Highlights
-
•
SPMs are promising substances and suggest a clinical trial be initiated to test their ability to activate resolution of lung inflammation.
-
•
SPMs could reduce tissue damage in COVID 19 patients to influence the cytokine storm.
-
•
The focus could be in the adjuvant management or for the improvement and resolution of chronic lung and heart inflammation in the post-acute phase of this disease.
Keywords: SARS Covid 19, Specialized pro‑resolving mediators, Inflammation, Resolution
Abstract
COVID-19 is a new disease caused by coronavirus SARS-CoV-2. It was first described in 2019, developed into an epidemic in January 2020 and has spread the global to the present COVID-19 pandemic. Specialized pro‑resolving mediators (SPMs) may play a new role in the management of this lung disease because SPM actively stimulate the resolution of infectious inflammation and are organ protective in animal disease models. Many tissues have been suitable targets for treating inflammation with SPMs or their active precursors 18-HEPE, 17-HDHA and the 14-HDHA, in order to elicit dynamic resolution of inflammation.
Here we discuss the possible mode of action of these substances in the management of SARS Covid 19.
The role of inflammation
In many chronic diseases, including vascular and neurological disorders, as well as metabolic syndrome, excessive inflammatory processes are manifested, thus representing a public health concern. If the endogenous control points within the inflammatory pathways were understood completely, the pathogenesis of the diseases might become more explicit, and new approaches for treatment might be found.
When a host experiences a trauma, barrier breakage, or microbial invasion, potential invaders must be eliminated, the location must be cleared, and affected tissue must be remodelled and regenerated. For the acute inflammatory response, several lipid mediators are crucial. They include eicosanoids (prostaglandins and leukotrienes), which derive from arachidonic acid, an essential fatty acid [1], [2], and different proteins like cytokines and chemokines [3], [4], [5]. These molecules interact with each other, thereby further intensifying the inflammatory process that may, in turn, be counteracted with pharmacological inhibitors and receptor antagonists. Since inflammatory processes are involved in many prevalent diseases, it is necessary to broaden the knowledge of all mechanisms involved in order to improve the therapeutic options.
Historically, the inflammatory response used to be separated into an active initiation and a passive resolution process [6]. Recently, however, mediators were identified which have pro-resolving capacities and can be synthesized from omega-3 (n-3) essential fatty acids (EFA). Studies have shown that the resolution process can be “switched on” in animal models and may thus rather be an active response in the self-limitation of acute inflammation than a passive dilution of chemo-attractants [7], [8].
Molecules, which are supposed to act as mediators, must be supplied in enough amounts in order to lead to reactions in vivo. For EPA and DHA, anti-inflammatory properties have been proposed for many years. These omega-3 fatty acids compete with arachidonic acid in reducing pro-inflammatory eicosanoids [9]. However, the underlying molecular mechanisms had remained obscure until recent results emerged, and whether EPA or DHA is more relevant for human health or therapeutic options is still under debate [9].
It has been shown for resolving inflammatory exudates that omega-3 fatty acids serve as substrates for the synthesis of specific signalling molecules – the so-called specialized pro-resolving mediators (SPMs), which comprise resolvins, protectins, lipoxins and maresins [10]. These findings triggered new studies concerning the resolution pathways and the immune mechanisms underlying homeostasis. It was shown in animal models that SPMs promote critical paths of the inflammatory resolution, as they limit the infiltration of polymorphonuclear neutrophils and the elimination of apoptotic cells by macrophages [11].
Active resolution of inflammation
Inflammations may be resolved entirely or become a chronic state. Formerly, resolution of active inflammation has been considered a passive event, upon which inflammatory mediators such as prostaglandins or cytokines were merely diluted, thus disappearing from the site of inflammation. This would finally lead to prevent leukocyte infiltration into the tissue. However, Serhan et al. provided new evidence to revise this theory by demonstrating the existence of an active resolution process mediated by so-called selective pro-resolving mediators (SPMs) in several studies. The SPM molecular superfamily contains subgroups named resolvins (Rvs), protectins, maresins, and lipoxins. The biosynthesis of the SPMs (with lipoxygenases and cyclooxygenases intervening both on the pathways of eicosanoids and SPMs), as well as the corresponding cell membrane receptors, have been described. SPMs are crucial for enough resolution of inflammatory processes, and based on these new findings, Serhan et al. described in detail novel pathways. They include the action of the SPMs, as well as crucial endogenous control mechanisms. [12].
Importantly, within this new perception of inflammatory processes, the resolution is an active mechanism, which does not start with a delay, but at experimental timepoint Zero.
Alfa signals Omega throughout the course of inflammation, mainly SPMs were found to repress inflammatory signals by ending tissue infiltration of neutrophils and preventing further recruitment of immune cells to the site of inflammation. Subsequently, phagocytic macrophages are stimulated, which further leads to increased clearance and elimination of apoptotic polymorphonuclear neutrophils (PMNs) by efferocytosis and phagocytosis [4].
SPMs are synthesized from eicosapentaenoic acid (EPA, C20:5n-3) and docosahexaenoic acid (DHA, C22:6–3). Both are omega-three polyunsaturated fatty acids (PUFAs) and serve as precursors in the biochemical pathways leading to SPMs via the metabolites 18-HEPE, 17-HDHA, or 14-HDHA [13].
Since the development of this new concept of inflammation, many tissues have been suitable targets for treating inflammation with SPMs or their active precursors 18-HEPE, 17-HDHA and the 14-HDHA, in order to elicit dynamic resolution of inflammation. In contrast to traditionally applied anti-inflammatory therapies, they do not act as immunosuppressors, and debris is cleared, thus being potentially useful for the treatment of chronic inflammation. Substances, which are applied nowadays, have distinct disadvantages: steroids may interfere with wound healing, can promote osteoporosis, and is immunosuppressive. NSAIDs may lead to stomach bleeding, are potentially toxic for the cardiovascular system and the kidneys and interfere with wound healing. Cyclooxygenase (COX)2 inhibitors constitute a risk factor for cardiovascular and thromboembolic events. Anti-TNF therapies for blocking cytokines lead to increased rates of infections and enhance the risk of lymphoma development.
SPMS, on the other hand, was shown to increase the killing of microbial invaders and their clearance by immunocytes. It was demonstrated that they down-regulate infiltration and recruitment of PMN, enhance phagocytosis, and efferocytosis (M1 to M2). Application of SPMs also decreased the level of pro-inflammatory chemical mediators, while increasing the number of anti-inflammatory mediators like IL-10, for example. Finally, they can reduce inflammatory pain, stimulate the regeneration of inflamed tissue, and promote wound healing [12].
COVID-19 is a new disease caused by coronavirus SARS-CoV-2. It was first described in 2019, developed into an epidemic in January 2020 and has spread the global to the present COVID-19 pandemic. It spreads mainly through droplet infection. On surfaces, virus particles remain infectious for hours to days, so that they can reach the mucous membranes of the mouth and nose from keyboards, tables, door handles and handles via the hands(lubricating infection). Infection via the conjunctiva of the eye is also possible [14].
The disease histories are non-specific and vary greatly. In addition to symptomless infections, mainly mild to moderate histories were observed, but also severe ones with pneumonia on both sides, including lung failure, multiorgan failure and death. Even as easily described disease histories can lead to long-term damage cannot be ruled out. Thus far, there are no specific therapeutic agents for coronavirus infections [15].
The global mortality rate of COVID-19 is 3.4% [16], whereas its mortality rate in Wuhan is 4.3% [17], as the proportion of severe cases in Wuhan is relatively high. A study has demonstrated that patient with hypertension and/or diabetes had a higher risk of contracting COVID-19 [18]. So far, there are no clinically proven effective antiviral drugs for COVID-19. 80% patients were moderate, without acute respiratory distress syndrome (ARDS) [18]. Without effective treatment, moderate patients could convert into severe patients and develop ARDS and multi-organ failure. Because most, if not all, death cases arise from severe patients, it is of great significance to carry out effective antiviral treatment in the 80% moderate patients with COVID-19, which can reduce the risk for moderate patients converting into severe cases.
Now, there is no specific treatment for COVID-19, so healthcare providers treat the clinical symptoms (e.g. fever, difficulty breathing) of patients. Supportive care (e.g. fluid management, oxygen therapy, etc.) can be highly effective for patients with symptoms. Specifically, there are currently no antiviral drugs recommended or licensed by WHO or any government regulatory department for COVID-19. Clinical studies of some drugs (human interferon alpha-2b, ribavirin, chloroquine phosphate, lopinavir and arbidol) were currently undergoing to test the efficacy and safety of these drugs in the treatment of COVID-19 [18].
SARS-CoV-2 and influenza viruses have a similar disease presentation. Both clinical manifestations are dominated by respiratory symptoms, which present as a wide range of illness from asymptomatic or mild through to severe disease and death. Importantly, both viruses are RNA virus and depends on RNA-dependent RNA polymerase (RdRp) to replicate. Although no antiviral drugs were proven effective for COVID-19, clinically assessible antiviral options exist for influenza.
Some pathogens, such as the influenza virus and the Gram-negative bacterium Francisella tularensis, do trigger life-threatening “cytokine storms” in the host which can result in significant pathology and ultimately death. For these diseases, it has been proposed that downregulating inflammatory immune responses may improve outcome [19].
As described previously, due to the lack of specific treatment, therapy has so far mainly been limited to supportive care [20], [21], [22]. Although acute respiratory distress syndrome (ARDS) is the central feature of disease severity, non-pulmonary organ damage has emerged as an important predictor of mortality [18].
Patients hospitalized for COVID-19 are at high risk for kidney failure and myocardial involvement is common in severe cases, both correlating with poor outcome [25,26,27,]. The underlying mechanisms of COVID-19 pulmonary and non-pulmonary tissue injury are insufficiently understood [23].
Preliminary data point to a prothrombotic state in COVID-19 patients [24], [25], with a possible beneficial effect of heparin. However, a thorough analysis of cellular and plasmatic coagulation of affected patients is missing [26], [27], [28]. It is unclear whether and how the inflammatory response to SARS-CoV-2 and its associated coagulopathy are intertwined.
Specialized pro‑resolving mediators (SPMs) may play a new role in the management of this lung disease because SPM actively stimulate the resolution of infectious inflammation and are organ protective in animal disease models [29]. SPM are produced by cells of the innate immune, which are formed via the stereoselective enzymatic conversion of essential fatty acids that include arachidonic acid, eicosapentaenoic acid, n‑3 docosapentaenoic acid and docosahexaenoic acid (DHA) [6]. SPMs are grouped into four families, lipoxins, resolvins, protectins, and maresins [10], [13]. These endogenous mediators share basic physiologic properties in regulating host responses to actively enhancing resolution of inflammatory response mechanisms, such as reducing the hosts’ production of proinflammatory cytokines and chemokines, limit the neutrophils trafficking, stimulating the macrophages phagocytosis of apoptotic cells, bacterial killing, and cellular debris via G‑protein coupled receptors (GPCRs) [10], [29].
Recent results [32] indicate that SPMs regulate the AFC in ARDS to protect lung function. Damage to the lung results in activation of the immune system, which not only leads to the release of several proinflammatory proteins and neutrophilic influx into the alveolar space but also leads to the local biosynthesis of pro‑resolution lipids mediators, such as lipoxins, resolvins, protectins, and maresins [30]. Along these lines, Cilloniz et al. [31] used a mouse model to investigate influenza A virus virulence, comparing host transcriptional responses to infection with reconstructed 1918 H1N1 virus to avian H5N1 virus (Vietnam/1203). They found that extra-pulmonary dissemination was associated with down-regulation of genes involved in mediating the pro-resolution impact of lipoxin on leucocyte recruitment and counter-regulation of pro-inflammatory cytokine induction and that loss of lipoxin’s pro-resolution actions may be associated with greater influenza A virus virulence. These findings suggest a protective role for SPM in this infection, possibly related to the reduction and counter-regulation of pro-inflammatory cytokines that are up-regulated during viral infections.
If the beneficial actions of these mediators translate from pre-clinical studies into human clinical trials, they represent promising new strategies in the management of infectious disease. The pro-resolution, anti-inflammatory and antimicrobial-enhancing actions of SPM such as the resolvins, protectin and potentially maresins make these appealing candidates for further study in humans and specifically in COVID19 patients. From a therapeutic perspective it is important to note that these pro-resolution mediators have a substantial advantage over steroids for use in the treatment of infectious inflammation, or other systemic inflammatory states, as they are not immunosuppressive agents. Acetylsalicylic acid-triggered lipoxins and resolvins epimers share these pro-resolution actions and act by the same intracellular pathways [32], [33]. This effect is unique to aspirin, and is not shared with non-steroidal anti-inflammatory drugs, which do not trigger the endogenous biosynthesis of these mediators.
Morita et al. [34] reported that the SPM, protectin D1 (aka neuroprotectin D1) markedly attenuated influenza virus replication via RNA export machinery. Production of this SPM was reduced during severe influenza and PD1 inversely correlated with the pathogenicity of H5N1 viruses. Importantly, treatment with the SPM improved both survival and pathology of severe influenza in mice, even under conditions where known antiviral drugs fail to protect from death.
Another new and important factor is that the infection with SARS-CoV2 may lead to significant inflammatory responses as measured by an increase in IL-6, C-reactive protein, fibrinogen and erythrocyte sedimentation rate [35]. Since the virus primarily attaches to ACE2 receptors, activation and damage of affected endothelial cells may lead to the above-described pro-thrombotic changes. Accordingly, enhanced plasma concentrations of proinflammatory cytokines have been found in Covid-19 patients with need for intensive care compared to non-ICU patients in early reports of COVID-19 patients [15]. These inflammatory conditions may lead to subsequent activation of the coagulation response as measured by increased levels of D-Dimers, a characteristic parameter for pro-coagulatory conditions. Elevated concentrations of D-Dimers have been linked to increased mortality of Covid-19 patients and both septic patients as well as those developing DIC conditions are at a higher risk for a fatal course of the disease. Although the mechanisms leading to coagulation during SARS-CoV-2 infection have not been elucidated yet, they rather seem to relate to the inflammatory response of the host instead of distinct viral pathogenic factors and in contrast to RNA-stranded viruses associated with haemorrhagic fever like Ebola, infections with SARS-CoV-2 do not result in excessive bleeding [15]. Data from Wuhan support the view that Covid-19- related coagulopathy rather results from the inflammatory host response that leads to exaggerated thrombotic processes through the above-described thrombo-inflammatory interrelations of different host signalling pathways.
Recent data of Nicolai et al. [36] present Covid-19 as a disease with a dysregulated immunothrombosis driven by activated neutrophils and immunogenic platelets that contribute to organ injury and a systemic thrombogenic state in COVID-19.
In addition, they found that plasmatic coagulation is skewed towards a procoagulant state correlating with disease severity, reflected by peripheral blood coagulation tests as well as histopathological evidence of microvascular thrombosis in affected organs. They stated that platelets, neutrophils, and the coagulation cascade are drivers of disease severity and might prove to be valuable pharmacological targets in Covid-19. In addition, SARS-CoV-2 infected patients are at risk for increased thrombotic events, making prophylactic anticoagulation and vigilant monitoring for thrombotic complications a central task in management of COVID-19 patients.
Cherpocova et al. could show that the administration of resolvin D4 (RvD4), an SPM that was enriched at the natural onset of thrombus resolution, significantly reduced thrombus burden, with significantly less neutrophil infiltration and more proresolving monocytes in the thrombus, as well as an increased number of cells in an early apoptosis state. Moreover, RvD4 promoted the biosynthesis of other D-series resolvins involved in facilitating resolution of inflammation, and they suggest that delivery of SPMs, specifically RvD4, modulates the severity of thromboinflammatory disease in vivo and improves thrombus resolution [37].
Serhan et al. also could show under a long-term treatment the safety of the SPMs. This trial was intended to improve de-clotting in coronary arterial disease and no severe adverse events were reported. Therefore, SPMs can be considered as highly safe [38].
Together these results with SPM in animal disease models are promising and suggest a clinical trial be initiated to test their ability to activate resolution of lung inflammation and reduce tissue damage in COVID 19 patients to stop the cytokine storm; namely, the adjuvant management of the Covid-19 disease or the in the management of the cured humans for the improvement and resolution of chronic lung and heart inflammation) in the post-acute phase of this disease (see Table 1 ). We should also consider means to increase endogenous production of SPM in these patients and test their association with outcomes of the disease? This pandemic brings urgent needs and suggest that we test whether activation of endogenous pro-resolving mechanisms in COVID 19 patients can expedite their recovery.
Table 1.
Viral infection cascade and immune response.
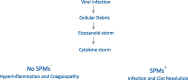 |
Funding
Not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Flower R.J. Prostaglandins, bioassay, and inflammation. Br J Pharmacol. 2006;147:82–192. doi: 10.1038/sj.bjp.0706506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuelsson B. Role of basic science in the development of new medicines: examples from the eicosanoid field. J Biol Chem. 2012;287(13):10070–10080. doi: 10.1074/jbc.X112.351437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinarello C.A., Simon A., van der Meer J.W.M. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan C.N., Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 5.Maderna P., Godson C. Lipoxins: resolutionary road. Br J Pharmacol. 2009;158:947–959. doi: 10.1111/j.1476-5381.2009.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabas I., Glass C.K. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339(6116):166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan C.N., Clish C.B., Brannon J., Colgan S.P., Chiang N., Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan C.N., Hong S., Gronert K., Colgan S.P., Devchand P.R., Mirick G., Moussignac R.-L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lands WE M. Fish, Omega-3 and Human Health 2nd edn (AOCS Press, 2005) p 34-45.
- 10.Serhan C.N., Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr Opin Pharmacol. 2013;13(4):632–640. doi: 10.1016/j.coph.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serhan C.N., Levy B.D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128(7):2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan C.N. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017;31(4):1273–1288. doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y.C., Chen C.S., Chan Y.J. Overview of the 2019 novel coronavirus (2019-nCoV): the pathogen of severe specific contagious pneumonia (SSCP) J Chin Med Assoc. 2020;11:217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [Google Scholar]
- 15.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y.i., Zhang L.i., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L.i., Xie J., Wang G., Jiang R., Gao Z., Jin Q.i., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Max Roser, Hannah Ritchie and Esteban Ortiz-Ospina. Coronavirus Disease (COVID-19)-Statistics and Research. https://ourworldindata.org/coronavirus.
- 17.Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel. Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020; published online Feb 07. DOI: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed]
- 18.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y.i., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Elia R.V., Harrison K., Oyston P.C., Lukaszewski R.A., Clark G.C. Targeting the “Cytokine storm” for therapeutic benefit. Clin Vaccine Immunol. 2013;20(3):319–327. doi: 10.1128/CVI.00636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2019;2020:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 21.Cao Bin, Wang Yeming, Wen Danning, Liu Wen, Wang Jingli, Fan Guohui, Ruan Lianguo, Song Bin, Cai Yanping, Wei Ming, Li Xingwang, Xia Jiaan, Chen Nanshan, Xiang Jie, Yu Ting, Bai Tao, Xie Xuelei, Zhang Li, Li Caihong, Yuan Ye, Chen Hua, Li Huadong, Huang Hanping, Tu Shengjing, Gong Fengyun, Liu Ying, Wei Yuan, Dong Chongya, Zhou Fei, Gu Xiaoying, Xu Jiuyang, Liu Zhibo, Zhang Yi, Li Hui, Shang Lianhan, Wang Ke, Li Kunxia, Zhou Xia, Dong Xuan, Qu Zhaohui, Lu Sixia, Hu Xujuan, Ruan Shunan, Luo Shanshan, Wu Jing, Peng Lu, Cheng Fang, Pan Lihong, Zou Jun, Jia Chunmin, Wang Juan, Liu Xia, Wang Shuzhen, Wu Xudong, Ge Qin, He Jing, Zhan Haiyan, Qiu Fang, Guo Li, Huang Chaolin, Jaki Thomas, Hayden Frederick G., Horby Peter W., Zhang Dingyu, Wang Chen. A Trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan Wei-jie, Ni Zheng-yi, Hu Yu, Liang Wen-hua, Ou Chun-quan, He Jian-xing, Liu Lei, Shan Hong, Lei Chun-liang, Hui David S.C., Du Bin, Li Lan-juan, Zeng Guang, Yuen Kwok-Yung, Chen Ru-chong, Tang Chun-li, Wang Tao, Chen Ping-yan, Xiang Jie, Li Shi-yue, Wang Jin-lin, Liang Zi-jing, Peng Yi-xiang, Wei Li, Liu Yong, Hu Ya-hua, Peng Peng, Wang Jian-ming, Liu Ji-yang, Chen Zhong, Li Gang, Zheng Zhi-jian, Qiu Shao-qin, Luo Jie, Ye Chang-jiang, Zhu Shao-yong, Zhong Nan-shan. Clinical Characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Zhe, Shi Lei, Wang Yijin, Zhang Jiyuan, Huang Lei, Zhang Chao, Liu Shuhong, Zhao Peng, Liu Hongxia, Zhu Li, Tai Yanhong, Bai Changqing, Gao Tingting, Song Jinwen, Xia Peng, Dong Jinghui, Zhao Jingmin, Wang Fu-Sheng. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respirat Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Tao, Chen Ruchong, Liu Chunli, Liang Wenhua, Guan Weijie, Tang Ruidi, Tang Chunli, Zhang Nuofu, Zhong Nanshan, Li Shiyue. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang Ning, Bai Huan, Chen Xing, Gong Jiale, Li Dengju, Sun Ziyong. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han H, Yang L, Liu R, Liu F, Wu KL, Li J, Liu XH, Zhu CL. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clinical chemistry and laboratory medicine. 2020:1116-1120. DOI: 10.1515/cclm-2020-0188. [DOI] [PubMed]
- 27.Cui Songping, Chen Shuo, Li Xiunan, Liu Shi, Wang Feng. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serhan C.N., Dalli J., Colas R.A., Winkler J.W., Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta. 2015;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Qian, Zheng Xia, Cheng Yang, Zhang Yi-Lan, Wen Hai-Xu, Tao Zhen, Li Hui, Hao Yu, Gao Ye, Yang Liang-Min, Smith Fang Gao, Huang Chang-Jiang, Jin Sheng-Wei. Resolvin D1 Stimulates Alveolar Fluid Clearance through Alveolar Epithelial Sodium Channel, Na,K-ATPase via ALX/cAMP/PI3K Pathway in Lipopolysaccharide-Induced Acute Lung Injury. JI. 2014;192(8):3765–3777. doi: 10.4049/jimmunol.1302421. [DOI] [PubMed] [Google Scholar]
- 30.Matthay Michael A., Ware Lorraine B., Zimmerman Guy A. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cilloniz Cristian, Pantin-Jackwood Mary J., Ni Chester, Goodman Alan G., Peng Xinxia, Proll Sean C., Carter Victoria S., Rosenzweig Elizabeth R., Szretter Kristy J., Katz Jacqueline M., Korth Marcus J., Swayne David E., Tumpey Terrence M., Katze Michael G. Lethal dissemination of H5N1 influenza virus is associated with dysregulation of inflammation and lipoxin signaling in a mouse model of infection. JVI. 2010;84(15):7613–7624. doi: 10.1128/JVI.00553-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sekheria M, El Kebirb, Ednerb N, Filepa JG. D15-Epi-LXA4 and 17-epi-RvD1 restore LR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation. Proc Natl Acad Sci U S A 2020; Mar 23[Online ahead of print]. DOI: 10.1073/pnas.1920193117. [DOI] [PMC free article] [PubMed]
- 33.Hachicha M., Pouliot M., Petasis N.A., Serhan C.N. Lipoxin (LX) A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1a-initiated neutrophil responses and trafficking: regulators of a cytokine–chemokine axis. J Exp Med. 1999;189:1923–1930. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morita Masayuki, Kuba Keiji, Ichikawa Akihiko, Nakayama Mizuho, Katahira Jun, Iwamoto Ryo, Watanebe Tokiko, Sakabe Saori, Daidoji Tomo, Nakamura Shota, Kadowaki Ayumi, Ohto Takayo, Nakanishi Hiroki, Taguchi Ryo, Nakaya Takaaki, Murakami Makoto, Yoneda Yoshihiro, Arai Hiroyuki, Kawaoka Yoshihiro, Penninger Josef M., Arita Makoto, Imai Yumiko. The Lipid Mediator Protectin D1 Inhibits Influenza Virus Replication and Improves Severe Influenza. Cell. 2013;153(1):112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019 [published online ahead of print 27 March 2020]. J Clin Invest. doi:10.1172/JCI137244. DOI: 10.1172/JCI137244.
- 36.Nicolai Leo, Leunig Alexander, Brambs Sophia, Kaiser Rainer, Weinberger Tobias, Weigand Michael, Muenchhoff Maximilian, Hellmuth Johannes C., Ledderose Stephan, Schulz Heiko, Scherer Clemens, Rudelius Martina, Zoller Michael, Höchter Dominik, Keppler Oliver, Teupser Daniel, Zwißler Bernhard, von Bergwelt-Baildon Michael, Kääb Stefan, Massberg Steffen, Pekayvaz Kami, Stark Konstantin. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation. 2020;142(12):1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cherpokova D, Jouvene CC, Libreros S, DeRoo EP, Chu L, de la Rosa X, Norris PC, Wagner DD, Serhan CN. Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood 2019 | VOLUME 00, DOI: 10.1182/blood.2018886317. [DOI] [PMC free article] [PubMed]
- 38.Elajami T.K., Colas R.A., Dalli J., Chiang N., Serhan C.N., Welty F.K. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodelling. FASEB J. 2016;30:2792–2801. doi: 10.1096/fj.201500155R. [DOI] [PMC free article] [PubMed] [Google Scholar]


