Abstract
Objective
Fear associated with medical vulnerability should be considered when assessing mental health among individuals with chronic medical conditions during the COVID-19 pandemic. The objective was to develop and validate the COVID-19 Fears Questionnaire for Chronic Medical Conditions.
Methods
Fifteen initial items were generated based on suggestions from 121 people with the chronic autoimmune disease systemic sclerosis (SSc; scleroderma). Patients in a COVID-19 SSc cohort completed items between April 9 and 27, 2020. Exploratory factor analysis (EFA) and item analysis were used to select items for inclusion. Cronbach's alpha and Pearson correlations were used to evaluate internal consistency reliability and convergent validity. Factor structure was confirmed with confirmatory factor analysis (CFA) in follow-up data collection two weeks later.
Results
787 participants completed baseline measures; 563 of them completed the follow-up assessment. Ten of 15 initial items were included in the final questionnaire. EFA suggested that a single dimension explained the data reasonably well. There were no indications of floor or ceiling effects. Cronbach's alpha was 0.91. Correlations between the COVID-19 Fears Questionnaire and measures of anxiety (r = 0.53), depressive symptoms (r = 0.44), and perceived stress (r = 0.50) supported construct validity. CFA supported the single-factor structure (χ2(35) = 311.2, p < 0.001, Tucker-Lewis Index = 0.97, Comparative Fit Index = 0.96, Root Mean Square Error of Approximation = 0.12).
Conclusion
The COVID-19 Fears Questionnaire for Chronic Medical Conditions can be used to assess fear among people at risk due to pre-existing medical conditions during the COVID-19 pandemic.
Keywords: Chronic medical condition, Coronavirus, COVID-19, Fear, Mental health, Scleroderma, Systemic sclerosis, Validation
Highlights
-
•
People with chronic diseases are at risk if infected with the COVID-19 virus.
-
•
The COVID-19 Fears Questionnaire assesses fear among people with medical conditions.
-
•
121 people with scleroderma from 10 countries provided item suggestions.
-
•
Validity was supported in a sample of 787 scleroderma patients.
-
•
The questionnaire is available for use in studies during the COVID-19 crisis.
1. Introduction
The COVID-19 outbreak has transformed the lives of people around the world through its rapid spread, number of deaths, social disruption, and devastating economic impact [1]. Fear of oneself or close relatives becoming infected is common among people exposed to any infectious disease outbreak [2]. During COVID-19, there may also be widespread fear that health care systems will not have adequate capacity and that appropriate medical care will not be available if one becomes infected, that isolation will be long-lasting with a heavy toll on mental health and social functioning, and that individual and public economic resources will not be sufficient or will not recover post-pandemic [[3], [4], [5]].
People with chronic diseases, particularly respiratory diseases, are at risk of severe complications from COVID-19 and may be more likely to experience negative mental health outcomes [2]. A Fear of COVID-19 Scale was developed for measuring fear of COVID-19 in the general population [5] and was translated into several languages and national contexts [[6], [7], [8], [9], [10], [11]]. No scales, however, have been developed and validated to assess the specific fears of vulnerable individuals due to pre-existing medical illnesses.
We solicited a list of fears during the COVID-19 outbreak from people living with the rare, chronic, autoimmune disease systemic sclerosis (SSc; scleroderma). People with SSc are representative of other groups of patients who are vulnerable due to a pre-existing medical condition; they are at risk of severe complications if infected due to lung involvement [12,13], general frailty [12], and the use of immunosuppressant drugs [14]. We used suggestions from 121 people with SSc and content analysis to develop a preliminary 15-item version of a fear measure for people with chronic medical conditions [15].
The objectives of the present study were to (1) evaluate items for inclusion in the final COVID-19 Fears Questionnaire for Chronic Medical Conditions; (2) evaluate the factor structure, internal consistency reliability, and convergent validity of the questionnaire; and (3) verify the factor structure and other validity indictors in follow-up data.
2. Methods
This was a cross-sectional study that analyzed, separately, two waves of data from participants enrolled in the Scleroderma Patient-centered Intervention Network (SPIN) COVID-19 Cohort [15,16]. We used baseline data (Wave 1) for initial validation and item selection, and data from Wave 2 (two weeks later) for verification. The SPIN COVID-19 Cohort study was approved by the Research Ethics Committee of the CIUSSS du Centre-Ouest-de-l'Île-de-Montréal.
2.1. Participants and procedures
The SPIN COVID-19 Cohort was open for enrolment between April 9, 2020 and April 27, 2020 [15]. Participants were recruited from the ongoing SPIN Cohort [17] and additionally via social media and patient organization advertisements. The SPIN Cohort includes over 1800 participants from 47 centres in Canada, the United States, the United Kingdom, France, Spain, Mexico, and Australia who complete regular 3-month online assessments. Eligible SPIN Cohort participants must be classified as having SSc based on 2013 American College of Rheumatology/European League Against Rheumatism criteria [18] by a SPIN physician; be ≥18 years old; be fluent in English, French or Spanish; and be able to respond to questionnaires online. Eligible SPIN Cohort participants are recruited at SPIN sites [19] during regular medical visits, and written informed consent is obtained. All participants consented to be contacted about additional SPIN studies. SPIN Cohort participants who complete measures in English or French were invited by email and by popups during visits to the SPIN Cohort online assessment portal to enroll in the SPIN COVID-19 Cohort. SPIN Cohort participants who consented to participate in the SPIN COVID-19 Cohort provided the email address linked with their SPIN Cohort account (to be able to link to SPIN Cohort demographic and medical data) [15].
Recruitment announcements for the SPIN COVID-19 Cohort were also posted on SPIN's Facebook page and Twitter account and distributed by patient organizations in countries with large English and French-speaking populations, including Canada, the United States, France, the United Kingdom, Australia, New Zealand, and the Philippines. Recruitment announcements directed potential participants to an online Qualtrics web page with information about the SPIN COVID-19 Cohort and instructions on how to consent to participate or decline. Potential participants who were not enrolled in the SPIN Cohort were asked to confirm that they were ≥ 18 years old, had been diagnosed with SSc by a physician, and were fluent in English or French. SPIN COVID-19 Cohort participants were invited to complete measures at baseline and every two weeks for the duration of the COVID-19 pandemic.
3. Measures
3.1. Demographic and disease characteristics
Patients provided demographic data including age, gender, education level, marital status, ethnicity, and country. For patients enrolled in the ongoing SPIN Cohort, the attending rheumatologist provided medical information, including time since diagnosis and SSc subtype (limited or diffuse). Participants enrolled in the SPIN COVID-19 Cohort who were not SPIN Cohort participants provided basic disease variables through self-report.
3.2. COVID-19 Fears Questionnaire for Chronic Medical Conditions
Prior to launching the SPIN COVID-19 Cohort, SPIN announced in English and French via its Facebook page and Twitter and by sharing with patient organization partners that it was seeking to identify fears experienced by people with scleroderma during the COVID-19 crisis [4]. The announcement was posted on March 26, 2020 and accessible for 72 h. Respondents were directed to an online Qualtrics survey that allowed them to enter between 1 and 10 fears. Respondents were instructed to, “Please list any fears you are experiencing, including things specific to scleroderma (e.g., that an infection would make my scleroderma worse) or not specific to scleroderma (e.g., that access to regular medical care will be limited or not available).” The survey was anonymous with only information on country collected. A total of 121 people provided between 1 and 10 fears. Respondents were from Canada (N = 77), the United States (N = 8), New Zealand (N = 4), Australia (N = 2), France (N = 2), the United Kingdom (N = 2), Colombia (N = 1), the Netherlands (N = 1), the Philippines (N = 1), and Turkey (N = 1); country was not provided by 22 respondents. Mean number of item suggestions was 4.0 (standard deviation = 2.3); median was 4 (25th percentile = 2; 75th percentile = 5). Original survey data can be found at https://osf.io/ka43f/ [4].
We employed content analysis to categorize fears into common themes to support item development [20]. One investigator (BDT) initially read all responses and generated a set of initial item themes. Then, that investigator and 12 members of the research team together reviewed fears listed by 10 respondents, classified them into initial item themes, and discussed a coding approach. Next, suggestions from the remaining 111 respondents were divided among research team members to complete classification. We involved 12 team members to review all responses carefully in a short time period so that the measure could be integrated into the SPIN COVID-19 Cohort. BDT reviewed all codes.
For each item theme, initial wording for an item was generated by BDT. The research team and the 9 members of the SPIN COVID-19 Patient Advisory Team then reviewed fear suggestions and proposed items. Item content and wording and whether to include an item in the questionnaire were discussed via email iteratively until all research team members and Patient Advisory Team members agreed on the final item list. Items were developed in English then translated into French using a well-accepted forward-backward translation method [21]. Only one item in the final list, related to the use of immunosuppressant drugs, was not applicable to all chronic medical conditions, generally. It was included for evaluation with the intent of potentially including as a SSc-specific addition to the measure, pending evaluation of measurement properties. See Appendix A for items included in the 15-item preliminary COVID-19 Fears Questionnaires for Chronic Medical Conditions and Appendix B for a 16-item preliminary version including the SSc-specific item.
Respondents are asked to select the response that reflects how much each statement describes their experience on a typical day in the last week on a 5-point numerical scale ranging from 1 (not at all) to 5 (extremely). The score for the scale is the total of all items, with higher scores reflecting greater fear.
3.3. Symptoms of anxiety
The 4-item PROMIS Anxiety 4a v1.0 scale [22,23] measures symptoms of anxiety in the past 7 days. Items are scored on a 5-point scale ranging from 1 (never) to 5 (always). Higher scores represent more anxiety. Total raw scores r are obtained by summing item scores for each domain. Raw scores are converted into T-scores standardized from the general US population (mean = 50, standard deviation = 10). PROMIS Anxiety 4a v1.0 has been validated in SSc in English and French [24,25].
3.4. Symptoms of depression
The 8-item Patient Health Questionnaire (PHQ-8) [26] measures depressive symptoms over the last 2 weeks on a 4-point scale, ranging from 0 (not at all) to 3 (nearly every day) with higher scores (range 0 to 24) indicating more depressive symptoms. The PHQ-8 performs equivalently to the PHQ-9 [27], which is a valid measure of depressive symptoms in patients with SSc [28]. The PHQ-8 is available in English and French [29].
3.5. Perceived stress
The 10-item Perceived Stress Scale (PSS) [30] measures the degree to which respondents appraise their life circumstances in the previous 4 weeks as unpredictable, uncontrollable, or overloaded. Items are scored on a 5-point scale from 0 (never) to 4 (very often). Total scores (range 0 to 40) are computed by summing individual item scores, and higher scores reflect greater perceived stress. The PSS has been validated in many medical and non-medical populations [31], including in France [32]. For the SPIN COVID-19 Cohort, the PSS was adapted to query about the perceived stress in the last week rather than the last 4 weeks.
4. Statistical analyses
Descriptive statistics were calculated as the mean and standard deviation (SD) for continuous variables and frequencies and percentages for categorical variables. Means, SDs, item intercorrelations, and corrected item-total correlations were calculated for each item of the COVID-19 Fears Questionnaire, and the mean and SD was calculated for the total score. Floor and ceiling effects were examined, defined as ≥15% of the participants having the lowest or highest possible score, respectively [33,34]. Cronbach's alpha was calculated to assess internal consistency.
Exploratory factor analysis (EFA) was conducted to identify the number of factors and assess item factor loadings to inform item selection [35]. EFA was done using weighted least squares mean with variance adjusted estimation, which accounts for the ordinal nature of the survey items, and with conventional standard errors, chi-square test statistic, and geomin oblique rotation [36]. Cattell's scree test on the sedimentation graph was examined. The number of factors was chosen based on the scree plot (eigenvalues), model adequacy, and overall interpretability. Model adequacy was assessed using a chi-square goodness-of-fit test and three fit indices, including the Tucker-Lewis Index (TLI) [37], the Comparative Fit Index (CFI) [38], and the Root Mean Square Error of Approximation (RMSEA) [39]. Since the chi-square test is highly sensitive to sample size and can lead to the rejection of well-fitting models, practical fit indices were emphasized [40]. Models with a TLI and CFI close to 0.95 or higher, and RMSEA close to 0.06 or lower are representative of good fitting models [41]. A CFI of 0.90 or above [42] and a RMSEA of 0.08 or more [43] may also be considered to represent reasonably acceptable model fit. Items were considered for removal if (1) the corrected item-total correlation was lower than 0.5;44,45 (2) the factor loading was lower than 0.6 [44,45]; or (3) there were high conceptual overlap/redundancy with other items [44].
To examine convergent validity, hypotheses on the direction and magnitude of Pearson's correlations with other psychological outcome measures were formulated a priori, based on existing evidence on fear during pandemic [2,3], generally, and for fear of progression, measured with a different scale, in SSc [46]. The magnitude of correlations was interpreted as small (|r| ≤ 0.3), moderate (0.3 < |r| < 0.5), or large (|r| ≥ 0.5). We expected to obtain moderate to large positive correlations of the COVID-19 Fears Questionnaire with anxiety symptoms, depressive symptoms, and perceived stress.
Confirmatory factor analysis (CFA) was performed to confirm the factor structure of the COVID-19 Fears Questionnaire using Wave 2 data. The CFA used the weighted least squares estimator with a diagonal weight matrix, robust standard errors, and a mean- and variance-adjusted chi-square statistic with delta parameterization in Mplus 7 [17]. Model adequacy was assessed using a chi-square test, TLI [37], CFI [38], and RMSEA [39].
For a one-factor CFA with 8 indicators, the minimum required sample size is estimated to be between 30 and 90, assuming factor loadings between 0.50 and 0.80 [47]. There were 10 indicators in the present study, and a model with more indicators requires even smaller sample relative to models with fewer indicators [47]. Stable estimates of correlations are typically achieved with a sample size of 250 or greater, although smaller correlations require larger samples. To assess a Pearson's correlation with 95% confidence and a precision of 0.10, a sample size of ≥403 is required for a correlation of 0.30, and ≥ 275 for a correlation of 0.50 [46]. Based on sample size requirements for CFA and correlation analyses, the available number of patients 563 from the Wave 2 dataset was more than sufficient.
EFA and CFA were conducted using Mplus 7, and all other statistical analyses were conducted using SPSS (Version 25).
5. Results
5.1. Sample characteristics
In total, 800 participants were included in the SPIN COVID-19 Cohort. Of these, 13 did not complete the COVID-19 Fear Questionnaire at baseline. All 787 patients who submitted responses for any COVID-19 Fear item completed the full scale. Demographic and disease characteristics for the baseline assessment and Wave 2 are shown in Table 1 . Most of the respondents were female (90%), White (84%), and married or living as married (69%); 248 (32%) were from the United States, 202 (26%) from France, and 194 (25%) from Canada. The mean (SD) time since diagnosis was 11.6 (8.0) years. Two weeks after the baseline assessment, 563 of 787 (72%) of participants completed the Wave 2 assessment. Characteristics of this subsample were similar to those of the baseline sample (Table 1).
Table 1.
Demographic Characteristics of the Item Analysis and Exploratory Factor Analysis Sample (N = 787) and the Confirmatory Factor Analysis Sample (N = 563)a.
| Variable | SPIN COVID-19 |
SPIN COVID-19 |
|---|---|---|
| Baseline value |
Wave 2 value |
|
| N = 787 | N = 563 | |
| Demographic | ||
| Age in years, mean (SD)b | 55.6 (12.6) | 56.7 (12.4) |
| Female gender, N (%)b | 706 (90.2) | 504 (89.5) |
| Education in years, mean (SD)c | 15.8 (3.5) | 15.8 (3.5) |
| Married or living as married, N (%)d | 537 (68.8) | 378 (67.7) |
| Race/ethnicity, N (%)e | ||
| White | 644 (82.9) | 452 (84.6) |
| Black | 53 (6.8) | 31 (5.8) |
| Other | 70 (10.3) | 51 (9.6) |
| Country, N (%)f | ||
| Canada | 194 (24.7) | 152 (27.1) |
| United States | 248 (31.6) | 167 (29.8) |
| France | 202 (25.7) | 145 (25.9) |
| United Kingdom | 68 (8.7) | 51 (9.1) |
| Australia | 43 (5.5) | 28 (5.0) |
| Italy | 1 (0.1) | 1 (0.2) |
| Other | 29 (3.7) | 16 (2.9) |
| Disease characteristics | ||
| Time since diagnosis in years, mean (SD)g | 11.6 (8.0) | 11.7 (8.1) |
| Diffuse disease subtype, N (%)h | 320 (41.5) | 231 (42.0) |
| Patient-reported outcome measures (mean ± SD (range)) | ||
| PROMIS Anxiety 4a v1.0 score | 58.5 ± 8.5 (40.3–81.6) | 56.4 ± 8.6 (40.3–81.6) |
| Patient Health Questionnaire-8 score | 7.0 ± 5.4 (0–24) | 6.3 ± 5.2 (0–24) |
| Perceived Stress Scale scorei | 15.9 ± 7.6 (0–37) | NA |
Due to missing data: bN (Baseline) = 783, N (Wave 2) = 560; cN (Baseline) = 774, N (Wave 2) = 552; dN (Baseline) = 780, N (Wave 2) = 558; eN (Baseline) = 777, N (Wave 2) = 534; fN (Baseline) = 785, N (Wave 2) = 560; gN (Baseline) = 758, N (Wave 2) = 542; hN (Baseline) = 771, N (Wave- 2) = 550; IN (Baseline) = 778. The PSS was only administered during baseline data collection.
The Wave 2 data of the SPIN COVID-19 cohort were used for the CFA of the final COVID-19 Fears Questionnaire. This was a subset of participants with baseline data.
5.2. Initial item analysis
Mean and SD of item scores are shown in Table 2 . Mean item scores ranged from 1.9 for Item 15 (Experience mental health problems from being isolated) to 3.4 for Item 7 (Be infected and experience more severe complications). Correlations between items (all p < 0.01) ranged from r = 0.24 for Items 1 (Become infected when getting supplies) and 3 (No sufficient financial resources) and Items 3 (No sufficient financial resources) and 14 (Be infected) to r = 0.82 for Items 6 (Be infected and not survive) and 7 (Be infected and experience more severe complications). In addition, correlations between Items 7 (Be infected and experience more severe complications) and 8 (Be infected and make condition worse) (r = 0.78), items 5 (Be infected and not be a priority for a ventilator) and 9 (Be infected and not receive medical treatment needed) (r = 0.78), items 5 (Be infected and not be a priority for a ventilator) and 6 (Be infected and not survive) (r = 0.74), items 6 (Be infected and not survive) and 14 (Be infected) (r = 0.74), items 9 (Be infected and not receive the medical treatment needed) and 10 (Be infected and healthcare professionals unfamiliar with needs) (r = 0.74), and items 6 (Be infected and not survive) and 8 (Be infected and make the condition worse) (r = 0.73) were all >0.70. Corrected item-total correlations ranged from r = 0.41 (Item 3; No sufficient financial resources) to r = 0.82 (Item 9; Be infected and not receive medical treatment needed) and were < 0.50 for only Items 3 (No sufficient financial resources) and 15 (Experience mental health problems from being isolated) (see Table 2).
Table 2.
Characteristics of the COVID-19 Fears Questionnaire for Chronic Medical Conditions.
| Itema | Mean (SD) Scoreb | Corrected Item-total Correlation | EFA Factor Loading | Mean (SD) Scoreb | CFAc Factor Loading |
|---|---|---|---|---|---|
| Items Included in Final 10-item Fears Questionnaire | |||||
| 1. I will become infected when I have to leave the house to get supplies or when supplies are brought to me | 3.0 (1.2) | 0.68 | 0.74 | 2.7 (1.1) | 0.71 |
| 2. I will not be able to access health care that I need for my condition | 2.6 (1.3) | 0.68 | 0.72 | 2.4 (1.2) | 0.77 |
| 4. I will need to be isolated for longer than others because of my condition | 3.1 (1.4) | 0.62 | 0.67 | 3.1 (1.4) | 0.72 |
| 7. I will be infected and experience more severe complications because of my condition | 3.4 (1.3) | 0.81 | 0.93 | 3.2 (1.2) | 0.89 |
| 9. I will be infected and will not receive the medical treatment I need | 2.6 (1.4) | 0.82 | 0.89 | 2.4 (1.3) | 0.86 |
| 10. I will be infected and healthcare professionals will not be familiar with the needs of a person with my condition | 3.1 (1.4) | 0.73 | 0.80 | 2.9 (1.4) | 0.74 |
| 11. People close to me (e.g., family, close friends) will be infected and become ill | 3.1 (1.3) | 0.63 | 0.69 | 2.8 (1.1) | 0.77 |
| 12. I will not be able to access medications I need for my condition due to shortages | 2.3 (1.3) | 0.64 | 0.70 | 2.1 (1.2) | 0.74 |
| 13. I will not be able to obtain basic supplies (e.g., food, other household necessities) | 2.0 (1.1) | 0.60 | 0.67 | 1.7 (1.0) | 0.68 |
| 14. I will be infected with the virus | 3.0 (1.2) | 0.77 | 0.84 | 2.7 (1.2) | 0.82 |
| Items in the preliminary 15-item removed after EFA | |||||
| 3. I or my family will not have sufficient financial resources | 2.1 (1.2) | 0.41 | 0.47 | – | – |
| 5. I will be infected and need a ventilator but there will be none available or I will not be considered a priority because of my pre-existing condition | 2.6 (1.5) | 0.78 | 0.86 | – | – |
| 6. I will be infected and not survive | 2.9 (1.4) | 0.80 | 0.91 | – | – |
| 8. I will be infected, and it will make my condition worse | 3.1 (1.4) | 0.76 | 0.85 | – | – |
| 15. I will experience mental health problems from being isolated | 1.9 (1.0) | 0.49 | 0.56 | – | – |
Item numbers are the original item numbers from the preliminary 15-item COVID-19 Fears Questionnaire for Chronic Medical Conditions.
On a 5-point scale, where 1 = not at all and 5 = extremely.
The Wave-2 data of the SPIN-COVID cohort were used for the CFA of the final COVID-19 Fears Questionnaire. This was a subset of participants with baseline data.
5.3. Exploratory factor analysis and questionnaire characteristics
EFA of the 15-item preliminary COVID-19 Fears Questionnaire yielded two eigenvalues greater than one (Factor 1 Eigenvalue 8.9 and Factor 2 Eigenvalue 1.2). Based on examination of the scree plot and item factor loadings, we judged that a one-factor solution provided the most interpretable model, as the two-factor model (inter-factor correlation = 0.66) had many items with substantial cross-loadings and was not readily interpretable. Model fit for the one-factor solution was good based on the CFI and TLI, although suboptimal based on the RMSEA (χ2(90) =1227.1, p < 0.001; CFI = 0.96; TLI = 0.95; RMSEA = 0.13). Based on inspection of the factor loadings, item correlations, and potential item redundancy due to conceptual overlap, items 3, 5, 6, 8 and 15 were removed, as per our predetermined criteria (Table 2).
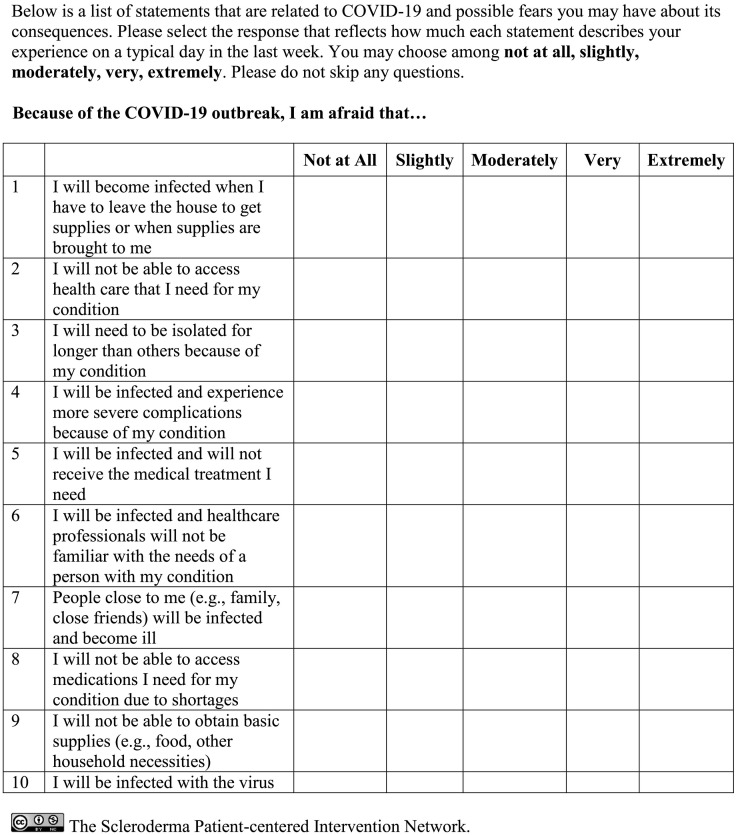
After item reduction, ten items were included in the final COVID-19 Fears Questionnaires for Chronic Medical Conditions (see Fig. 1 ). The mean (SD) of the 10-item COVID-19 Fears Questionnaire total score was 28.0 (9.7) (median = 28.0, range 10.0 to 50.0, skewness = 0.16, kurtosis = −0.85). As shown in Table 2, mean item scores ranged from 2.0 for Item 13 (Not be able to obtain basic supplies) to 3.4 for Item 7 (Be infected and experience more severe complications). Correlations between items ranged from r = 0.35 (p < 0.01; Items 4, Be isolated for longer than others and 11, People close to me be infected) to r = 0.74 (p < 0.01; Items 9, Be infected and not receive medical treatment needed and 10, Be infected and healthcare professionals unfamiliar with needs). Corrected item-total correlations ranged from r = 0.59 (Item 13, Not be able to obtain basic supplies) to r = 0.78 (Items 7, Be infected and experience more severe complications and 9, Be infected and not receive medical treatment needed). Cronbach's alpha was 0.91. There were 12 participants (1.5%) who had the lowest possible score (10.0) on the scale and 4 (0.5%) with the highest possible score (50.0), suggesting that there were no substantive floor or ceiling effects. The distribution of the item responses for each item are presented in Table 3 .
Fig. 1.
Final 10-item COVID-19 Fears Questionnaire for Chronic Medical Conditions.
Table 3.
Item response distribution for each item of the 10-item COVID-19 Fears Questionnaire for Chronic Medical Conditions (Baseline, N = 787).
| Item Responses |
|||||
|---|---|---|---|---|---|
| Item | 1 |
2 |
3 |
4 |
5 |
| Not at All |
Slightly |
Moderately |
Very |
Extremely |
|
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| 1. I will become infected when I have to leave the house to get supplies or when supplies are brought to me | 78 (9.9) | 232 (29.5) | 216 (27.4) | 173 (22.0) | 88 (11.2) |
| 2. I will not be able to access health care that I need for my condition | 217 (27.6) | 176 (22.4) | 176 (22.4) | 148 (18.8) | 70 (8.9) |
| 4. I will need to be isolated for longer than others because of my condition | 135 (17.2) | 139 (17.7) | 154 (19.6) | 202 (25.7) | 157 (19.9) |
| 7. I will be infected and experience more severe complications because of my condition | 69 (8.8) | 163 (20.7) | 143 (18.2) | 221 (28.1) | 191 (24.3) |
| 9. I will be infected and will not receive the medical treatment I need | 260 (33.0) | 148 (18.8) | 147 (18.7) | 127 (16.1) | 105 (13.3) |
| 10. I will be infected and healthcare professionals will not be familiar with the needs of a person with my condition | 143 (18.2) | 154 (19.6) | 156 (19.8) | 161 (20.5) | 173 (22.0) |
| 11. People close to me (e.g., family, close friends) will be infected and become ill | 89 (11.3) | 201 (25.5) | 195 (24.8) | 176 (22.4) | 126 (16.0) |
| 12. I will not be able to access medications I need for my condition due to shortages | 283 (36.0) | 190 (24.1) | 155 (19.7) | 94 (11.9) | 65 (8.3) |
| 13. I will not be able to obtain basic supplies (e.g., food, other household necessities) | 363 (46.1) | 208 (26.4) | 135 (17.2) | 57 (7.2) | 24 (3.0) |
| 14. I will be infected with the virus | 76 (9.7) | 242 (30.7) | 211 (26.8) | 146 (18.6) | 112 (14.2) |
5.4. Convergent validity
As shown in Table 4 , there were moderate to large correlations between the COVID-19 Fears Questionnaire and measures of anxiety (r = 0.53), depressive symptoms (r = 0.44), and perceived stress (r = 0.50). All correlations were consistent with convergent validity hypotheses. All hypotheses were also confirmed in the 11-item COVID-19 Fears Questionnaire for Systemic Sclerosis (Appendix C), including the additional item on immunosuppressant drugs use.
Table 4.
Hypotheses and correlation of variables with the COVID-19 Fears Questionnaire for Chronic Medical Conditions (N = 787).
| Variables (measures) | Pearson's correlation | p-Value | Confirmed |
|---|---|---|---|
| Moderate to large positive correlation | |||
| Anxiety: PROMIS Anxiety 4a v1.0 | 0.53 | <0.01 | Yes |
| Depressive symptoms: Patient Health Questionnaire-8 | 0.44 | <0.01 | Yes |
| Stress: Perceived Stress Scale | 0.50 | <0.01 | Yes |
5.5. Confirmatory factor analysis in the wave 2 dataset
CFA was performed on the remaining 10 items to confirm the single-factor structure of the fear questionnaire using Wave 2 data. In the initial CFA, in which measurement errors between all items were specified as uncorrelated, model fit for the hypothesized single-factor model was suboptimal (χ2(35) = 541.6, p < 0.001, TLI = 0.94, CFI = 0.95, RMSEA = 0.16). Inspection of the modification indices indicated that model fit would be improved if the error terms of Items 1 and 14, Items 9 and 10, and Items 12 and 13 were freed to covary. Items 1 (“I will become infected when I have to leave the house to get supplies or when supplies are brought to me”) and 14 (“I will be infected with the virus”) both measure fear of being infected with COVID-19. Items 9 (“I will be infected and will not receive the medical treatment I need”) and 10 (“I will be infected and healthcare professionals will not be familiar with the needs of a person with my condition”) both evaluate the fear of medical treatment not meeting (disease-specific) needs. Items 12 (“I will not be able to access medications I need for my scleroderma due to shortages”) and 13 (“I will not be able to obtain basic supplies (e.g., food, other household necessities)” both assess fear of shortages in supplies. Therefore, the model was refitted to the data, allowing the error terms of these items to covary. These changes resulted in improvements in model fit (χ2(32) = 311.2, p < 0.001, TLI = 0.96, CFI = 0.97, RMSEA = 0.12). All factor loadings were adequate, with factor loadings ranging from 0.68 (item 13) to 0.89 (item 7). Results of the 10-item CFA are shown in Table 2.
CFA was also performed to confirm the single-factor structure of the 11-item COVID-19 Fear Questionnaire for Systemic Sclerosis (model including the error terms freed to covary as in the general version). Model fit for the single-factor structure was comparable to the 10-item Chronic Medical Conditions version (χ2(41) = 346.6, p < 0.001, TLI = 0.96, CFI = 0.97, RMSEA = 0.12). Factor loadings were similar for both versions as well, with factor loadings ranging from 0.63 (Item 16) to 0.88 (Item 7). Results are shown in Appendix D.
6. Discussion
The COVID-19 Fears Questionnaire for Chronic Medical Conditions is the first measure assessing pandemic-related fears among patients vulnerable due to pre-existing illnesses [4]. The main findings of this study were that the 10-item measure can be scored with a total score reflecting a single dimension and that the scale had good internal consistency reliability and convergent validity.
In addition to the COVID-19 Fears Questionnaire for Chronic Medical Conditions, we tested a SSc-specific version, which included an additional item that reflected fears of having to discontinue the use of immunosuppressant medications, which are used by approximately half of people with SSc [48]. The measurement properties did not change meaningfully by inclusion or exclusion of the item, but some patient advisors and team members believed that content validity of a measure for people with SSc required coverage of this topic. It may be the case that investigators who conduct studies of people with other medical conditions with disease-specific fears may also explore whether there are disease-specific aspects that may be added; however, in the present study, we do not believe that this is a requirement for valid measurement of fears in COVID-19.
A recently published paper reported on the development and initial validation of a general measure for fear in COVID-19 [5]. For that measure, items were derived from multiple existing fear measures and selected by experts for inclusion. There was no input, however, from members of the public. Items from general fear measures were adapted by adding that manifestations of fear during COVID-19 and included items on being afraid; discomfort thinking about the pandemic; clammy hands; being afraid of losing life; nervousness and anxiety when watching news; inability to sleep; heart racing or palpitating. Except for one item on fear that life could be lost, items reflect cognitive and physiological manifestations of general anxiety and fear not specific to COVID-19. The COVID-19 Fears Questionnaire for Chronic Medical Conditions, on the other hand, was designed to evaluate level of fear about specific aspects of the pandemic, such as fear for long-term social isolation, shortage of basic supplies, potential medical complications, and inability to access health care or medication needed for pre-existing conditions. As such, it is modelled generally on a disease- or vulnerability-specific approach; we referred to a measure on fear of progression for the general structure [46], and we developed items based on patient input. Because of these core differences in the approach and focus of the measures, we believe that the COVID-19 Fears Questionnaire for Chronic Medical Conditions may be a more appropriate and specific measure for evaluating fear due to COVID-19 and its consequences for people with pre-existing medical diseases, although the two measures should be compared in future studies.
The COVID-19 Fears Questionnaire for Chronic Medical Conditions can be used to better evaluate behaviours and other outcomes among people with medical conditions during the current pandemic situation and as the situation develops over time. For example, fear due to COVID-19 is one of the outcome measures in trial of a mental health intervention designed to reduce anxiety among people with SSc [15]. Having a valid fear measure specifically designed for people with chronic diseases will allow exploration of how COVID-19-related fear is associated with other mental health outcomes, such as anxiety, which will provide information on how best to tailor future interventions for people living with pre-existing medical conditions during pandemic situations.
An important strength of the present study was that items were generated based on fears shared by over 100 people with SS, even though the measure was developed quickly and there was a short time (72 h) for people to provide suggestions. Furthermore, members of the SPIN Patient Advisory Team were directly involved in conceiving the ideas incorporated into the scale and in the development and wording of items. It was validated in a large sample of people with SSc.
Additionally, there are limitations to consider. First, we used convenience samples to develop the questions and evaluate the measurement properties of the COVID-19 Fears Questionnaire. The demographic (including gender, ethnicity, and country) and medical characteristics of SPIN-COVID-19 Cohort participants are similar, though, to our ongoing SPIN Cohort, which is comparable with other large international SSc cohorts [13]. Second, this study only included patients with SSc, and item development did not include participants with other chronic medical conditions. Ideally, the scale will undergo further evaluation in other patient groups. In addition, although the study was conducted in a longitudinal cohort, we confined our analyses to cross-sectional analyses. We did not evaluate consistency over time because the natural history and degree of stability of fear during COVID-19 were unknown. Finally, in different settings, it is possible that some questionnaire items may be perceived differently than in SSc or that parts of items may overlap. Future studies could further test the applicability of items and consider reformulation based on setting and population.
To conclude, results of the present study demonstrate that the 10-item COVID-19 Fears Questionnaire for Chronic Medical Conditions is a valid instrument for the measurement of COVID-19-related fears in patients with chronic medical diseases. This is an important contribution since fears during pandemics may be more severe in individuals with pre-existing medical conditions, who are at high risk of complications from COVID-19.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Financial support
The study was supported with funding from the Canadian Institutes of Health Research (CIHR); the McGill Interdisciplinary Initiative in Infection and Immunity Emergency COVID-19 Research Fund; Scleroderma Canada, made possible by an educational grant for patient support programming from Boehringer Ingelheim; Scleroderma Society of Ontario; Scleroderma Manitoba; Scleroderma Atlantic; Scleroderma Australia; Scleroderma New South Wales; Scleroderma Victoria; Scleroderma Queensland; Scleroderma SASK; Scleroderma Association of BC. Drs. Wu and Levis were supported by a postdoctoral fellowship training award from the Fonds de recherche du Québec – Santé (FRQ-S); Dr. Henry was supported by a Mitacs postdoctoral fellowship award; and Mr. Harb and Ms. Carboni-Jiménez were supported by CIHR Canada Graduate Scholarship-Master's awards; Mr. Bhandari was supported by a studentship from the Research Institute of the McGill University Health Centre; Ms. Gagarine was supported by a Mitacs internship award; Ms. Neupane was supported by G.R. Caverhill Fellowship from the Faculty of Medicine, McGill University; Drs. Benedetti and Thombs were supported by FRQ-S researcher salary awards, all outside of the present work.
Declaration of Competing Interest
None.
Acknowledgements
We thank the 121 people with scleroderma who provided item suggestions for the questionnaire.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychores.2020.110271.
Contributor Information
on behalf of the Scleroderma Patient-centered Intervention Network (SPIN) COVID-19 Investigators:
Nicole Culos-Reed, Ghassan El-Baalbaki, Shannon Hebblethwaite, Scott Patten, John Varga, Laura Bustamante, Delaney Duchek, Kelsey Ellis, and Danielle Rice
SPIN COVID-19 Patient Advisory Team:
Laura Dyas, Catherine Fortuné, Amy Gietzen, Geneviève Guillot, Nancy Lewis, Karen Nielsen, Michelle Richard, Maureen Sauvé, and Joep Welling
Appendix A. Supplementary data
Supplementary material
References
- 1.Callaway E., Cyranoski D., Mallapaty S., Stoye E., Tollefson J. The coronavirus pandemic in five powerful charts. Nature. 2020;579:482–483. doi: 10.1038/d41586-020-00758-2. [DOI] [PubMed] [Google Scholar]
- 2.Brooks S.K., Webster R.K., Smith L.E. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salisbury H. Helen Salisbury: fear in the time of covid. BMJ. 2020;368:m1286. doi: 10.1136/bmj.m1286. https://doi [DOI] [PubMed] [Google Scholar]
- 4.Thombs D.B., Tao L., Wu Y. Preliminary COVID-19 fears questionnaire: systemic sclerosis and chronic medical conditions versions. OSF Preprints. 2020, April 7 doi: 10.31219/osf.io/m2ybt. [DOI] [Google Scholar]
- 5.Ahorsu D.K., Lin C.Y., Imani V. The fear of COVID-19 scale: development and initial validation. Int. J. Ment. Health Ad. 2020 doi: 10.1007/s11469-020-00270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakib N., Bhuiyan A.K.M.I., Hossain S. Psychometric validation of the Bangla fear of COVID-19 scale: confirmatory factor analysis and Rasch analysis. Int. J. Ment. Heal. Addict. 2020:1–12. doi: 10.1007/s11469-020-00289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzur Bitan D., Grossman-Giron A., Bloch Y., Mayer Y., Shiffman N., Mendlovic S. Fear of COVID-19 scale: psychometric characteristics, reliability and validity in the Israeli population. Psychiatry Res. 2020;289:113100. doi: 10.1016/j.psychres.2020.113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reznik A., Gritsenko V., Konstantinov V., Khamenka N., Isralowitz R. COVID-19 fear in Eastern Europe: validation of the fear of COVID-19 scale. Int. J. Ment. Heal. Addict. 2020:1–6. doi: 10.1007/s11469-020-00283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soraci P., Ferrari A., Abbiati F.A. Validation and psychometric evaluation of the Italian version of the fear of COVID-19 scale. Int. J. Ment. Heal. Addict. 2020:1–10. doi: 10.1007/s11469-020-00277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haktanir A., Seki T., Dilmaç B. Adaptation and evaluation of Turkish version of the fear of COVID-19 scale. Death Stud. 2020:1–9. doi: 10.1080/07481187.2020.1773026. [DOI] [PubMed] [Google Scholar]
- 11.Satici B., Gocet-Tekin E., Deniz M.E., Satici S.A. Adaptation of the fear of COVID-19 scale: its association with psychological distress and life satisfaction in Turkey. Int. J. Ment. Heal. Addict. 2020:1–9. doi: 10.1007/s11469-020-00294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allanore Y., Simms R., Distler O., Trojanowska M., Pope J., Denton C.P., Varga J. Systemic sclerosis. Nat. Rev. Dis. Prim. 2015;1:15002. doi: 10.1038/nrdp.2015.2. [DOI] [PubMed] [Google Scholar]
- 13.Dougherty D.H., Kwakkenbos L., Carrier M.E. The scleroderma patient-centered intervention network cohort: baseline clinical features and comparison with other large scleroderma cohorts. Rheumatology. 2018;57:1623–1631. doi: 10.1093/rheumatology/key139. [DOI] [PubMed] [Google Scholar]
- 14.Herrick A.L., Pan X., Peytrignet S. Treatment outcome in early diffuse cutaneous systemic sclerosis: the European scleroderma observational study (ESOS) Ann. Rheum. Dis. 2017;76:1207–1218. doi: 10.1136/annrheumdis-2016-210503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thombs B.D., Kwakkenbos L., Carrier M.E. Protocol for a partially nested randomised controlled trial to evaluate the effectiveness of the Scleroderma Patient-Centered Intervention Network COVID-19 Home-Isolation Activities Together (SPIN-CHAT) Program to reduce anxiety among at-risk scleroderma patients. J. Psychosom. Res. 2020 doi: 10.1016/j.jpsychores.2020.110132. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thombs B.D., Kwakkenbos L., Henry R.S. Protocol for the scleroderma patient-centered intervention network (SPIN) COVID-19 cohort and analysis of changes in symptoms of anxiety and depression from Pre-COVID-19 to COVID-19. OSF Preprints. 2020, June 13 doi: 10.31219/osf.io/m2ybt. [DOI] [Google Scholar]
- 17.Kwakkenbos L., Jewett L.R., Baron M. The scleroderma patient-centered intervention network (SPIN) cohort: protocol for a cohort multiple randomised controlled trial (cmRCT) design to support trials of psychosocial and rehabilitation interventions in a rare disease context. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-003563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Hoogen F., Khanna D., Fransen J. Classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scleroderma Patient-centered Intervention Network https://www.spinsclero.com/ Accessed March 31, 2020.
- 20.Mayring P. Qualitative content analysis. Forum Qual. Soc. Res. 2000;1(2) http://www.qualitative-research.net/index.php/fqs/article/view/1089/2385 Art. 20. Accessed April 4, 2020. [Google Scholar]
- 21.World Health Organization Process of translation and adaptation of instruments. https://www.who.int/substance_abuse/research_tools/translation/en/ Accessed April 10, 2020.
- 22.PROMIS Measure Development and Research http://www.healthmeasures.net/explore-measurement-systems/promis/measure-development-research Accessed March 31, 2020.
- 23.Cella D., Riley W., Stone A. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinchcliff M., Beaumont J.L., Thavarajah K. Validity of two new patient-reported outcome measures in systemic sclerosis: patient-reported outcomes measurement information system 29-item health profile and functional assessment of chronic illness therapy–dyspnea short form. Arthritis Care Res. 2011;63:1620–1628. doi: 10.1002/acr.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwakkenbos L., Thombs B.D., Khanna D. Validation of the patient-reported outcomes measurement information System-29 (PROMIS-29) in scleroderma and associations with clinical characteristics: a scleroderma patient-centered intervention network (SPIN) cohort study. Rheumatology. 2017;56(8):1302–1311. doi: 10.1093/rheumatology/kex055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroenke K., Strine T.W., Spitzer R.L. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y., Levis B., Riehm K.E. Equivalency of the diagnostic accuracy of the PHQ-8 and PHQ-9: a systematic review and individual participant data meta-analysis. Psychol. Med. 2019;50:1368–1380. doi: 10.1017/S0033291719001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milette K., Hudson M., Baron M., Thombs B.D., Canadian Scleroderma Research Group Comparison of the PHQ-9 and CES-D depression scales in systemic sclerosis: internal consistency reliability, convergent validity and clinical correlates. Rheumatology. 2010;49:789–796. doi: 10.1093/rheumatology/kep443. [DOI] [PubMed] [Google Scholar]
- 29.Arthurs E., Steele R.J., Hudson M. Are scores on English and French versions of the PHQ-9 comparable? An assessment of differential item functioning. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 31.Lee E.-H. Review of the psychometric evidence of the Perceived Stress Scale. Asian Nurs. Res. 2012;6:121–127. doi: 10.1016/j.anr.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Lesage F.X., Berjot S., Deschamps F. Psychometric properties of the French versions of the Perceived Stress Scale. Int. J. Occup. Med. Environ. Health. 2012;25:178–184. doi: 10.2478/S13382-012-0024-8. [DOI] [PubMed] [Google Scholar]
- 33.Terwee C.B., Bot S.D.M., de Boer M.R. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007;60:34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Terwee C.B., Prinsen C.A.C., Chiarotto A., De Vet H.C.W., Westerman M.J., Patrick D.L., Alonso J., Bouter L.M., Mokkink L.B. COSMIN standards and criteria for evaluating the content validity of health-related Patient-Reported Outcome Measures: a Delphi study. Qual. Life Res. 2018;27:1159–1170. doi: 10.1007/s11136-018-1829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muthén L., Muthén B.O. Seventh edition. Muthén & Muthén; Los Angeles, CA: 1998-2012. Mplus user's Guide Version. [Google Scholar]
- 36.Fabrigar L.R., Wegener D.T., MacCallum R.C., Strahan E.J. Evaluating the use of exploratory factor analysis in psychological research. Psychol. Methods. 1999;4:272. [Google Scholar]
- 37.Tucker L.R., Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38(1):1–10. [Google Scholar]
- 38.Bentler P.M. Comparative fit indexes in structural models. Psychol. Bull. 1990;107:238. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 39.Steiger J.H. Structural model evaluation and modification: an interval estimation approach. Multivar. Behav. Res. 1990;25:173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- 40.Reise S.P., Widaman K.F., Pugh R.H. Confirmatory factor analysis and item response theory: two approaches for exploring measurement invariance. Psychol. Bull. 1993;114:552. doi: 10.1037/0033-2909.114.3.552. [DOI] [PubMed] [Google Scholar]
- 41.Hu L.T., Bentler P.M. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Equ. Model. 1999;6:1–55. [Google Scholar]
- 42.Kline R.B. In: Principles and Practice of Structural Equation Modeling. Little T.D., editor. The Guilford Press; New York (NY): 2015. [Google Scholar]
- 43.Browne M.W., Cudeck R., Bollen K.A., Long J.S. Alternative ways of assessing model fit. In: Bollen K.A., Long J.S., editors. Testing Structural Equation Models. Sage; Beverly Hills (CA): 1993. pp. 136–162. [Google Scholar]
- 44.Nunnally J.C. 2nd Ed. McGraw-Hill; 1978. Psychometric Theory; p. 281. [Google Scholar]
- 45.Hair J.F., Black W.C., Babin B.J., Anderson R.E. Pearson Prentice Hall; New Jersey: 2010. Multivariate Data Analysis: A Global Perspective; pp. 661–699. [Google Scholar]
- 46.Kwakkenbos L., van den Hoogen F.H., Custers J., Prins J., Vonk M.C., van Lankveld W.G., Becker E.S., van den Ende C.H. Validity of the fear of progression questionnaire-short form in patients with systemic sclerosis. Arthritis Care Res. 2020;64:930–934. doi: 10.1002/acr.21618. [DOI] [PubMed] [Google Scholar]
- 47.Wolf E.J., Harrington K.M., Clark S.L., Miller M.W. Sample size requirements for structural equation models: an evaluation of power, bias, and solution propriety. Educ. Psychol. Meas. 2013;76:913–934. doi: 10.1177/0013164413495237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thombs B., Kwakkebos L., Henry R.S. Comparison of mental health symptoms prior to and during COVID-19 among patients with systemic sclerosis from four countries: a Scleroderma Patient-centered Intervention Network (SPIN) Cohort study. medRxiv. 2020, June 13 doi: 10.1101/2020.06.13.20128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material