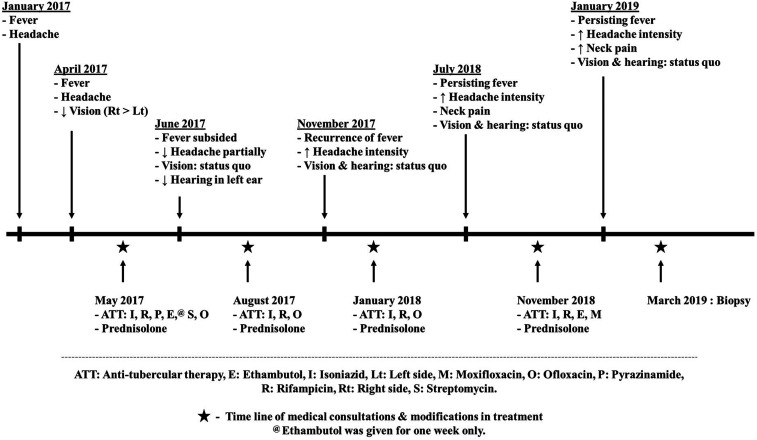
A 48-year-old man, a farmer by occupation, from the eastern state of India (Bengal), developed headache which was holocranial, but worse in the occipital and neck region. This was associated with mild intermittent fever. About 3 months later, he developed bilateral painless impairment in vision, which was worse in the right than the left eye. He was not on any medications that were likely to suppress the immunity before the onset of his neurological symptoms including steroids. He was seen at a local center where he underwent brain MRI and lumbar cerebrospinal fluid (CSF) study. Brain MRI showed evidence of ring-enhancing lesions in the sylvian fissures. Cerebrospinal fluid showed lymphocyte predominant pleocytosis. He had been provisionally diagnosed with tubercular meningitis and empirically administered antitubercular therapy (ATT) (isoniazid, rifampicin, pyrazinamide, and ethambutol) and steroids. He presented to our center after 1 week of starting ATT for second opinion. The clinical course, treatment given, and response to the same are depicted in Figure 1.
Figure 1.
Time line of the clinical course of the patient till biopsy.
At the time of first evaluation at our center, neurological examination was normal, except for reduced visual acuity. Brain MRI showed persisting multiple ring-enhancing lesions in the sylvian fissure. Repeat CSF analysis showed lymphocytic pleocytosis with elevated protein and decreased glucose (Table 1). The visual evoked potential study showed prolonged latency of P100 on the right side. Evaluation for alternative etiologies showed that antinuclear antibody, antineutrophil cytoplasmic antibody, and angiotensin-converting enzyme in serum were normal or negative. Serological testing for HIV was negative. Cluster of differentiation 4 count was within the normal range (1,856 cells/μL and 32% of lymphocytes). Computed tomography scan of the thorax was normal. Sputum analysis was not carried out in our patient because he did not have any cough or expectoration. Because no other etiology could be established, steroids were continued, and ATT was modified. Ethambutol was stopped in view of impaired vision. The possibility of multidrug-resistant tuberculosis was considered. Streptomycin and ofloxacin were added.
Table 1.
Serial CSF examination in the patient
| Parameter | May 2017* | August 2017 | September 2018 | November 2018 | January 2019 | March 2019 |
|---|---|---|---|---|---|---|
| Cell count (per mm3) | 102 | 115 | 350 | 140 | 1,000 | 120 |
| Cell type | L: 70% and P: 30% | L: 70%, P: 29%, and degenerated cells: 1% | L: 80% and P: 20% | L: 70% and P: 30% | L: 20% and P: 80% | L: 20%, P: 70%, and degenerated cells: 5% |
| Protein (mg/dL) | 74 | 47 | 155 | 105 | 338 | 177 |
| Glucose (mg/dL) | 42 | 47 | 73 | 175 | 297 | 216 |
| Chloride (mmol/L) | NA | NA | 120 | 119 | 114 | 119 |
| CSF lactate (mg/dL) | NA | NA | NA | 28 | NA | NA |
| Cartridge-based nucleic acid amplification test | – | – | – | Negative | – | Negative |
| Acid-fast bacilli culture | – | No growth | No growth | – | – | No growth |
| India ink | NA | Negative | Negative | NA | NA | Negative |
| Cryptococcal capsular polysaccharide antigen by latex agglutination test | – | – | Negative | – | – | – |
| Fungal PCR | – | – | – | – | – | Positive |
| Fungal culture | – | – | – | – | – | No growth |
| Cytospin for malignant cells | – | Transformed lymphocytes, reactive monocytes, RBCs, and few polymorphonuclear cells | Transformed lymphocytes, few reactive monocytes, polymorphonuclear cells, and RBCs | Predominantly transformed lymphocytes, few reactive monocytes, and polymorphonuclear cells | Predominantly polymorphonuclear cells, few lymphocytes, occasional reactive monocytes, eosinophils, and plasmacytoid cells | Inflammatory smear with numerous degenerating polymorphonuclear and few reactive lymphocytes, and monocytoid cells. No malignant cells |
CSF = cerebrospinal fluid; L = lymphocyte; NA = not available; P = polymorph; RBC = red blood cells.
Cerebrospinal fluid analysis was carried out at another center.
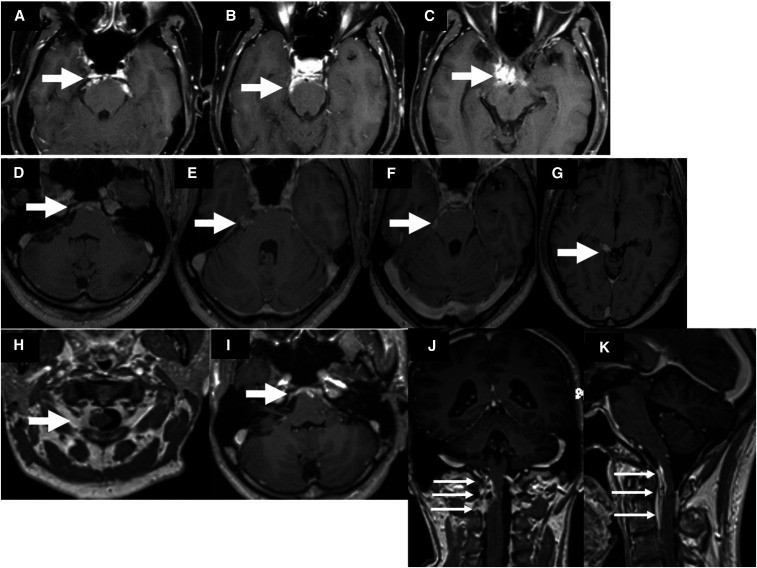
During follow-up over the next 2 months, his symptoms persisted with intermittent exacerbations of fever and headache. He also developed progressive deafness. Audiometry showed severe sensorineural hearing loss on the left side and normal findings in the right ear were observed. Serial MRIs of the brain showed appearance of enhancing exudates in the basal cisterns, fresh lesions in the sella, and perichiasmatic and cerebellopontine angle regions along with enhancement of cranial nerves and leptomeninges (Figure 2). Cerebrospinal fluid analysis performed on several occasions showed persisting pleocytosis with mildly elevated protein and normal glucose levels (Table 1). Workup for tubercular meningitis using cartridge-based nucleic acid amplification test (CBNAAT) and culture (the Mycobacteria Growth Indicator Tube and Löwenstein–Jensen medium) drew negative results. The standard agglutination test for Brucella was negative. India ink and latex agglutination test for cryptococcal capsular polysaccharide antigen were negative in the CSF. Anti-tubercular therapy was continued along with steroids.
Figure 2.
Contrast-enhanced MRIs of the brain at 6 and 11 months of illness show enhancing exudates in the basal cisterns, sella, and perichiasmatic and cerebellopontine angle regions. At 26 months of illness, there is extension of the lesions into the craniovertebral junction.
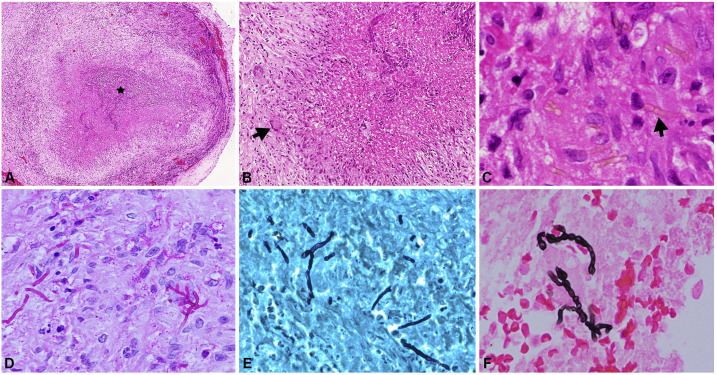
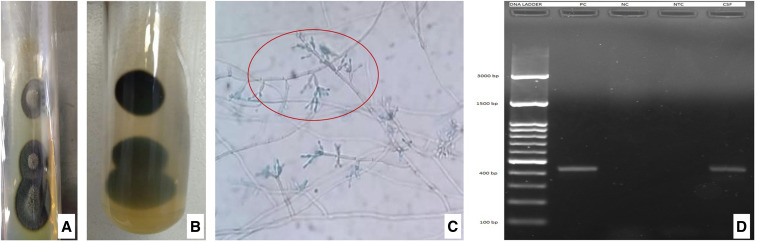
At 21 months of follow-up, there was recurrence of fever and headache. Brain MRI showed extension of the lesions into the interpeduncular fossa and craniovertebral junction (Figure 2). Glutamic acid decarboxylase antibodies were detected on one occasion, but the result could not be replicated on repeat testing. ELISA for anti-cysticercal antibodies was positive in the CSF, and albendazole was added to the ATT and steroid therapy. There was no clinical or radiological improvement despite regular and optimal therapy. Cerebrospinal fluid pan-fungal PCR was performed using a set of primers, that is, fungus I (5′-GTT AAA AAG CTC GTA GTT G-3′) and fungus II (5′-TCC CTA GTC GGC ATA GTT TA-3′) which target 429 bp of highly conserved region and 18S rDNA of pathogenic fungi. A positive band was seen at the 429-bp level indicative of fungal infection (Figure 4D). However, CSF fungal culture remained sterile. Biopsy of dura mater at the cervicomedullary junction was performed through posterior cervical approach. The dura appeared gray white to gray brown, and no abnormalities were detected on gross examination. Light microscopy of the dural tissue revealed necrotizing granulomatous lesion composed of central zones of necrosis with neutrophilic abscesses. Numerous pigmented fungal hyphae which were slender, pauci-septate, pigmented, and engulfed by giant cells were seen. These zones were surrounded by lymphocytes, plasma cells, and histiocytes. Periodic acid Schiff and Gomori methenamine silver stains highlighted the fungal hyphae (Figure 3). The biopsied tissue was subjected to pan-fungal PCR which was positive. Fungal culture from the dural tissue yielded Fonsecaea pedrosoi (Figure 4). The isolate was referred to the Mycology Division (WHO Collaborating Center, Center of Advanced Research in Medical Mycology), Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh, India, for further confirmation. Based on these features, a diagnosis of cerebral phaeohyphomycosis was made. Antitubercular therapy and steroids were discontinued. He was initiated on antifungal treatment, that is, combination of intravenous amphotericin B (0.7 mg/kg/day) and oral voriconazole (8 mg/kg/day). He was followed up telephonically after 3 months of initiating antifungal therapy. The patient reported that his headache had improved by 50%.
Figure 3.
Photomicrograph showing necrotizing granulomatous inflammation with central necrosis (asterisk, A, H&E, ×150) surrounded by palisading histiocytes and multinucleate giant cells (arrow, B, H&E, ×40). Within the granulomas septate and pigmented fungal hyphae (arrow, C, H&E, ×400) are observed. The fungi are highlighted by periodic acid Schiff (D, ×200), Gomori methenamine silver (E, ×200), and Masson Fontana (F, ×200) stains. This figure appears in color at www.ajtmh.org.
Figure 4.
Fungal culture of dural tissue shows dark gray-colored colonies with cone-shaped protrusion in the center with a black reverse on Sabouraud dextrose Agar medium resembling Fonsecaea pedrosoi (A and B). Microculture shows brown-colored septate, branched hyphae, and conidiophores with primary, secondary, and tertiary conidia resembling F. pedrosoi (C). Pan-fungal PCR shows a positive band at the 429-bp level in the cerebrospinal fluid (CSF) indicative of fungal infection (lane 1: 100 bp DNA ladder, lane 2: positive control showing band at 429 bp, lane 3: negative control, lane 4: non-template control, and lane 5: CSF sample of the patient). This figure appears in color at www.ajtmh.org.
DISCUSSION
Diagnosis of chronic meningitis poses significant challenges to clinicians in day-to-day clinical practice, particularly in a patient who has received empirical treatment before the determination of etiology. Because of poor sensitivity of the available diagnostic tests, patients in areas endemic for tuberculosis are often given empirical ATT.3 Cerebral phaeohyphomycosis is rare, but there are more than 100 species and 60 genera of dematiaceous fungi that are implicated in human disease worldwide. The genus Fonsecaea is an extremely rare cause of cerebral phaeohyphomycosis. The most common organism involved is Cladophialophora bantiana. Other species include Ramichloridium mackenziei, Ochroconis gallopava, Bipolaris spicifera, Exophiala dermatitidis, and Chaetomium strumarium.1,2 There are sporadic reports of Fonsecaea monophora causing cerebral infection. However, cerebral phaeohyphomycosis caused by F. pedrosoi is uncommon, and there are very few cases reported in the literature.
Fonsecaea is a dematiaceous (melanized) fungus belonging to the ascomycetous family Herpotrichiellaceae of order Chaetothyriales. The genus Fonsecaea comprises three sibling species, namely, F. pedrosoi, F. monophora, and Fonsecaea nubica.4 Fonsecaea monophora has recently been separated from F. pedrosoi as a distinct species by molecular studies.4,5 Fonsecaea pedrosoi is found in rotten wood, decaying plant materials, and soil. They are distributed worldwide, particularly in tropical Asia, South America, and Africa.6 Hence, farmers are reported to be at an increased risk of developing infection.7 It is one of the important causes for chromoblastomycosis. The exact pathogenesis of cerebral phaeohyphomycosis is not known. It is thought to be due to hematogenous dissemination of inhaled spores, accidental skin inoculation, or direct extension from adjacent paranasal sinuses or ears.1,8,9 Melanin in the cell walls acts as a virulence factor by scavenging free radicals and hypochlorite produced by phagocytic cells during the oxidative burst that normally kills most microorganisms.10,11 Melanin may also bind to the hydrolytic enzymes in the phagocytic cells and prevent their action on plasma membrane.
Fukushiro reported several cases of cerebral infections caused by F. pedrosoi that had cutaneous and pulmonary involvement.12 Al-Hedaithy et al.13 described infection with Fonsecaea in an elderly man with multiple frontal abscesses. There are a few case reports of cerebral abscess and enhancing fungal masses caused by Fonsecaea from India.14–16
Central nervous system infection with Fonsecaea usually results in black. necrotic brain tissue, black pus and black CSF.17 Classically, it leads to the formation of brain abscesses that can be life threatening. However, there are no reported cases of chronic meningitis due to this fungus. Revankar et al.,2 in a review of 101 cases with fungal infection, included four patients with Fonsecaea infection, and none presented with meningitis. Other manifestations of Fonsecaea infection include myelitis or arachnoiditis.18,19 Here, our patient presented with features of chronic meningitis without any features of cutaneous lesions. It is interesting to note that our patient developed unilateral deafness. Because the onset of deafness was temporally related to the introduction of streptomycin, this was withdrawn. It is possible that deafness was a manifestation of cranial nerve involvement by the meningitic process.
The CSF picture in cerebral phaeohyphomycosis is similar to that of chronic tubercular meningitis; however, hypoglycorrhachia is uncommon.2 Our case is atypical, as the patient’s predominant symptom was headache which had a protracted course. He did not have features to suggest immunocompromised state: HIV serology was negative, and he was not on any chronic medications that are known to suppress the immunity before the onset of neurological symptoms. He had a rather indolent course despite being treated with ATT and steroids sans antifungal therapy. It is very unusual for a patient with F. pedrosoi infection to survive for 2 years despite prolonged steroid therapy without antifungal agents. Apart from CSF pleocytosis, tests for tuberculosis such as CBNAAT and culture were negative. The patient received ATT for a long duration empirically because of high endemicity of tuberculosis in India, without clinical or radiological improvement.
The diagnosis of phaeohyphomycosis can be difficult because dematiaceous fungi are common soil inhabitants and are often considered to be contaminants when identified in culture. As with other filamentous fungi, identification in culture, the gold standard for the diagnosis, requires expert interpretation of colony and microscopic morphology. But cultures are slow and depend on adequate representative clinical specimen and the presence of viable fungal elements. Pan-fungal PCR through the use of universal fungal primers facilitates rapid detection of fungal infection directly from the clinical specimens. Fungal PCR has a sensitivity of 90% and specificity of 100% for formalin-fixed paraffin-embedded tissue.20 Sensitivity is also high when hyphae are seen in a biopsy specimen. It may form a part of the diagnostic workup in any patient with chronic meningitis in whom the etiology is not established. In our patient, early performance of fungal PCR in the CSF when he did not respond to ATT might have accelerated the diagnosis and thereby appropriate treatment. Molecular techniques are presently available to rapidly and reliably identify these fungi to even the genus level.10 Hence, tissue examination is useful to identify the irregularly swollen hyphae with yeast-like structures. Masson Fontana stain which is specific for melanin can be used to confirm the presence of dematiaceous hyphae.2,21 Histopathological studies in our patient showed the presence of numerous pigmented fungal hyphae which were slender, pauci-septate, and pigmented and engulfed by giant cells, which were positive on the Masson Fontana stain. There was no contamination as there was an inflammatory response to the fungal hyphae, and they were noted within the dural biopsy.
Because of the rarity, there are no standard treatment guidelines for cerebral phaeohyphomycosis. Combined surgical and medical treatment is generally recommended.2,18,22 A combination of antifungal agents viz amphotericin B, 5-flucytosine, and itraconazole is generally used as it is associated with improved survival rates. Posaconazole and ravuconazole are the newer azole derivatives that are reported to have broad-spectrum antifungal activity against pigmented fungi.10,23 The prognosis of cerebral phaeohyphomycosis is dismal. The mortality rate approaches 100% in untreated patients, whereas that of treated cases is high as 65–73% despite the aggressive treatment.10,21 Our patient had an uncommon presentation with chronic indolent course with minimal neurological deficits throughout the illness and despite not receiving antifungal therapy.
CONCLUSION
Chronic meningitis caused by F. pedrosoi is very rare, but can be life threatening despite adequate antifungal and surgical measures. This patient highlights a very major point which is the importance of early brain biopsy in the setting of lack of therapeutic response to an adequate trial of empirical ATT. Microbiological CSF culture including fungal plates and tubercular culture and ideally PCR before starting ATT is crucial. Chronic course of illness in an immunocompetent person does not exclude fungal etiology. Resource limitations locally may sometimes preclude workup for uncommon fungal pathogens. Targeted biopsy aids in establishing diagnosis and providing treatment.
Acknowledgments:
We gratefully thank M. R. Shivaprakash, Mycology Division, WHO collaborating center, Center of Advanced Research in Medical Mycology, Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India, for his kind inputs in confirming the isolate as Fonsecaea pedrosoi. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1.Revankar SG, Patterson JE, Sutton DA, Pullen R, Rinaldi MG, 2002. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin Infect Dis 34: 467–476. [DOI] [PubMed] [Google Scholar]
- 2.Revankar SG, Sutton DA, Rinaldi MG, 2004. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin Infect Dis 38: 206–216. [DOI] [PubMed] [Google Scholar]
- 3.Modi M, Goyal MK, Jain A, Sawhney SS, Sharma K, Vyas S, Ahuja CK, 2018. Tuberculous meningitis: challenges in diagnosis and management: lessons learnt from Prof. Dastur’s article published in 1970. Neurol India 66: 1550–1571. [DOI] [PubMed] [Google Scholar]
- 4.de Hoog GS, Attili-Angekis D, Vicente VA, Gerrits Van Den Ende AHG, Queiroz-Telles F, 2004. Molecular ecology and pathogenic potential of Fonsecaea species. Med Mycol 42: 405–416. [DOI] [PubMed] [Google Scholar]
- 5.Najafzadeh MJ, Gueidan C, Badali H, Gerrits van den Ende AHG, Xi L, de Hoog GS, 2009. Genetic diversity and species delimitation in the opportunistic genus Fonsecaea. Med Mycol 47: 17–25. [DOI] [PubMed] [Google Scholar]
- 6.Revankar SG, Sutton DA, 2010. Melanized fungi in human disease. Clin Microbiol Rev 23: 884–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suri P, Chhina DK, Kaushal V, Kaushal RK, Singh J, 2014. Cerebral phaeohyphomycosis due to Cladophialophora bantiana–a case report and review of literature from India. J Clin Diagn Res 8: DD01–DD05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandt ME, Warnock DW, 2003. Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. J Chemother 15 (Suppl 2): 36–47. [DOI] [PubMed] [Google Scholar]
- 9.Ochiai H, Kawano H, Minato S, Yoneyama T, Shimao Y, 2012. Cerebral phaeohyphomycosis: case report. Neuropathology 32: 202–206. [DOI] [PubMed] [Google Scholar]
- 10.Ravisankar S, Chander RV, 2013. Cerebral pheohyphomycosis: report of a rare case with review of literature. Neurol India 61: 526–528. [DOI] [PubMed] [Google Scholar]
- 11.Revankar SG, 2007. Dematiaceous fungi. Mycoses 50: 91–101. [DOI] [PubMed] [Google Scholar]
- 12.Fukushiro R, 1983. Chromomycosis in Japan. Int J Dermatol 22: 221–229. [DOI] [PubMed] [Google Scholar]
- 13.Al-Hedaithy SS, Jamjoom ZA, Saeed ES, 1988. Cerebral phaeohyphomycosis caused by Fonsecaea pedrosoi in Saudi Arabia. APMIS Suppl 3: 94–100. [PubMed] [Google Scholar]
- 14.Madhugiri VS, et al. 2013. Opportunistic Fonsecaea pedrosoi brain abscess in a patient with non-cirrhotic portal fibrosis-induced hypersplenism–a novel association. Br J Neurosurg 27: 690–693. [DOI] [PubMed] [Google Scholar]
- 15.Madhugiri VS, Bhagavatula ID, Mahadevan A, Siddaiah N, 2011. An unusual infection, an unusual outcome–Fonsecaea pedrosoi cerebral granuloma. J Neurosurg Pediatr 8: 229–232. [DOI] [PubMed] [Google Scholar]
- 16.Santosh V, Khanna N, Shankar SK, Pal L, Das S, Chandramukhi A, Kolluri VR, 1995. Primary mycotic abscess of the brain caused by Fonsecaea pedrosoi. Case report. J Neurosurg 82: 128–130. [DOI] [PubMed] [Google Scholar]
- 17.Góralska K, Blaszkowska J, Dzikowiec M, 2018. Neuroinfections caused by fungi. Infection 46: 443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li DM, de Hoog GS, 2009. Cerebral phaeohyphomycosis–a cure at what lengths? Lancet Infect Dis 9: 376–383. [DOI] [PubMed] [Google Scholar]
- 19.Rosow L, Jiang JX, Deuel T, Lechpammer M, Zamani AA, Milner DA, Folkerth R, Marty FM, Kesari S, 2011. Cerebral phaeohyphomycosis caused by Bipolaris spicifera after heart transplantation. Transpl Infect Dis 13: 419–423. [DOI] [PubMed] [Google Scholar]
- 20.Valero C, de la Cruz-Villar L, Zaragoza Ó, Buitrago MJ, 2016. New panfungal real-time PCR assay for diagnosis of invasive fungal infections. J Clin Microbiol 54: 2910–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gongidi P, Sarkar D, Behling E, Brody J, 2013. Cerebral phaeohyphomycosis in a patient with neurosarcoidosis on chronic steroid therapy secondary to recreational marijuana usage. Case Rep Radiol 2013: 191375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sood S, Vaid VK, Sharma M, Bhartiya H, 2014. Cerebral phaeohyphomycosis by Exophiala dermatitidis. Indian J Med Microbiol 32: 188–190. [DOI] [PubMed] [Google Scholar]
- 23.Cristini A, Garcia-Hermoso D, Celard M, Albrand G, Lortholary O, 2010. Cerebral phaeohyphomycosis caused by Rhinocladiella mackenziei in a woman native to Afghanistan. J Clin Microbiol 48: 3451–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]