Hospitalisation with COVID-19 infection has been associated with an increased incidence of thrombosis, particularly in the critical care setting. Our two centres have previously described the early in-patient incidence of venous thromboembolism (VTE) at the peak of the COVID-19 outbreak in the United Kingdom [1,2]. The cohort from Cambridge showed 6 patients out of a total of 63 who developed VTE with a median follow-up of 8 days whereas London showed 10 patients of 66 had VTE with a median follow-up of 30 days. The risk of hospital-associated VTE (HAT) for patients has been shown to extend from admission to 90 days following discharge with an early peak within the first weeks of this period [3]. However, it has not yet been established whether the risk of VTE following COVID-19 pneumonia also persists to 90 days.
We conducted an observational study of our previous cohorts with a minimum of 90 days follow-up from their critical care admission at our centres. Our methods have previously been described and approval was obtained from the Research and Development departments at both Trusts [1,2]. The composite endpoint was image-proven pulmonary embolism (PE) and deep vein thrombosis (DVT) including catheter-associated thrombi. The index date was admission to critical and censorship data was 17th July 2020. We assessed the D-dimer levels at the time of imaging in those with and without thrombosis, which we and others have previous shown to be significantly higher in those developing VTE [2]. Cumulative incidence was adjusted for the competing risk of death but not for hospital discharge although the majority of patients had been discharged in this study [4].
In total, 129 patients with COVID-19 infection confirmed by polymerase chain reaction on nasopharyngeal swab or bronchoalveolar lavage were included. Both centres performed VTE risk assessments using the Department of Health tool [5]. Our guidance for thromboprophylaxis was based originally on NICE guidance for medical patients using dalteparin that was prescribed according to weight and renal function unless contraindicated in all patients. Post-discharge (i.e. extended thromboprophylaxis) was not used. The demographics of these patients are described fully in the previous articles. To summarise, there was predominance of males in both cohorts (69% Cambridge and 73% London), high rates of mechanical ventilation use (83% and 79%) and the mean ages was 62 and 56 years, respectively.
The median number of days of follow-up was 113 (range 96–138 days) for those who were alive at review. The median duration of critical care admission was 13.5 days (range 1–130 days). At the censorship date, 40/129 patients had died (31%), 6/129 patients (5%) remained in critical care, 7/129 (5%) were in-patients but had been discharged from critical care, 3/129 (2%) were transferred to their local hospitals and 73/129 (57%) were alive and discharged from hospital. 48/73 (66%) of the patients discharged alive at follow-up had a hospital discharge duration of ≥90 days.
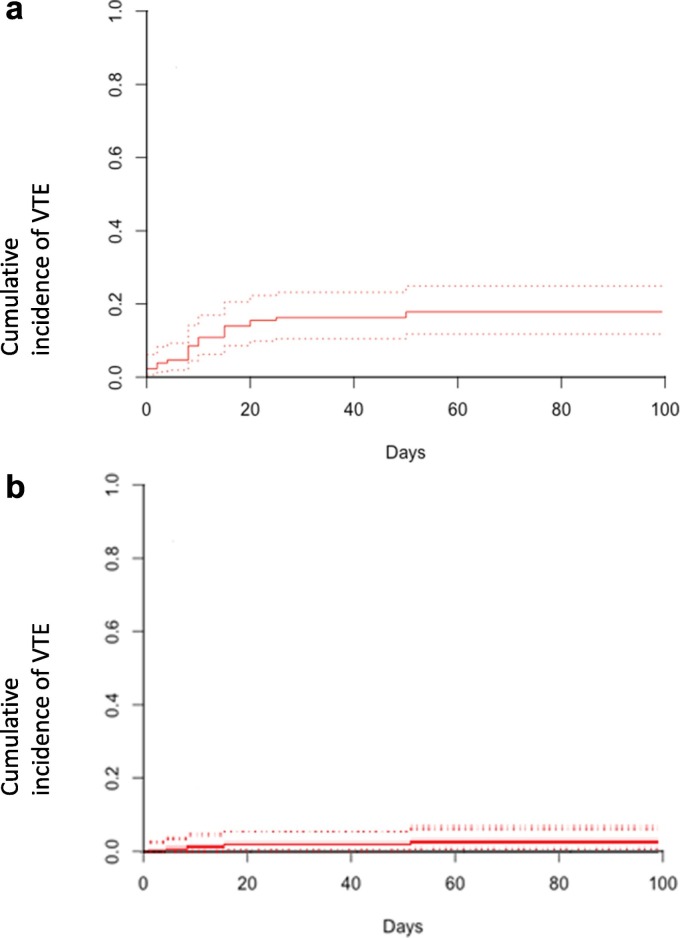
24/129 (19%) patients developed 26 VTE events. The estimated cumulative incidence of VTE over a minimum of 90 days following critical care admission was 18.6% shown in Fig. 1(a) (95% confidence interval 12.4–25.8). When segmental/sub-segmental pulmonary embolism was excluded, the cumulative incidence of VTE was 4.1% (95% CI 1.5–8.8%), which is displayed in Fig. 1(b). All VTE events occurred during the initial hospitalisation with COVID-19 infection with no VTE events occurring as an out-patient or further rehospitalisation. The median time from ICU admission to VTE diagnosis was 9.5 days (range 0–60 days). 17/129 (13%) had PE of which the most proximal vessels were: 1 main pulmonary artery, 4 lobar, 8 segmental and 4 subsegmental. 12/17 (71%) could therefore be considered as small-vessel pulmonary thrombi (‘immunothrombosis’). 9/129 (7%) patients had DVT, all of which were related to indwelling lines: 3 proximal lower limb, 4 proximal upper limb and 3 neck. Two patients had both PE and DVT and one patient had two DVT. One patient developed a further DVT at an indwelling line site following the diagnosis of a PE although the patient had major bleeding, so anticoagulation was not given.
Fig. 1.
a. Cumulative incidence of all VTE (the composite endpoint) with point estimate shown as solid line with dashed line the 95% confidence interval. b. Cumulative incidence of VTE when segmental/subsegmental pulmonary embolism are excluded with the point estimate shown as solid line with dashed line the 95% confidence interval. Abbreviation: VTE – venous thromboembolism.
Overall, 15 ultrasound Doppler scans or computer tomography (CT) were performed to assess for DVT with 60% being positive; and 35 CT pulmonary angiography scans were performed with 49% being positive for a PE. 5/9 DVTs and 3/17 PEs were incidental findings. D-dimers performed at the time of imaging in those diagnosed with VTE had a median level of 5.9 FEU mg/L (range 0.7–80.0, interquartile range 2.4–9.3) in comparison to a median of 0.8 FEU mg/L without VTE (range 0.0–5.4, interquartile range 0.51–0.97, p < 0.001). There was no statistical difference in Clauss fibrinogen levels at the time of imaging in those with and without VTE. 47/50 (94%) of imaging was performed during the initial in-patient admission.
All VTE events occurred in hospital, mainly within the first few days of admission to critical care. The site was predominantly catheter-associated thrombosis or small segmental/subsegmental changes on CTPA, which are increasingly recognised as immunothrombosis [2]. 10% of our initial cohorts remained in hospital after 90 days of admission.
Rates of critical care in-patient hospital-associated VTE have been reported to be between 17–27% at other centres with a significantly higher rate if routine screening Doppler scans are used [[6], [7], [8]]. Our prolonged follow-up shows that although some patients remained hospitalised after 90 days, there are minimal rates of further VTE in hospital and following discharge. Our data is in line with Roberts et al who have described HAT in 9/1877 (0.5%) in all hospitalisation discharges with COVID-19 infection of varying disease severity with up to 42 days follow-up [9].
Due to the concerns of an extended prothrombotic risk with COVID-19, particularly in those with severe disease, the use of extended thromboprophylaxis has been considered although there is no clinical trial data yet. In a meta-analysis in medically unwell patients who did not have COVID-19 infection, extended chemical thromboprophylaxis compared to standard duration thromboprophylaxis resulted in no overall change in mortality rates but a 32% relative risk reduction in VTE and a 104% relative risk increase in major haemorrhage [10]. At present, we feel that this data however cannot be extrapolated to the setting of patients with COVID-19. The International Society on Thrombosis and Haemostasis (ISTH) and American College of Chest Physicians (ACCP) in their expert-led guidance advocated the use of low molecular weight heparin during hospitalisation with COVID-19 infection and ISTH advocated consideration of using extended thromboprophylaxis while ACCP did not [11,12].
Our results are limited by the inclusion of only two centres. Additionally, the diagnosis of VTE was based principally upon symptomatic presentation unless incidental VTE were found. HAT is defined by the development of VTE 90 days following discharge and in those 10% with continued hospitalisation, we were unable to assess their full period of possible HAT.
Given the absence of VTE following discharge, our data does not support the use of extended thromboprophylaxis post-hospitalisation for patients with COVID-19 following critical care admission. Further larger studies are required.
Funding
Nil.
References
- 1.Thomas W., Varley J., Johnston A. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb. Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desborough M.J.R., Doyle A.J., Griffiths A., Retter A., Breen K.A., Hunt B.J. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom [published online ahead of print, 2020 May 29] Thromb. Res. 2020;193:1–4. doi: 10.1016/j.thromres.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lester W., Freemantle N., Begaj I., Ray D., Wood J., Pagano D. Fatal venous thromboembolism associated with hospital admission: a cohort study to assess the impact of a national risk assessment target. Heart. 2013;99(23):1734–1739. doi: 10.1136/heartjnl-2013-304479. [DOI] [PubMed] [Google Scholar]
- 4.Borjas-Howard J.F., Bhoelan S., van Miert J. Beware overestimation of thrombosis in ICU: mortality is not the only competing risk! [published online ahead of print, 2020 May 30] Thromb. Res. 2020;193:78. doi: 10.1016/j.thromres.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health VTE Risk Assessment Tool. https://www.nice.org.uk/guidance/ng89/resources/department-of-health-vte-risk-assessment-tool-pdf-4787149213
- 6.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middeldorp S., Coppens M., van Haaps T.F. Incidence of venous thromboembolism in hospitalized patients with COVID-19 [published online ahead of print, 2020 May 5] J Thromb Haemost. 2020 doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llitjos J.F., Leclerc M., Chochois C. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts L.N., Whyte M.B., Georgiou L. Post-discharge venous thromboembolism following hospital admission with COVID-19 [published online ahead of print, 2020 Aug 3] Blood. 2020 doi: 10.1182/blood.2020008086. blood.2020008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiasakul T., Evans C.R., Spyropoulos A.C., Raskob G., Crowther M., Cuker A. Extended vs. standard-duration thromboprophylaxis in acutely ill medical patients: a systematic review and meta-analysis. Thromb. Res. 2019;184:58–61. doi: 10.1016/j.thromres.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., Levi M., Samama C.M., Thachil J., Giannis D., Douketis J.D. Subcommittee on perioperative, critical care thrombosis, haemostasis of the scientific, standardization committee of the international society on thrombosis, haemostasis. scientific and standardization committee communication: clinical guidance on the diagnosis, prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moores L.K., Tritschler T., Brosnahan S. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report [published online ahead of print, 2020 Jun 2] Chest. 2020 doi: 10.1016/j.chest.2020.05.559. S0012-3692(20)31625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]