Sir,
We read with interest that antibody testing using a rapid immunochromatographic assay is reliable in the diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.1 However, the accuracy of antibody testing and RT-PCR does not meet the need for a large number of screening tests. False negative RT-PCR and false positive antibody tests are a concern.
Coronavirus disease 2019 (COVID-19), which is caused by SARS-Cov-2, was first detected at the end of 2019, and was named by the World Health Organization on January 12, 2020. COVID-19 is now a pandemic. It took only 25 days for newly confirmed cases to decrease to zero in Beijing in June, which revealed that timely discovery, accurate diagnosis, early isolation and treatment of COVID-19 are the most effective measures.
Suspected cases were judged by epidemiological history and clinical manifestations. Confirmed cases were diagnosed by real-time fluorescent reverse transcription-polymerase chain reaction (RT-PCR) which identified the new coronavirus nucleic acid and serum IgM/IgG antibody tests. Infected persons with false negative RT-PCR results may not be isolated and can infect others.2 False positive results can cause panic among patients and doctors. Furthermore, unnecessary crowd isolation wastes human and material resources.
Different types of clinical specimens and thermal inactivation may cause false negative RT-PCR results. Nucleic acid detection has the limitation of a low positive rate in different types of clinical specimens. Wenling Wang's research revealed that bronchoalveolar lavage fluid specimens showed the highest positive rates (14 of 15; 93%), followed by sputum (72 of 104; 72%), nasal swabs (5 of 8; 63%), fibrobronchoscope brush biopsy (6 of 13; 46%), pharyngeal swabs (126 of 398; 32%), feces (44 of 153; 29%), and blood (3 of 307; 1%).3 We recommend that upper respiratory tract specimens be collected in the acute phase and lower respiratory tract specimens or feces samples be collected in the non-acute phase.
Based on the knowledge of SARS-CoV and Middle East respiratory syndrome (MERS)-CoV, thermal inactivation at 56 °C was recommended to inactivate SARS-CoV-2 before nucleic acid testing. However, Pan Y's research demonstrated that thermal inactivation affected the efficiency of RT-PCR for SARS-CoV-2 detection and chemical inactivators, such as guanidinium-based lysis, are suggested. His study showed that approximately half of the weakly positive samples (7 of 15 samples, 46.7%) were RT-PCR negative after heat inactivation in at least one parallel test.4
At the initial stage of the SARS-CoV-2 epidemic in February 2020, we observed 57 suspected COVID-19 infected patients. Pharyngeal swabs were tested for nucleic acid and serum samples were obtained for 2019-nCOV IgM and IgG tests. The positive rate of COVID-19 nucleic acid was 42.10%. The positive detection rate of combined 2019-nCOV IgM and IgG for patients with COVID-19 negative and positive nucleic acid tests was 72.73% and 87.50%, respectively.5 These data demonstrated that the detection of novel coronavirus antibodies is an important supplementary method for the diagnosis of COVID-19. In China's trial version 7 of the diagnosis and treatment guideline for the novel coronavirus disease (COVID-19), serum novel coronavirus specific IgM antibody and IgG antibody were regarded as criteria for confirming COVID-19 in patients on March 3, 2020.
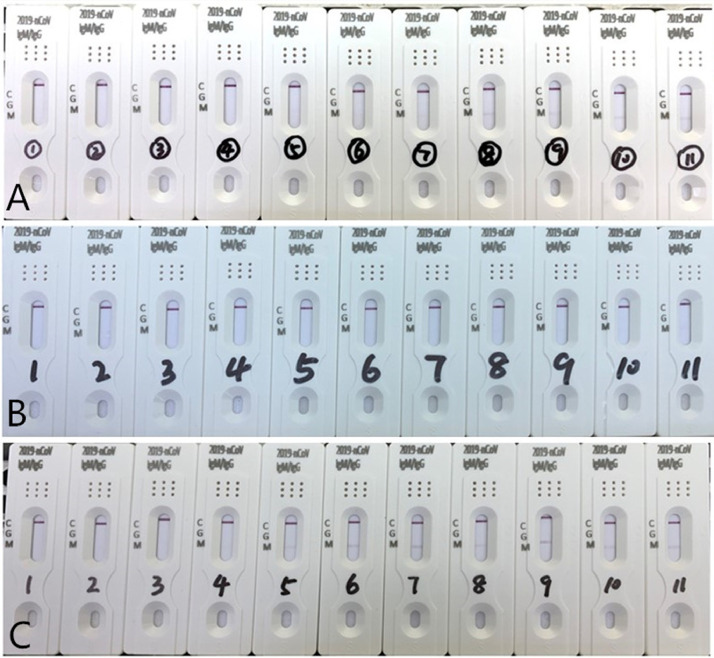
A study involving 1779 patients in Iceland, showed that 91.1% of those who recovered from SARS-COV-2 infection tested seropositive.6 It was found that the antibody test may be affected by other factors such as rheumatoid factor (RF) in the serum.7 Rheumatoid patients often have different concentrations of RF, and RF has five types of immunoglobulins including IgM, IgG, IgD, IgA and IgE. A high concentration of RF-IgM may interfere with novel coronavirus IgM/IgG antibody detection. The effects of RF concentration from 16.1 IU/mL to 539.7 IU/mL on the detection of 2019-nCoV IgM and IgG tests were observed. It was found that RF may lead to false results when the serum RF level was higher than 231.7 IU/mL. The false positive antibody results could be eliminated after five times dilution with normal human serum, when the RF level was lower than 10 IU/mL. It was not eliminated after five times dilution with physiological saline[Fig. 1 ]. We also identified five patients with false antibody results, who had nasopharyngeal carcinoma, colon cancer, duodenal carcinoma, diabetes, and diffuse bronchitis, respectively. Serum RF level in these patients was lower than 100 IU/mL. The false positive antibody results could also be eliminated after 5 times dilution with normal human serum. Thus, further studies are needed to investigate the false results of this test.
Fig. 1.
Influence and elimination experiment resulting in a false positive test result, which was affected by serum rheumatoid factor levels.
Eleven different serum concentrations of rheumatoid factor were tested for 2019-nCoV IgM and IgG. From left to right, RF was 16.1, 85.0, 133.0, 187.5, 231.7, 284.3, 331.0, 390.5, 450.0, 490.6, and 539.7 (IU/mL). A is the original result, B and C are the results diluted five times with normal human serum and physiological saline, respectively.
We believe that no diagnostic technique has 100% sensitivity and specificity. Although the RT-PCR test has become the standard method for the diagnosis of SARS-CoV-2 infection, false-negative rates have been reported. For the serological antibody test, the detection time needs to consider the window period. Moreover, several factors should be considered when diagnosing COVID-19, including epidemiology, history of exposure and clinical symptoms, such as fever or respiratory disease.
Therefore, the combination of serum IgM/IgG antibody detection, the nucleic acid test, CT scan and clinical features improves the accuracy of COVID-19 diagnosis.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
Ethical approval
This study was approved by the Ethics Committee of Shenzhen Hospital, Southern Medical University (NYSZYYEC20200009).
Acknowledgments
We thank Lijun Zhang and Qing Liu of the Department of Clinical Laboratory Medicine Center, Shenzhen Hospital, Southern Medical University who collected the serum samples.
References
- 1.Spicuzza L., Montineri A., Manuele R. Reliability and usefulness of a rapid IgM-IgG antibody test for the diagnosis of SARS-CoV-2 infection: a preliminary report [published online ahead of print, 2020 Apr 23] J Infect. 2020 doi: 10.1016/j.jinf.2020.04.022. S0163-4453(20)30231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection–challenges and implications [published online ahead of print, 2020 Jun 5] N Engl J Med. 2020 doi: 10.1056/NEJMp2015897. 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 3.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens [published online ahead of print, 2020 Mar 11] JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan Y., Long L., Zhang D. Potential false-negative nucleic acid testing results for severe acute respiratory syndrome coronavirus 2 from thermal inactivation of samples with low viral loads. Clin Chem. 2020;66(6):794–801. doi: 10.1093/clinchem/hvaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.X. Jia, P. Zhang, Y. Tian, et al. Clinical significance of IgM and IgG test for diagnosis of highly suspected COVID-19 infection. medRxiv 2020.02.28.20029025; doi: https://doi.org/ 10.1101/2020.02.28.20029025.
- 6.Gudbjartsson D.F., Norddahl G.L., Melsted P. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020 Sep 1 doi: 10.1056/NEJMoa2026116. Epub ahead of print. PMID: 32871063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Sun S., Shen H. Cross-reaction of SARS-CoV antigen with autoantibodies in autoimmune diseases. Cell Mol Immunol. Aug 2004;1(4):304–307. PMID: 16225774. [PubMed] [Google Scholar]