Abstract
Except for Remdesivir® no other drug or vaccine has yet been approved to treat the coronavirus disease (COVID-19) caused by the virus known as, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Remdesivir® an small molecule and nucleic acid analogue, it is used to treat adults and children with laboratory confirmed COVID-19, only administrated in hospital settings. Small molecules and particularly natural products count for almost fifty percent of the commercially available drugs, several of them are marketed antiviral agents and those can be a potential agent to treat COVID-19 infections. This short review rationalized different key natural products with known activity against coronaviruses as potential leads against COVID-19.
Keywords: 2019-nCoV, Natural products, SARS-CoV-2, Coronaviruses
1. Introduction
Coronaviruses (CoVs) are the largest group of viruses belonging to the Nidovirales order, which includes Coronaviridae, Arteriviridae, and Roniviridae families. The Coronavirinae comprises one of two subfamilies in the Coronaviridae family, with the other being the Torovirinae [1]. Coronaviruses are named for the crown-like spikes on their surface and there are four main sub-groupings of coronaviruses, known as alpha, beta, gamma, and delta [2]. Human coronaviruses were first identified in the mid-1960′s [3]. Coronaviruses cause acute and chronic respiratory, enteric and/or central nervous system diseases in many species, including humans [4].
There are seven coronaviruses that can infect human worldwide, four common types are 229E and NL63 (alpha coronavirus), while OC43 and HKU1 (beta coronavirus). Sometimes animal coronaviruses can cross-species transmitted and infect humans [5]. Three recent examples of this are beta coronaviruses; SARS-CoV (causes severe acute respiratory syndrome, or SARS), MERS-CoV (causes Middle East respiratory syndrome, or MERS), and the recently named by World Health Organization (WHO) as SARS-CoV-2 coronavirus causing the disease called COVID-19 [6]. Porcine transmissible gastroenteritis virus (TGEV), human coronavirus (HCoV) 229E, mouse hepatitis virus (MHV), bovine coronavirus (BCoV), and porcine epidemic diarrhea virus (PEDV) all belong to the coronaviridae family. These coronaviruses are encompassed, positive single-abandoned RNA infections containing 27–31 kb genomes, which cause respiratory and enteric maladies in people and animals [7].
Currently, it is difficult to generate animal models capable to simulate the key features for the human disease. These models are aiming to find the pathogenic mechanism, search for avenues to identify targets on the virus suitable for attack and design successful treatments. SARS-CoV is capable to readily infect laboratory mice, but, it does not cause any significant disease symptoms unless the virus is transmitted for adaptation into the mouse host [8]. Moreover, infection of primates produces a milder disease than that observed in humans, although fever and pulmonary inflammation were noted [9].
On March 11, 2020, The World Health Organization (WHO) has declared the novel coronavirus (COVID-19) outbreak a global pandemic. On March 28, 2020, United States Food and Drug Administration (FDA) issued an emergency use authorization of hydroxychloroquine sulfate (Plaquenil®, Quineprox®), chloroquine phosphate (Aralen®), in which the physicians can prescribe those to seriously hospitalized COVID-19 patients. Later in June 15, 2020 FDA revoked their use based on serious side effects and no real benefits on clinical trials [10]. As of September 28, 2020, the coronavirus disease 2019 (COVID-19) pandemic had resulted in 33,270,108 cases and 1,001,497 deaths worldwide, including 7,317,312 cases and 209,423 deaths in the United States (US) [11].
Drugs currently being investigated for the possibility of use against CoV disease include monoclonal antibodies, direct-acting antivirals (DAAs) such as protease, helicase, and polymerase inhibitors, and immunomodulators such as interferons and corticosteroids. Challenges in the development of CoV antivirals are both general to RNA viruses and specific to CoVs. The process of replication of positive-sense RNA virus genomes is generally characterized by high error rates, high viral yields, short replication times, and abundant homologous and nonhomologous recombination [12]. Given the urgency of the COVID-19 outbreak, we focus here in this report, on the potential to repurpose existing natural antiviral and anticancer agents with established activity against various coronaviruses such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) [13].
1.1. Approved small molecules to treat COVID-19
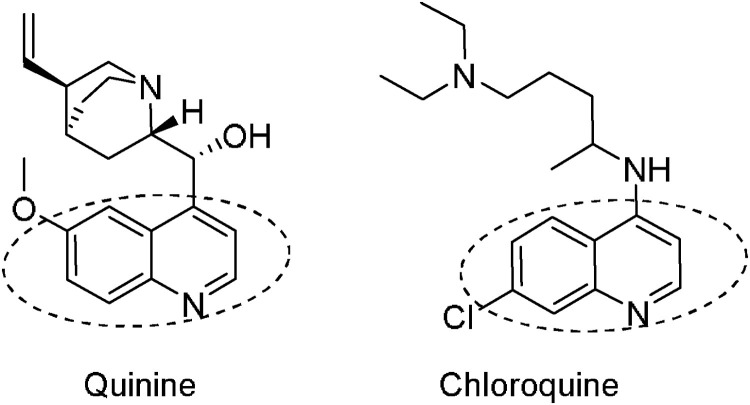
Natural compounds are potential sources for the development of antiviral agents. Various medicinal herbs producing anti-inflammatory, antifungal, and antitumor activities have been extensively studied in order to identify the herbs possessing antiviral properties. Indeed, chloroquine, the first small molecule Food and Drug Administration (FDA) approved to treat COVID-19, later revoked, was inspired and developed from quinine sharing the same quinoline core (Fig. 1 ). Quinine is the bioactive component, an old antimalarial agent, it was isolated from the bark of Cinchona officinalis used for centuries by the Inca empire in South America to treat malaria and other illness [14].
Fig. 1.
Quinine and chloroquine chemical structures, dashed ellipse corresponded to the quinoline core.
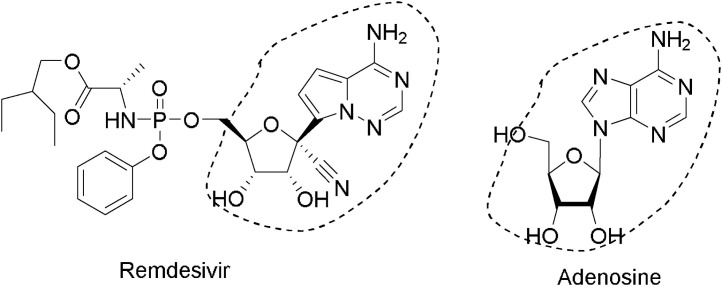
Remdesivir® possessed broad activity against RNA viruses; many research groups assessed its antiviral activity both in vitro and in vivo, validating its activity against coronaviruses. Its antiviral activity was confirmed against SARS, MERS zoonotic coronaviruses, as well as the circulating human coronaviruses HCoV−OC43 and HCoV-229E, which cause common human cold. In vitro and preclinical in vivo animal models supported the effectiveness of Remdesivir® against SARS-CoV-2 and related coronaviruses. These include a recent in vitro study of Remdesivir® assessing antiviral activity against SARS-CoV-2 using qRT-PCR quantification of viral copy number in infected Vero E6 cells. This study demonstrated an IC50 of 770 nM and an IC90 equal to 1760 nM (with cytotoxic concentration >100 mM). The mechanism of action of Remdesivir® is attacking a weak point of viral replication within the host, such as targeting the divergent RNA-dependent RNA polymerase (RdRp). The chemical structure for Remdesivir® resembles adenosine one (Fig. 2 ). Imagining under cryo-electron microscopy confirm Remdesivir® latches onto the primer RNA of the virus shutting down the viral reproduction [15].
Fig. 2.
Chemical structures of Remdesivir® and adenosine.
Recently, Y. Wang et al. reported randomized, double-blind, placebo-controlled trial in hospitalized adults with severe COVID-19 in China. Adverse effects such as constipation, hypoalbuminemia, hypokalemia, anemia, and thrombocytopenia as well as increased total bilirubin concentrations were reported in 66 % of patients who received Remdesivir®. Serious adverse events reported in 18 %, and drug discontinued because of the adverse events [16].
Next section is a compilation of the natural products that have been shown to be active against the coronavirus affecting the human health. The compounds are grouped according to their biosynthetic origin.
1.2. Natural Products showing activity against coronaviruses
1.2.1. Alkaloids
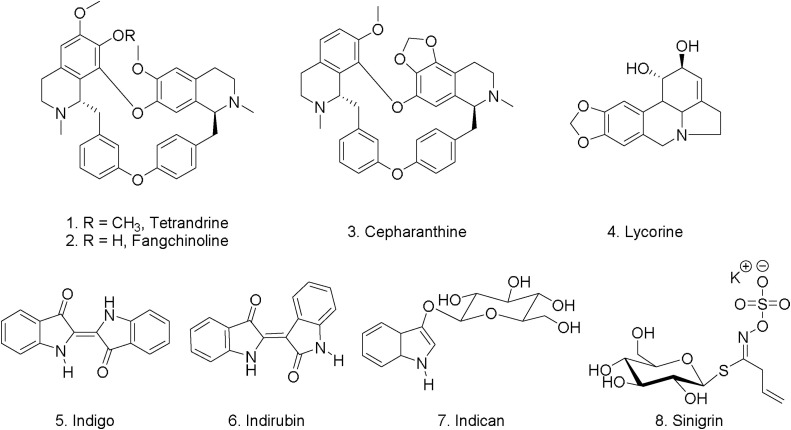
Several alkaloids have shown antiviral activity, for example, Kim et al. 2019, showed that bis-benzylisoquinoline alkaloids such as, tetrandrine (1), fangchinoline (2), and cepharanthine (3) isolated from Stephania tetrandra and other related species of Menispermaceae, (Fig. 3 ) to have potent activity against human coronavirus OC43 infection. Interestingly, bis-benzylisoquinoline alkaloids are enriched in several well-known edible plants such as Nelumbo nucifera (lotus) which is widely cultivated in Asia [17]. Tetrandrine (1) and fangchinoline (2) showed in vitro antiviral activity against human coronavirus [18]. The expression levels of the S- and N-proteins in MRC-5 lung human cells infected with HCoV−OC43 at two days’ post-infection were significantly diminished and virus replication was suppressed by the action of alkaloids 1-3. These alkaloids did not show cytotoxic effects on MRC-5 cells up to 10 μM (CC50 > 10 μM). The IC50 values of tetrandrine (1), fangchinoline (2), and cepharanthine (3) were 0.33 ± 0.03, 1.01 ± 0.07, and 0.83 ± 0.07 μM, respectively [19]. This in turn demonstrated that tetrandrine (1), fangchinoline (2), and cepharanthine (3) inhibited HCoV−OC43 at the early stage of infection which is mainly due to suppression of the replication of HCoV−OC43 virus [19].
Fig. 3.
Chemical structure for selected alkaloids and sinigrin.
Tetrandrine (1) has been known as antagonist of calmodulin, has anti-tumor, anti-inflammatory effects, and can effectively inhibit fibroblasts and thereby inhibiting pulmonary fibrosis [20]. Since the 1950s, tetrandrine (1) has been used in China as an antihypertensive drug. In 1970s, tetrandrine (1) underwent a phase I clinical trial as an antitumor drug in US. Although tetrandrine is not in clinical use in countries other than mainland China, it has been used in research worldwide showing to have multiple pharmacological activities including immunosuppression, antihypertensive, and antitumor activities. Tetrandrine had a synergistic effect on the cytotoxicity of the chemotherapeutic agents; 5-fluorouracil, oxaliplatin, and docetaxel in two gastric cancer cell lines [21]. Fangchinoline (2) is known to have inhibitory effects on histamine release and on the production of IL-1 and tumor necrosis factor-a (TNF-α). Also, fangchinoline was identified as capable of inhibiting PI3K and its downstream signaling pathways and suppressing PI3K-mediated SGC7901 behavior including growth, migration, and invasion. Further testing in experimental in vivo models is warranted [22].
In an extensive study, more than 200 Chinese medicinal herb extracts were screened for antiviral activities against (SARS-CoV) using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS) assay for virus-induced cytopathic effect (CPE) [19]. The extracts of Lycoris radiata, Artemisia annua, Pyrrosia lingua, and Lindera aggregata showed potent antiviral activities against SARS-CoV strain BJ001 with 50 % effective concentration (EC50) 2.4 ± 0.2, 34.5 ± 2.6, 43.2 ± 14.1, and 88.2 ± 7.7 μg/mL, respectively. Among those extracts, L. radiata extract showed the best inhibition effect in SARS-CoV bioassay guided fractionation yielded lycorine (4) as the main bioactive components (Fig. 3). The EC50 value for lycorine (4) was found to be 15.7 ± 1.2 nM. The CC50 of lycorine (4) in Vero E6 and HepG2 cells were 14980.0 ± 912.0 and 18810.0 ± 1322.0 nM, respectively [23]. Lycorine (4) can cause toxic effects at low doses in canines’ model (∼ 1 mg/kg), further development of this compound as a potential drug candidate is needed, and the mechanism of its antiviral activity is still not clear [24].
Isatis indigotica root is a Chinese herb frequently used for the prevention of SARS during the SARS outbreaks in China, Hong Kong, and Taiwan. The main components of I. indigotica root are the alkaloids; indigo (5), indirubin (6), and indican (indoxyl-β-d-glucoside) (7), and the allyl glucosinate, sinigrin (8) (Fig. 3). Indigo (5) and indirubin (6) were identified as the promiscuous chymotrypsin inhibitors [25]. C.W. Lin et al. [26] characterized the anti-SARS-CoV 3CLpro effect of the water extract of I. indigotica root-derived compounds. Of the five compounds that were tested in vitro, sinigrin (8) and indigo (5) showed dose-dependently inhibited cleavage activities of the 3CLpro in cell-free and cell-based assays. The IC50 values in the cell-free assays were 121 μM for sinigrin and 300 μM for indigo. The cell-based assay indicated that sinigrin (IC50 = 217 μM) was more efficient in blocking the cleavage processing of the 3CLpro than indigo (IC50 = 752 μM). Sinigrin (8) is a component in many edible plants of the Brassicaceae family, such as broccoli and Brussels sprouts. In seeds of Brassica nigra (mustard seeds) sinigrin (8) is found in high concentration, mustard has been used for centuries by mankind for its culinary, as well as various medicinal properties [27].
1.2.2. Flavonoids
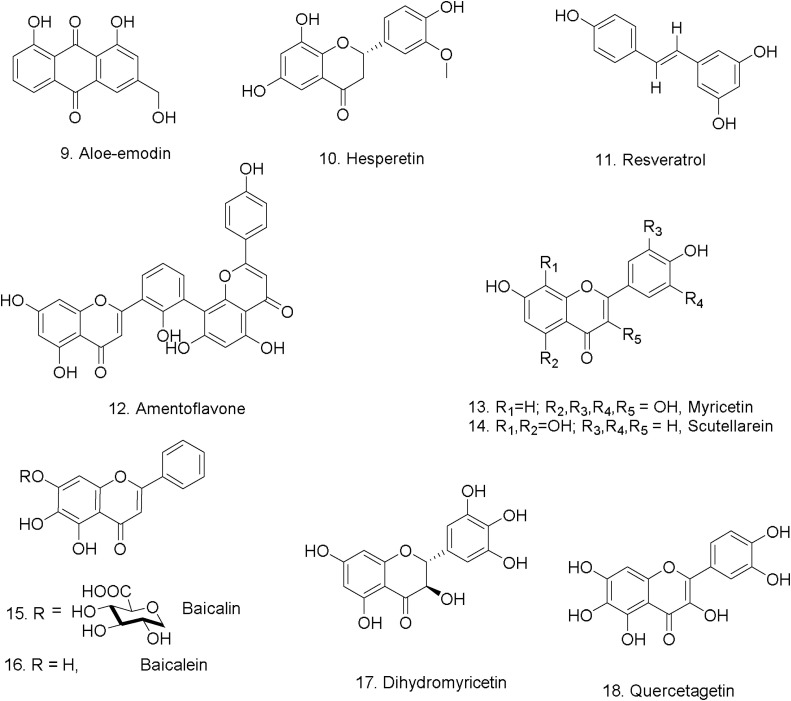
In 2005, Lin et al. studied phenolic compounds which were evaluated for their inhibitory effects on the SARS- CoV 3CLpro. Aloe-emodin (9) and hesperetin (10) dose-dependently inhibited cleavage activity of the 3CLpro in in vitro cell-free and cell-based assays, the IC50 values of aloe-emodin (9) and hesperetin (10) were 132 μM and 60 μM, respectively [26].
The expression of nucleocapsid (N) protein essential for MERS-CoV replication was decreased after resveratrol treatment, down regulating the apoptosis induced by MERS-CoV in vitro assay [28]. Resveratrol also has been found to inhibit herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) replication in a dose-dependent, and reversible manner [29]. In several toxicity studies, resveratrol was orally administrated at its maximum tolerated doses to access its adverse effects; the results show the lack of carcinogenicity. Reports revealed the absence of acute skin and eye irritation or other allergenicity signs that could be caused by the compound. Despite being an estrogen-like compound, studies provide further evidence that trans-resveratrol has low estrogenic potency in vivo [30]. A large amount of resveratrol is produced in the skin of grapes to protect the plant against fungal diseases and sun damage [31]. Resveratrol is widely available on red wine extract, grape seed extract, and Japanese knotweed extract and others. Most supplements on the market are derived from Japanese knotweed as it has the highest concentrations of resveratrol in nature [32]. Commercial dietary supplements contain an average between 50–500 mg of trans-resveratrol (11), human clinical studies have also been performed up to single doses of 5 g of resveratrol without observing adverse effects [30]. These data suggest that trans-resveratrol (11) is well tolerated in humans and that 450 mg/day can represent a safe dose for a 70 kg individual [33]. Resveratrol has low systemic bioavailability and is available in solution form and as a transdermal patch, however, its safety and effectiveness have not been approved by the FDA [34].
Amentoflavone (12) a bi-flavonoid isolated from Selaginella sinensis, Torreya nucifera, and other medicinal plants has shown in vitro potent antiviral activity against respiratory syncytial virus (RSV), with an IC50 of 5.5 μg/mL [35], as well as significant activity against influenza A and B viruses [36]. Additionally, amentoflavone (12) revealed moderate anti-herpes simplex virus HSV-1 and anti-HSV-2 activities with EC50 values of 17.9 μg/mL (HSV-1) and 48.0 μg/mL (HSV-2), and also described as SARS-CoV 3CL protease inhibitor with an IC50 of 8.3 μM [37]. This compound has shown high cytotoxicity against MCF-7 and HeLa cancer cell lines [38].
Myricetin (13) and scutellarein (14) (Fig. 4 ) are strong inhibitors of SARS-CoV helicase and their effect is mediated through inhibition of ATPase activity. The IC50 values of myricetin and scutellarein in vitro were 2.71 ± 0.19 μM and 0.86 ± 0.48 μM, respectively [39]. It has been noticed that neither myricetin (13) nor scutellarein (14) can affect the growth of MCF10A cells at cellular concentrations close to their IC50′s [40].
Fig. 4.
Phenolic compounds tested against coronaviruses.
Myricetin (13) is a typical plant-derived flavonoid produced fundamentally by individuals from the families Myricaceae, Anacardiaceae, Polygonaceae, Pinaceae, and Primulaceae and considered as one of the key elements of different beverages [40].
Myricetin (13) shows a wide spectrum of activities that incorporate anti-oxidant, anticancer, antidiabetic and anti-inflammatory activities [41]. It shows a few activities that are identified with the central nervous system (CNS) and the compound is suggested to be useful against Parkinson's and Alzheimer's diseases [42]. According to some reports myricetin (13) has the capability to modify the immune response or functioning of the immune system [42]. This compound has cytotoxic and apoptosis-promoting effects in prostate cancer (PCa) cells [43].
Scutellaria baicalensis roots have been widely used in traditional Chinese medicine to treat viral infection [44]. In vitro and in vivo replication of SARS-CoV can be inhibited by the flavonoid baicalin (15), which is isolated from S. baicalensis. Numerous inhibitory activities were confirmed for baicalin (EC50 at 48 h is 12.5–25 μg/mL and at 72 h is 25–50 μg/mL) by virtue of neutralization-based testing of the nine SARS coronavirus isolates [45].
The most potent anti-SARS-CoV-2 3CLpro activity was shown by an aglycon of baicalin, baicalein (16), with an IC50 of 0.39 μM. Studies of several analogs have demonstrated the potentiality of additionally four flavonoids as potent inhibitors of SARS-CoV-2 3CLpro. These potent inhibitors include scutellarein (14), which is largely distributed in genera Scutellaria and Erigerontis, and shows IC50 value of 5.8 μM against SARS-CoV-2 3CLpro. The other three remaining flavonoid compounds are myricetin (13), dihydromyricetin (17), and quercetagetin (18). They were respectively constituents from Ampelopsis japonica (Bailian in Chinese), Eriocaulon buergerianum (Gujingcao in Chinese) and Polygoni avicularis (Bianxu in Chinese), and possess inhibitory activity against SARS-CoV- 2 3CLpro with IC50 values of 1.20, 1.24, and 2.86 μM [46]. Baicalin has been shown to possess potential cytotoxicity [47].
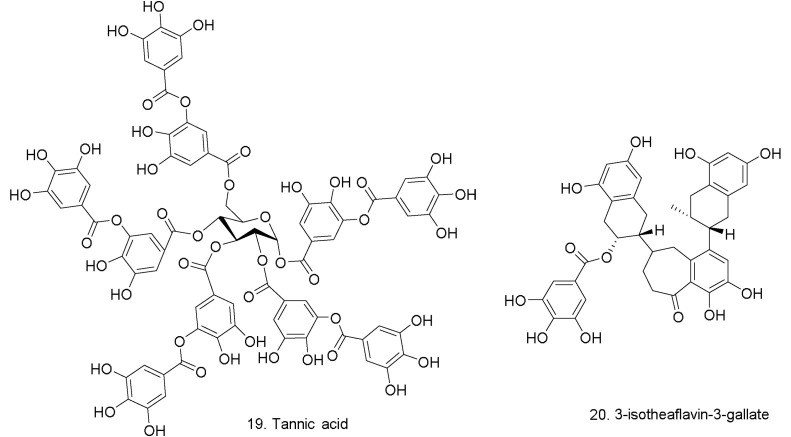
Tannic acid (19) (IC50 = 3 μM) and 3-isotheaflavin-3-gallate (20) (IC50 = 7 μM) (Fig. 5 ) were shown to be potent inhibitors of SARS 3CLpro by virtue of high-throughput screening of a natural product library (approx. 720 compounds) [48]. These two compounds form part to a group of natural polyphenols found in tea [45]. According to the results obtained, the extracts from Pu-erh tea and black tea were the most potent inhibitors of SARS protease [48]. It was also reported that bovine coronavirus and rotavirus infections could be neutralized by theaflavins extracted from black tea [49] with EC50 of 34.7 μg/mL. The findings also corroborate the presence of an inactivation activity (in vitro) of theaflavin and theaflavin gallate derivatives (20) against both rotavirus and coronavirus.
Fig. 5.
Chemical structure of theaflavins extracted from black.
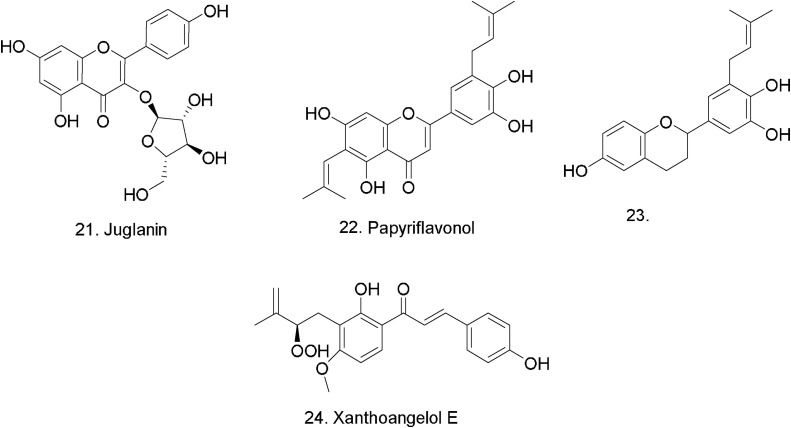
The activity of polyphenols derived from Broussonetia papyrifera against 3-chymotrypsin-like and papain-like coronavirus cysteine proteases were discussed by Ji-Young Park et al. [50]. Many polyphenols were discovered to be more potent as inhibitors of papain-like protease (PLpro) than those of 3-chymotripsin-like protease (3CLpro) [50]. Among these polyphenols, papyriflavonol (22) (Fig. 6 ) was discovered to manifest the most potent inhibitory activity against PLpro with an IC50 value of 3.7 μM while C-5-alkyl group (prenyl)-substituted flavan (23) was the most potent against SARS- CoV 3CLpro which was proven to be more effective than quercetin derivative (IC50 = 52.7 μM).
Fig. 6.
Phenolic compounds tested against coronaviruses.
Recently, it has been suggested by Stevens et al. that polyphenols inhibition of viral proteases associated with viral replication is due to their protein affinity via hydrogen bonding [51].
Houttuynia cordata Thunb. (Saururaceae) or HC is a medicinal plant commonly used in folk medicine in a number of Asian countries. It is effective in the treatment of pneumonia, infectious diseases, refractory hemoptysis, and malignant pleural effusion [52]. It was the main herbal component of heat-removing and detoxifying formula during the severe acute respiratory syndrome (SARS) outbreak in 2002–2003 [53]. Considerable inhibitory effects were demonstrated in vitro and in vivo by HC water extract on both SARS-CoV 3C-like protease (3CLpro) and RNA-dependent RNA polymerase [54], where HC extract was discovered to be an effective inhibitor of [α-32P] UTP incorporation at 50 μg/mL. Interestingly, oral acute toxicity analysis demonstrated that HC was non-toxic to laboratory animals after an oral administration at 16 g/kg.
The inhibitory activities of chalcones and coumarins isolated from the edible Angelica keiskei against SARS-CoV proteases (3CLpro and PLpro) were determined (cell-free/based) [55]. Xanthoangelol E (24) (Fig. 6), exhibited the most inhibitory activity against 3CLpro and PLpro with IC50 values of 11.4 and 1.2 μM [56]. Data suggested that chalcones exhibited competitive inhibition to the SARS-CoV 3 CLpro and noncompetitive inhibition to the SARS-CoV PLpro.
1.2.3. Terpenoids
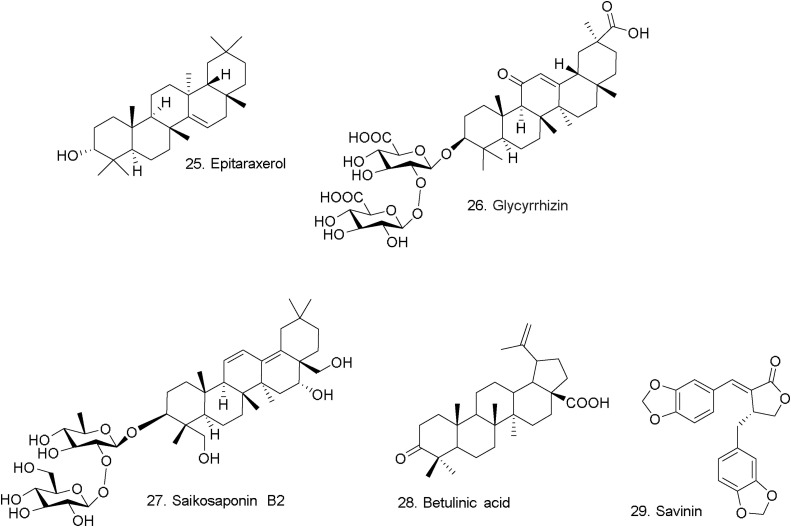
Terpenoids represent diverse class of molecules that provide a wealth of opportunities to address many human health and societal issues. Euphorbia neriifolia L. is an herb local to Southeast Asia. From leaves of E. neriifolia, 23 compounds were isolated, including 22 triterpenoids and one flavonoid glycoside [57]. The antiviral activity of all the isolated compounds was evaluated. The assay results indicated the highly influence of the antiviral activity with small differences in the structural features of the tested compounds. Among the friedelane derivatives tested, two epimers, 3β- friedelanol and 3α-friedelanol, with difference orientation at C-3 that affected dramatically their antiviral activity while epitaraxerol (25) (Fig. 7 ), a taraxerane derivative, was the most active derivative [57].
Fig. 7.
Chemical structure of triterpenoids and Savinin.
Glycyrrhizin (26) (Fig. 7), the active component of liquorice roots, has been reported to possess moderate antiviral activity against SARS-CoV in vitro with an EC50 of 300 g/mL [58], however its full mechanism is unclear. Glycyrrhizin affects cellular signaling pathways such as protein kinase C; casein kinase II; and transcription factors such as activator protein 1 and nuclear factor kB (NF-κB).
Saikosaponins isolated from medicinal plants such as Bupleurum spp., Heteromorpha spp., and Scrophularia scorodonia have been reported to produce various biological activities including antihepatitic, antinephritic, antihepatomic, anti-inflammatory, immunomodulatory, and antibacterial effects [[59], [60], [61]]. The cytotoxicity of saikosaponins A, B2, C, D and actinomycin D on MRC-5 cells using the XTT assay indicated no cytotoxic effect on the cells at concentrations of 2.5 μM/L. Saikosaponins (A, B2, C and D) were examined for their anticoronaviral activity, where saikosaponin B2 (27) (Fig. 7) has potent anticoronaviral activity [62]. The percentage viral inhibition for 0.25, 2.5, and 25 μM/L saikosaponin B2 was 35.7 ± 0.7, 63.0 ± 0.8, and 100.0 ± 0.2 %, respectively. The mechanistic studies show that saikosaponin B2 inhibits HCoV-229E infection in a dose- and time-dependent manner, block viral penetration into cells, and interfere with the early stage of viral replication, such as virus absorption and penetration.
In another study, 221 phytocompounds were evaluated for their activity against severe acute respiratory syndrome associated coronavirus (SARS-CoV) using a cell-based assay measuring SARS-CoV-induced cytopathogenic effect on Vero E6 cells. Twenty tested compounds exhibited significant levels of anti-SARS-CoV activity at 10 μM with no cytotoxicity against Vero E6 cells. Due to its pivotal role in the SARS-CoV life cycle, the 3CL protease is a key target for discovery of anti-SARS- CoV agents [63]. The inhibitory effects of compounds on SARS-CoV 3CL protease activity were investigated. Only betulinic acid (28) (IC50 = 10 μM, Ki = 8.2 ± 0.7 μM) and savinin (29) (IC50 = 25 μM, Ki = 9.1 ± 2.4 μM) (Fig. 7) exhibited significant inhibition on 3CL protease. The mechanism of action of betulinic acid and savinin on 3CL protease was shown to be competitive inhibition via molecular docking [63,64].
1.2.4. Fatty acids and polyketides- diarylheptanoids
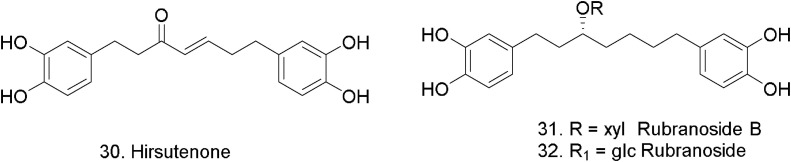
Alnus japonica and its constituents exhibit various biological properties, including anti-inflammatory, anticancer, and anti-influenza activities. The ethanol extract of the stem bark of A. japonica exhibited PLpro inhibitory activity. Nine diarylheptanoids were identified; platyphyllenone, hirsutenone (30), platyphyllone, platyphyllonol-5-xylopyranoside, hirsutanonol, oregonin, rubranol, rubranoside B (31), and rubranoside (32) (Fig. 8 ) [65]. The isolated compounds were tested against SARS-CoV PLpro using a continuous fluorometric assay, where six diarylheptanoids showed a dose-dependent inhibitory effect against the PLpro. Compounds 30 and 31 exhibited modest activity, with IC50 values of 8.0 and 9.1 μM. These compounds also were tested using recombinant 3CLpro expressed in vitro, where compound 32 exhibited stronger 3CLpro inhibition than the other derivatives with IC50 = 36.2 μM.
Fig. 8.
Diarylhepatanoids from Alnus japonica (Betulaceae).
The diarylheptanoids were found to be reversible inhibitors, where an increase in concentration rapidly reduced enzyme activity [65]. Structural-activity relationship (SAR) of diarylheptanoids established that α, β-unsaturated carbonyl and catechol groups may play a pivotal role in SARS-CoV PLpro inhibition by interacting with the PLpro nucleophiles [65]. The mechanism of inhibition of cysteine protease may involve the formation of a covalent bond between the carbonyl group located at the warhead of the inhibitor and the cysteine active site residue in the enzyme.
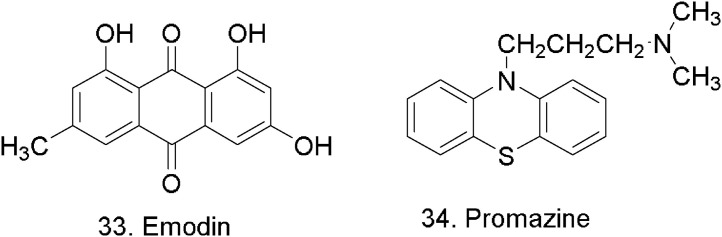
A number of experiments aimed at the evaluation of the inhibitory activity and the effects of Chinese medicinal herbs on the S protein and ACE2 interaction have been carried out by T.-Y. Ho et al. [66]. The herbs were divided by the team into 32 families in order to compare the levels of inhibition through taxonomic characterization. Up to 60–90 % of the binding of S protein to Angiotensin-converting enzyme 2 (ACE2) at 1 μg was blocked by six herb families, including Nelumbonaceae, Labiatae, Magnoliaceae, Oleaceae, Lauraceae, and Polygonaceae. Among the given families, the highest inhibitory effect on the S protein and ACE2 interaction was demonstrated by Polygonaceae, with t inhibitory percentage of 86.33 ± 4.55 %, where Radix et Rhizoma Rheim, Radix Polygoni multiflora, and Caulis Polygoni multiflori pertain to Polygonaceae [67]. Emodin, the major components of the genus Rheum and Polygonum, is the likely active constituent responsible for blocking both the binding of SARS-CoV S protein to ACE2. Emodin (33) (Fig. 9 ) blocked the binding of S protein to ACE2 in a dose-dependent manner. The IC50 value of emodin is 200 μM. Emodin is an anthraquinone compound consists of three cyclic rings. The anti-psychotic drug promazine (34) (Fig. 9), which has been shown to exhibit the significant effect in inhibiting the replication of SARS-CoV [68], shared a similar structure with emodin. As compared to emodin, promazine exhibited the highest inhibition. However, the differences between emodin and promazine were not significant.
Fig. 9.
Chemical structure of emodin and promazine.
1.2.5. Current trends and clinical trials
Many anti-immune treatments, investigational clinical trials using repurposed drugs for evaluation of direct antiviral activity have already been launched, including multiple antiviral and antimalarial medicines [68]. A great effort has been addressed towards sharing information on biomolecular simulation data to reveal models of how the coronavirus could potentially infect human, as well as how to prevent and/or treat COVID-19 [69].
Recently, Riva et al., reported a high-throughput analysis of approximately 12,000 known drugs evaluated for their activity against SARS-CoV-2 replication which afforded 30 known drugs capable to inhibit viral SARS-CoV-2 replication [69]. Six drugs were further characterized for cellular dose-activity relationships, and showed effective concentrations likely to be commensurate with therapeutic doses in patients. The mechanism of those drugs includes the PIKfyve kinase inhibitor Apilimod, cysteine protease inhibitors MDL-28170, Z LVG CHN2, VBY-825, and ONO 5334, and the CCR1 antagonist MLN-3897 [70].
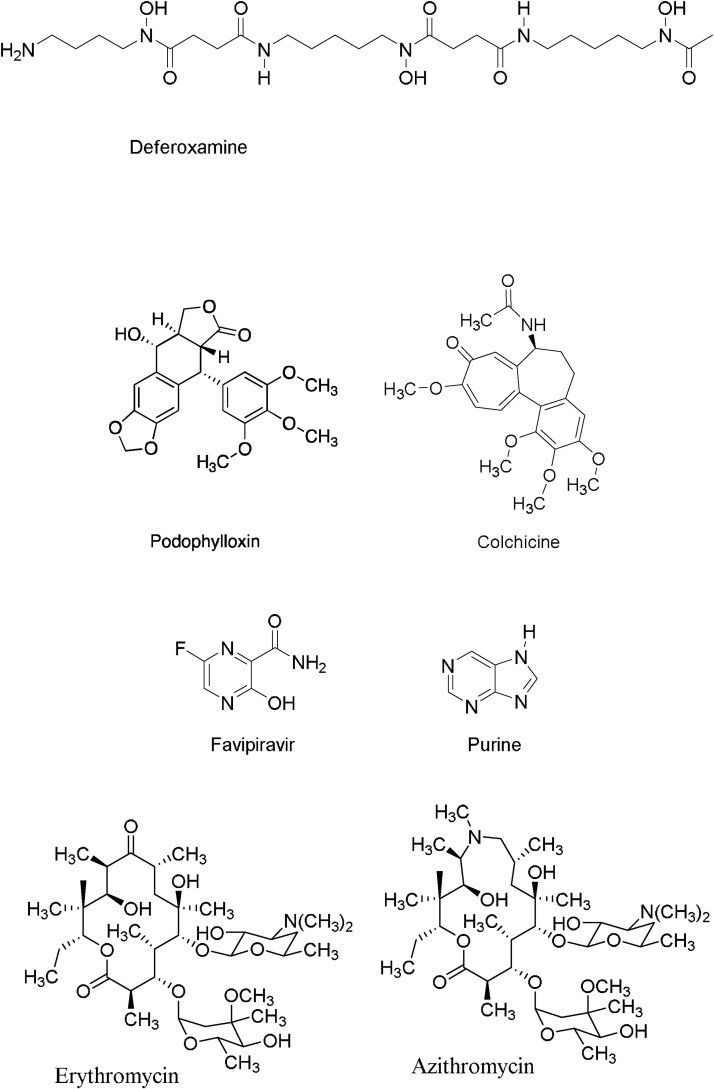
There are several ongoing clinical trials using repurposed clinical-stage or approved drugs such as remdesivir, favipiravir, lopinavir/ritonavir, hydroxychloroquine, and natural or semisynthetic products like quercetin [71,72], colchicine [73], tetrandrine [74], desferrioxamine B [75], azithromycin [[76], [77], [78]] (Fig. 10 ) are under investigation for treating COVID-19 patients, as well as several vaccines are in fast phases of clinical trials. Selected examples of natural products which are in current clinical trials to treat COVID-19 are shown below.
Fig. 10.
Natural and semisynthetic compounds that are under ongoing clinical trials.
Desferrioxamine B (Desferal®) is produced by Streptomyces pilosus and the only siderophore currently marketed (DB00746). It is produced by the fermentation of S. pilosus and is used therapeutically in patients with iron and aluminum overload [79]. Desferrioxamine B is also known for its antiproliferative activity against leukemia and neuroblastoma cells in vitro and in vivo and in current clinical trials for its antitumor activity [80]. Replication of human immunodeficiency virus type 1 (HIV-1) can be influenced by iron, hence, decreasing the availability of iron may inhibit HIV-1 replication, where deferoxamine is capable of forming catalytically inactive iron-chelator complexes [81].
Quercetin is reported to be effective on treatment and prophylaxis of other SARS like coronavirus infections, as a strong antioxidant and scavenger flavonoid without any serious reported adverse effects. This in turn motivates investigators to consider quercetin as an effective lead on both prophylaxis and treatment of COVID-19 cases [82].
Favipiravir (T-705; 6-fluoro-3-hydroxy-2-pyrazinecarboxamid, Avigan®) is an antiviral drug that selectively inhibited the RdRP of influenza virus [83]. It showed specific activity against all three influenza A, B, and C [84,85]. The primary mechanism of action of favipiravir against the influenza virus was through specific inhibition to vRNA polymerase [86]. Favipiravir functions as a purine homologue, where vRNA polymerase mistakenly recognizes favipiravir-RTP as a purine nucleotide resulted in inhibition of viral RNA synthesis [86].
Colchicine (Colcrys®) is an alkaloid isolated from the plant Colchicum autumnale. It is a drug used to treat gout and Behçet's disease with narrow therapeutic-toxicity window and a marked variability between individuals in drug disposition [87]. Colchicine binds in an equimolar and poorly reversible manner to soluble nonpolymerized tubulin with high activation energy, forming a tubulin-colchicine complex [88].
It appears through this short review that natural products with the unique pharmacophores and high safety margin will present key element in dealing with the pandemic 2019-nCoV.
2. Conclusions
There is an urgent and critical need to identify novel medical countermeasures both for prophylactic and treatment use. Since the production of a vaccine could take 12–18 months, and de novo development of therapies usually requires 10–17 years, repositioning clinically evaluated drugs represents one of the most practicable strategies for the rapid identification and deployment of treatments for emerging infectious diseases such as COVID-19 [65].
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
This work is partially supported by the Agricultural Research Service - United States Department of Agriculture (USDA ARS), Specific Cooperative Agreement No. 58-6060-6-015.
References
- 1.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. In: Maier H., Bickerton E., Britton P., editors. Coronaviruses. Methods in Molecular Biology. 2015. p. 1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Payne S. 2017. Family Coronaviridae. Viruses; pp. 149–158. [DOI] [Google Scholar]
- 3.Myint S.H. Human coronaviruses: a brief review. Rev. Med. Virol. 1994;4(1):35–46. doi: 10.1002/rmv.1980040108. [DOI] [Google Scholar]
- 4.Liang P.H. Characterization and inhibition of SARS-coronavirus main protease. Curr. Top. Med. Chem. 2006;6(4):361–376. doi: 10.2174/156802606776287090. [DOI] [PubMed] [Google Scholar]
- 5.Rachel L.G., Ralph S.B. Recombination, reservoirs, and the modular spike, mechanisms of coronavirus cross-species transmission. J. Virol. 2010;84(7):3134–3146. doi: 10.1128/jvi.01394-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi Y. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020;16(10):1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Hoek L. Identification of a new human coronavirus. Nat. Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gralinski L.E., Menachery V.D. Return of the coronavirus: 2019-nCoV return of the coronavirus: 2019-nCoV. Viruses. 2020;12:135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Wit E. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or#:∼:text=FDA%20cautions%20against%20use%20of%20hydroxychloroquine%20or%20chloroquine,treat%20hospitalized%20patients%20with%20COVID-19%20is%20now%20available, accessed on 09/27/2020.
- 11.https://coronavirus.jhu.edu/us-map, accessed on 07/13/2020.
- 12.Pruijssers A.J., Denison M.R. Nucleoside analogues for the treatment of coronavirus infections. Curr. Opin. Virol. 2019;35:57–62. doi: 10.1016/j.coviro.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haagmans B.L., Osterhaus D.M. Coronaviruses and their therapy. Antiviral Res. 2006;71:397–403. doi: 10.1016/j.antiviral.2006.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev., Drug Discover. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 15.Yin W. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by Remdesivir. Science. 2020;368(6498):1499–1504. doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeming W. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-31029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D.E. Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules. 2019;9(11):696. doi: 10.3390/biom9110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng X.L. Bisbenzylisoquinoline alkaloids of lotus (Nelumbo nucifera Gaertn.) seed embryo inhibit lipopolysaccharide-induced macrophage activation via suppression of Ca2+-CaM/CaMKII pathway. Food Agric. Immunol. 2019;30(1):878–896. doi: 10.1080/09540105.2019.1638889. [DOI] [Google Scholar]
- 19.Yang Y. Traditional Chinese medicine in the treatment of patients infected with 2019-New coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S. The status of Chinese medicine in reversing multi-drug resistance of hepatocellular carcinoma. Chin. -Ger. J. Clin. Oncol. 2011;10:541. doi: 10.1007/s10330-011-0828-1. [DOI] [Google Scholar]
- 21.Huang Yi-Tsau, Hong Chuang-Ye. Tetrandrine. Cardiovasc. Drug Rev. 1998;16(1):1–15. doi: 10.1111/j.1527-3466.1998.tb00341.x. [DOI] [Google Scholar]
- 22.Tian F., Ding D., Li D. Fangchinoline targets PI3K and suppresses PI3K/AKT signaling pathway in SGC7901 cells. Int. J. Oncol. 2015;46:2355–2363. doi: 10.3892/ijo.2015.2959. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Cinatl J. W. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kretzing S. Dose-dependent emetic effects of the Amaryllidaceous alkaloid lycorine in beagle dogs. Toxicon. 2011;57:117–124. doi: 10.1016/j.toxicon.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Li S.Y. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67:18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Ch.W. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Res. 2005;68:36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazumder A. Sinigrin and its therapeutic benefits. Molecules. 2016;21(4):416. doi: 10.3390/molecules21040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chafekar A., Fielding B.C. MERS-CoV: understanding the latest human coronavirus threat. Viruses. 2018;10(2):93. doi: 10.3390/v10020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Docherty J.J. Resveratrol inhibition of herpes simplex virus replication. Antiviral Res. 1999;43(3):145–155. doi: 10.1016/s0166-3542(99)00042-x. [DOI] [PubMed] [Google Scholar]
- 30.Neves A.R. Resveratrol in medicinal chemistry: a critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr. Med. Chem. 2012;19:1663–1681. doi: 10.2174/092986712799945085. [DOI] [PubMed] [Google Scholar]
- 31.Frémont L. Biological effects of resveratrol. Life Sci. 2000;66(8):663–673. doi: 10.1016/S0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 32.Henning S.M. Variability in the antioxidant activity of dietary supplements from pomegranate, milk thistle, green tea, grape seed, goji, and acai: effects of in vitro digestion. J. Agric. Food Chem. 2014;62:4313–4332. doi: 10.1021/jf500106r. [DOI] [PubMed] [Google Scholar]
- 33.Weston L.A. Review of the biology and ecology of three invasive perennials in New York state: japanese knotweed (Polygonum cuspidatum), mugwort (Artemisia vulgaris) and pale swallow-wort (Vincetoxicum rossicum) Plant Soil. 2005;277:53–69. doi: 10.1007/s11104-005-3102-x. [DOI] [Google Scholar]
- 34.Timmers S. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y.M. Antiviral activities of biflavonoids. Planta Med. 1999;65:120–125. doi: 10.1055/s-1999-13971. [DOI] [PubMed] [Google Scholar]
- 36.Ma Shuang-Cheng. Antiviral Amentoflavone from Selaginella sinensis. Biol. Pharm. Bull. 2001;24(3):311–312. doi: 10.1248/bpb.24.311. [DOI] [PubMed] [Google Scholar]
- 37.Ryu Y.B. Biflavonoids from Torreya nucifera displaying SARS‐CoV 3CL (pro) inhibition. Bioorg. Med Chem. 2010;18:7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee Eunjung. Cytotoxic activities of amentoflavone against human breast and cervical cancers are mediated by increasing of PTEN expression levels due to peroxisome proliferator-activated receptor γ activation. Bull. Korean Chem. Soc. 2012;33(7):2219–2223. doi: 10.5012/bkcs.2012.33.7.2219. [DOI] [Google Scholar]
- 39.Yu M.S. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg. Med. Chem. Lett. 2012;22:4049–4054. doi: 10.1016/j.bmcl.2012.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semwal D. Myricetin: a dietary molecule with diverse biological activities. Nutrients. 2016;8(2):90. doi: 10.3390/nu8020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan Interactions between traditional Chinese medicines and Western therapeutics. Curr. Opin. Drug Discov. Devel. 2010;13(1):50–65. [PubMed] [Google Scholar]
- 42.Hyman B.T. Alzheimer’s disease: cell - specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 43.Ye C. The natural compound myricetin effectively represses the malignant progression of prostate Cancer by inhibiting PIM1 and disrupting the PIM1/CXCR4 interaction. Cell. Physiol. Biochem. 2018;48:1230–1244. doi: 10.1159/000492009. [DOI] [PubMed] [Google Scholar]
- 44.Qing Zh. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Science bulletin. 2016;61(18):1391–1398. doi: 10.1007/s11434-016-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen F. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. clin. Virol. off. Pub. Pan American Soc. Clin. Virol. 2004;31(1):69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Hongbo. 2020. Scutellaria Baicalensis Extract and Baicalein Inhibit Replication of SARS-CoV-2 and its 3C-like Protease in Vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu X. Baicalin induces human mucoepidermoid carcinoma Mc3 cells apoptosis in vitro and in vivo. Invest. New Drugs. 2011;29:637–645. doi: 10.1007/s10637-010-9402-x. [DOI] [PubMed] [Google Scholar]
- 48.Chen C.N. Inhibition of SARS-CoV 3C-like protease activity by Theaflavin-3,3-digallate (TF3) Evid. Based Complement. Altern. Med. 2005;2:209–215. doi: 10.1093/ecam/neh081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark K.J. An in vitro study of theaflavins extracted from black tea to neutralize bovine rotavirus and bovine coronavirus infections. Vet. Microbiol. 1998;63:147–157. doi: 10.1016/S0378-1135(98)00242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park J.-Y. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzyme Inhib. Med. Chem. 2017;32(1):504–512. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paraiso I.L. Potential use of polyphenols in the battle against COVID-19. Curr. Opin. Food Sci. 2020 doi: 10.1016/j.cofs.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anon herba Houttuynia. Modern Study Trad. Chinese Med. 1997;3:2983–3003. [Google Scholar]
- 53.Thiel Mechanisms and enzymes involved in SARS coronavirus genome expression. J. Gen. Virol. 2003;84:2305–2315. doi: 10.1099/vir.0.19424-0. [DOI] [PubMed] [Google Scholar]
- 54.Lau K.M. Immunomodulatory and anti‐SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008;118:79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park J.-Y. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg. Med. Chem. 2012;20:5928–5935. doi: 10.1016/j.bmc.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park J.-Y. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J. Enzyme Inhib. Med. Chem. 2016;31(1):23–30. doi: 10.3109/14756366.2014.1003215. [DOI] [PubMed] [Google Scholar]
- 57.Chang F.R. Anti-Human Coronavirus (anti-HCoV) triterpenoids from the leaves of Euphorbia neriifolia. Nat. Prod. Commun. 2012;7:1–3. doi: 10.1177/1934578X1200701103. [DOI] [PubMed] [Google Scholar]
- 58.Cinatl J. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2059. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guinea M.C. Biologically active triterpene saponins from Bupleurum fruticosum. Planta Med. 1994;60:163–167. doi: 10.1055/s-2006-959442. [DOI] [PubMed] [Google Scholar]
- 60.Recio M.C. Anti-inflammatory activity of Saikosaponins from Heteromorpha trifoliate. J. Nat. Prod. 1995;58:140–144. doi: 10.1021/np50115a023. [DOI] [PubMed] [Google Scholar]
- 61.Bermejo Benito P. In vivo and in vitro anti-inflammatory activity of Saikosaponins. Life Sci. 1998;63:1147–1156. doi: 10.1016/S0024-3205(98)00376-2. [DOI] [PubMed] [Google Scholar]
- 62.Cheng P.W. Antiviral effects of Saikosaponins on human coronavirus 229E in vitro. Clin. Exp. Pharmacol. Physiol. 2006;33:612–616. doi: 10.1111/j.1440-1681.2006.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wen Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 64.Yang H. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. PNAS. 2003;100(23):13190–13195. doi: 10.1073/pnas.1835675100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park J.-Y. Diarylheptanoids from Alnus japonica inhibit papain-like protease of severe acute respiratory syndrome coronavirus. Biol. Pharm. Bull. 2012;35(11):2036–2042. doi: 10.1248/bpb.b12-00623. [DOI] [PubMed] [Google Scholar]
- 66.Ho T.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X.W., Yap Y.L. Old drugs as lead compounds for a new disease? Binding analysis of SARS coronavirus main proteinase with HIV, psychotic and parasite drugs. Bioorg. Med. Chem. 2004;12(10):2517–2521. doi: 10.1016/j.bmc.2004.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kiplin G. Rapid repurposing of drugs for COVID-19. Science. 2020;368(6493):829–830. doi: 10.1126/science.abb9332. [DOI] [PubMed] [Google Scholar]
- 69.Riva Laura. A large-scale drug repositioning survey for SARS-CoV-2. Antivirals. 2020 doi: 10.1101/2020.04.16.044016. [DOI] [Google Scholar]
- 70.Amaro Rommie E., Adrian Mulholland J. A community letter regarding sharing biomolecular simulation data for COVID-19. J. Chem. Inf. Model. 2020;60(6):2653–2656. doi: 10.1021/acs.jcim.0c00319. [DOI] [PubMed] [Google Scholar]
- 71.https://clinicaltrials.gov/ct2/show/NCT04377789?cond=COVID-19&draw=14, accessed on 06/13/2020.
- 72.https://clinicaltrials.gov/ct2/show/NCT04377789?cond=COVID-19&draw=3&rank=184, accessed on 05/15/2020.
- 73.https://clinicaltrials.gov/ct2/show/NCT04375202?cond=COVID-19&draw=2&rank=61, accessed on 05/15/2020.
- 74.https://clinicaltrials.gov/ct2/show/NCT04308317?cond=COVID-19&draw=4&rank=298, accessed on 05/15/2020.
- 75.https://clinicaltrials.gov/ct2/show/NCT04333550?cond=COVID-19&draw=5&rank=3, accessed on 05/15/2020.
- 76.https://clinicaltrials.gov/ct2/show/NCT04359316?cond=COVID-19&draw=2&rank=78, accessed on 05/15/2020.
- 77.https://clinicaltrials.gov/ct2/show/NCT04344444?cond=COVID-19&draw=4&rank=244, accessed on 05/15/2020.
- 78.https://clinicaltrials.gov/ct2/show/NCT04332094?cond=COVID-19&draw=4&rank=273, accessed on 05/15/2020.
- 79.Porter J.B. A risk-benefit assessment of iron-chelation therapy. DrugSafety. 1997;17:407–421. doi: 10.2165/00002018-199717060-00006. [DOI] [PubMed] [Google Scholar]
- 80.Mortazavi M., Akbarzadeh A. Improvement of desferrioxamine B production of Streptomyces pilosus ATCC 19797 with use of protease inhibitor and minerals related to its activity. Indian J. Clin. Biochem. 2012;27(3):274–277. doi: 10.1007/s12291-012-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Georgiou N.A. Inhibition of human immunodeficiency virus type 1 replication in human mononuclear blood cells by the iron chelators deferoxamine, deferiprone, and bleomycin. J. Infect. Dis. 2000;181(2):484–490. doi: 10.1016/j.antiviral.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 82.Naesens Role of human hypoxanthine guanine phosphoribosyl transferase in activation of the antiviral agent T-705 (favipiravir) Mol. Pharmacol. 2013;84:615–629. doi: 10.1124/mol.113.087247. [DOI] [PubMed] [Google Scholar]
- 83.Takahashi In vitro and in vivo activities of T-705 and oseltamivir against influenza virus. Antivir. Chem. Chemother. 2003;14:235–241. doi: 10.1177/095632020301400502. [DOI] [PubMed] [Google Scholar]
- 84.Tanaka T. T-705 (Favipiravir) suppresses tumor necrosis factor α production in response to influenza virus infection: a beneficial feature of T-705 as an anti-influenza drug. Acta Virol. 2017;61(1):48–55. doi: 10.4149/av_2017_01_48. [DOI] [PubMed] [Google Scholar]
- 85.Furuta Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015\. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shiraki K., Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020;209 doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robert A., Terkeltaub M.D. Colchicine Update: 2008. Semin. Arthritis Rheum. 2009;38(6):411–419. doi: 10.1016/j.semarthrit.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 88.Newman D.J. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000;17:215–234. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]