Abstract
Background
Prognostic tools are required to guide clinical decision-making in COVID-19.
Methods
We studied the relationship between the ratio of interleukin (IL)-6 to IL-10 and clinical outcome in 80 patients hospitalized for COVID-19, and created a simple 5-point linear score predictor of clinical outcome, the Dublin-Boston score. Clinical outcome was analysed as a three-level ordinal variable (“Improved”, “Unchanged”, or “Declined”). For both IL-6:IL-10 ratio and IL-6 alone, we associated clinical outcome with a) baseline biomarker levels, b) change in biomarker level from day 0 to day 2, c) change in biomarker from day 0 to day 4, and d) slope of biomarker change throughout the study. The associations between ordinal clinical outcome and each of the different predictors were performed with proportional odds logistic regression. Associations were run both “unadjusted” and adjusted for age and sex. Nested cross-validation was used to identify the model for incorporation into the Dublin-Boston score.
Findings
The 4-day change in IL-6:IL-10 ratio was chosen to derive the Dublin-Boston score. Each 1 point increase in the score was associated with a 5.6 times increased odds for a more severe outcome (OR 5.62, 95% CI -3.22–9.81, P = 1.2 × 10−9). Both the Dublin-Boston score and the 4-day change in IL-6:IL-10 significantly outperformed IL-6 alone in predicting clinical outcome at day 7.
Interpretation
The Dublin-Boston score is easily calculated and can be applied to a spectrum of hospitalized COVID-19 patients. More informed prognosis could help determine when to escalate care, institute or remove mechanical ventilation, or drive considerations for therapies.
Funding
Funding was received from the Elaine Galwey Research Fellowship, American Thoracic Society, National Institutes of Health and the Parker B Francis Research Opportunity Award.
Keywords: COVID-19, Interleukin-6, Interleukin-10, Prognostic score, Clinical outcome, Inflammation, Cytokines
Research in Context.
Evidence before this study
Patients with coronavirus disease 2019 (COVID-19) demonstrate elevated levels of circulating cytokines and accelerated progression to acute respiratory distress syndrome. As the burden of COVID-19 on healthcare resources grows, tools that help caregivers predict the clinical course of the COVID-19 patient address an area of need.
Added value of this study
The Dublin-Boston score is based on changes in the ratio of interleukin (IL)−6 to interleukin-10 over time, and identifies hospitalized patients at risk of impending poor outcome. In this study the score, and the change in IL-6:IL-10 from which it is derived, significantly outperformed the predictive capabilities of IL-6 alone.
Implications of all the available evidence
Alterations in cytokine balance predict clinical progression, and can be used to guide decision making. More informed prognosis could help determine when to escalate or de-escalate care, a key component of the efficient allocation of resources during the current pandemic.
Alt-text: Unlabelled box
1. Introduction
Coronavirus disease 2019 (COVID-19) is a global threat to health. As of mid-August 2020, more than 22 million laboratory-confirmed cases have been documented worldwide, with over 770,000 deaths [1]. In-hospital studies have described a febrile pro-inflammatory syndrome with accelerated progression to acute respiratory distress syndrome (ARDS), acute renal failure, shock and arrhythmia [2,3]. While the overall clinical phenotype amongst those hospitalized is heterogeneous, marked arterial hypoxaemia at initial presentation is common.
In the intensive care unit (ICU) setting, the overall burden of disease and prolonged duration of mechanical ventilation have fuelled concerns regarding potential ventilator shortages. Knowing when to institute mechanical ventilation - and similarly when to remove it – represents a key component of the efficient allocation of resources during the current pandemic. Clinical management is further complicated by COVID-associated hyperinflammatory syndromes and by the off-label administration of both non-specific and targeted anti-infective and anti-inflammatory therapies to COVID-19 patients without an established evidence base for their use.
Circulating levels of the master pro-inflammatory cytokine IL-6 are elevated in patients with COVID-19 [4,5], and IL-6 has subsequently been suggested as a potential biomarker to help identify patients who may benefit from proposed anti-inflammatory therapies, such as steroids or monoclonal antibodies. IL-6 is secreted by a wide range of cell types in response to a variety of pathological states, including infection, inflammation and cancer [6,7]. Its gene expression is controlled by activating nuclear factors such as nuclear factor (NF)–IL-6 [8], hypoxia-inducible factor (HIF)−1α [9], [10], [11] and, in particular, NF-κB [12], [13], [14]. Indeed, IL-6 can be induced by engagement of bacterial lipopolysaccharide (LPS) [15], pro-inflammatory cytokines such as TNF-α and IL-1 [16,17], or viral infections [18], all of which activate NF-κB, and comprehensive mutational analyses have identified the NF-κB binding site as being crucial for IL-6 gene induction [12,13]. HIF-1α is typically elevated in response to hypoxia [19], but increased HIF-1α-mediated transcription of IL-6 may also be observed in normoxia following a metabolic shift towards increased aerobic glycolysis, known as a Warburg effect [11,20]. Although this phenomenon was originally described in tumour cells it is not limited to cancer, and occurs in immune cells in response to LPS and severe inflammation [9,10,[21], [22], [23], [24]]. In addition to transcribing pro-inflammatory cytokines, HIF-1α also acts to suppress production of the anti-inflammatory and pro-resolution cytokine IL-10 by regulatory T-cells (Tregs). It does this by directly binding FOXP3 - a transcription factor vital for Treg development - and marking it for ubiquitination and proteasomal degradation [9,23,24].
Despite high levels in blood, use of isolated IL-6 measurement as a COVID-19 prognostic tool, or as a means of evaluating clinical response to treatment, is hindered by several factors. First, IL-6 levels within the same patient vary significantly over the course of any given day, the most conspicuous effect being a trough in the morning [25]. Second, the magnitude of the IL-6 response to infection is, in absolute terms, also variable between patients [26]. Furthermore, the presence of immunometabolic comorbidities such as obesity can also influence circulating IL-6 levels and IL-6 release [27], [28], [29].
Rather than focusing solely on increased baseline levels of IL-6 in COVID-19, it may be more useful to view longitudinal inflammatory biomarker levels as features of a more comprehensive shift in metabolic and inflammatory balance, in which the ability of anti-inflammatory mediators to keep pace with pro-inflammatory ones is compromised.
Therefore, in this study, we evaluated longitudinal changes in IL-6 and the ratio of IL-6:IL-10 as they related to clinical trajectory in 80 patients hospitalized for COVID-19. We aimed to determine whether changes in IL-6:IL-10 ratio are superior to changes in IL-6 in identifying those at highest risk of clinical deterioration, and thus useful in guiding clinical decision-making.
2. Methodology
2.1. Ethics
Ethical approval was received from the Beaumont Hospital Ethics Committee (REC #18/52, #17/06). All patients provided informed consent.
2.2. Patient selection
Patients hospitalized for COVID-19 (n = 80) were selected at random from a list of medical record numbers corresponding to patients with a confirmed diagnosis of COVID-19. A confirmed case of COVID-19 was defined by a positive result on a reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of a specimen collected on a nasopharyngeal swab. Patients were excluded if they were chronically immunosuppressed, receiving long-term oral corticosteroids, antivirals, hydroxychloroquine, anti-IL-1, anti-IL-6 or anti-TNF therapy, known to be pregnant, on dialysis for chronic kidney disease, had active neoplasia, or had a history of vasculitis or connective tissue disease.
2.3. Assessment of clinical outcome
Clinical outcome was based on clinical change from day of study entry (day 0) to day 7 of the study. Clinical improvement was defined as a decrease of ≥2 points on a six-point ordinal scale [30] endorsed by the World Health Organization (Table S1) or live discharge or both, with clinical decline defined as an increase of ≥2 points or death or both. Patients were “unchanged” if they did not meet the criteria for having “improved” or “declined”. All subjects were evaluated for their WHO clinical status each day on the study.
2.4. Cytokine measurements
Levels of IL-6 and IL-10 were measured in plasma by ELISA (R&D systems, Minneapolis MN, USA) in accordance with the manufacturer's instructions. Plasma was obtained every two days by centrifugation of whole blood at 250 x g for 5 min at room temperature. Cytokine measurements were undertaken by a blinded investigator not involved in the clinical care of the patients. Similarly, results were not shared with the treating physicians so as not to bias or influence the clinical outcomes assessed. To minimize the potential for inter-assay variability, samples were run in triplicate en bloc, with a selection of samples run on every plate as points of reference. Absolute values are provided in the Supplementary Appendix.
The decision to study these cytokines was based on work recently published by our group and others regarding the COVID-19 cytokinaemia [4,5]. Although IL-1β, IL-8, TNF-α and other pro-inflammatory cytokines were increased in those with severe illness, the most prominent elevations observed in these studies were for IL-6. Central to the present investigation was the concept of a loss of balance between pro-inflammatory and anti-inflammatory mediators in COVID-19. IL-10 is the most widely studied anti-inflammatory and pro-resolution cytokine in ARDS [31], and was therefore used as an anti-inflammatory comparator to IL-6. Severe COVID-19 is also associated with increased intracellular HIF-1α [4], which further prompted consideration of whether the IL-10 response would become blunted as patients with COVID-19 got sicker. An additional reason for choosing IL-6 ahead of other pro-inflammatory cytokines was that it is currently being measured in a substantial number of hospital laboratories, unlike its alternatives, which are largely confined to the research lab setting. While we were aware that the inclusion of multiple cytokines would almost certainly produce a more precise score, this would also potentially render the score impractical and more difficult to replicate.
2.5. Association of predictors with clinical outcomes
Our analysis focused on the every-two-day IL-6 and IL-6:IL-10 ratio measurements. We analysed clinical outcome as a three-level ordinal variable (“Improved”, “Unchanged”, or “Declined”). For both IL-6 and IL-6:IL-10 ratio, we associated clinical outcome with a) baseline biomarker levels, b) change in biomarker level from day 0 to day 2, c) change in biomarker from day 0 to day 4, and d) slope of biomarker change throughout the time spent by a patient on the study. The IL-6 and IL-6:IL-10 ratio slopes were determined from a linear regression of biomarker level (y) on hospital days (x). Untransformed IL-6 and IL-6:IL-10 ratio levels were used for all analyses. “Day 0″ was defined as the day of first sample. The associations between ordinal clinical outcome (“Improved”/“Unchanged”/“Declined”) and each of the different predictors (a-d above for both IL-6 alone and the IL-6:IL-10 ratio) were performed with proportional odds logistic regression using the polr() function in the MASS package in R [32]. The cumulative odds ratios (OR) for the proportional odds logistic regression represents the cumulative odds of a more severe clinical outcome (“Improved” or “Unchanged” vs. “Declined”; “Improved vs. “Unchanged” or “Declined”). The range of baseline IL-6 values is approximately 100 times greater than the baseline range of IL-6:IL-10 ratio values. Due to differences in scale of IL-6 and IL-6:IL-10 ratio values, the ordinal logistic regression OR is in reference to a 10-unit increase in all IL-6 predictors and a 0.1 unit increase for IL-6:IL-10 ratio predictors. All ordinal logistic regression associations were run “unadjusted” and were then adjusted for age and sex. A Bonferroni-adjusted P value <0.00625 (0.05/8 predictors) was considered statistically significant and P value <0.05 was considered “nominally” significant. The Akaike information criterion (AIC) regression metric was used to evaluate the relative quality of logistic regression models.
2.6. Calculating an easy-to-use clinical prediction score: the Dublin-Boston score
In addition to the IL-6 and IL-6:IL-10 ratio predictors above, we created a simple 5-point linear score predictor of clinical outcome (“Dublin-Boston score”). This linear score was generated by multiplying the day 0 to day 4 change in IL-6:IL-10 ratio by two, rounding to whole numbers, and then restricting the score to a 5-point scale ranging from −2 to 2 (i.e. the possible values were −2, −1, 0, 1, 2), with a higher score giving a worse prognosis. The distribution of the Dublin-Boston score was evaluated and its prediction accuracy was compared to the various IL-6 and IL-6:IL-10 ratio predictors including baseline levels, two-point change (day 0 to day 2, day 0 to day 4), and slope over the course of the study. The Dublin-Boston score was associated with ordinal clinical outcome (“Improved”/“Unchanged”/“Declined”) with the OR representing the increased odds of a more severe clinical outcome for each 1 point increase in the Dublin-Boston score.
2.7. Process for selection of a prediction model
A nested cross-validation (CV) approach was applied to select the best model for prediction and to obtain unbiased estimates of prediction accuracy. In the outer loop of the nested CV, an 8-fold CV was conducted by splitting the data to a training set and a test set (containing ⅛ of the entire data) eight times. In the inner loop of the nested CV, for each CV fold, another 5-fold CV was conducted by splitting the training set to another training set and a test set. The strongest models selected in each CV fold were then evaluated using the test set that was not used in the selection of the model. It has been proposed that such nested CV provides almost unbiased performance estimates [33]. We considered a number of models for prediction of clinical outcome (“improved”/“unchanged”/“declined”) as noted in the main manuscript and also in the Supplementary Appendix. Prediction accuracy was determined using multiple metrics, including mean squared error (MSE), mean absolute error (MAS), ranked probability score (RPS), and area under the curve (AUC, using the pROC package in R). The final prediction model was obtained by training our “best” model – specifically, the one that best combined predictive performance with ease of use – on the entire dataset.
2.8. Role of funders
Funders had no role in study design, data collection, data analyses, interpretation, or writing of this manuscript.
3. Results
3.1. Characteristics of the patients
The baseline clinical characteristics of the patients are shown in Table 1. The mean (±SD) age of the patients was 58±17 years; 65% were male. The mean duration of symptoms before hospital admission was 2 ± 2 days. The most common symptoms on admission to the hospital were fever, dyspnoea, fatigue and cough. The mean length of stay at the time of study entry was 4 ± 3 days.
Table 1.
Baseline clinical characteristics of the cohort.
| Total number | 80 |
| Age in years | 58 +/- 17 |
| Male/female | 52 (65) / 28 (35) |
| Duration of symptoms before admission in days | 2 +/- 2 |
| Duration of hospitalization at time of study entry in days | 4 +/- 3 |
| Symptoms at admission | |
| Fever | 58 (73) |
| Dyspnoea | 52 (65) |
| Cough | 33 (41) |
| Sputum production | 17 (21) |
| Myalgia | 32 (40) |
| Sore throat | 20 (25) |
| Nasal congestion | 4 (5) |
| Headache | 18 (23) |
| Fatigue | 51 (64) |
| Anorexia | 14 (18) |
| Nausea | 12 (15) |
| Diarrhoea | 14 (18) |
| Chest pain | 17 (21) |
| Anosmia | 10 (13) |
| Comorbidities | |
| Hypertension | 36 (45) |
| Ischaemic heart disease | 20 (25) |
| Diabetes mellitus | 13 (16) |
| Obesity | 37 (46) |
| Chronic lung disease | 22 (28) |
| Chronic kidney disease | 16 (20) |
| Smoking history | |
| Current | 15 (19) |
| Former | 19 (24) |
| Never | 46 (58) |
| Vaping history | |
| Current | 7 (9) |
| Former | 0 (0) |
| Never | 73 (91) |
Data presented as mean +/- SD or absolute number (percentage of group total).
Note: percentages rounded to nearest whole number.
At the time of study entry, 88% of the total cohort were receiving oxygen support therapy; 24 were invasively ventilated in the intensive care unit (ICU), 19 were non-invasively ventilated or on high-flow oxygen, and 27 were receiving low-flow oxygen via nasal cannula. The ICU subgroup included all patients meeting the study criteria who were admitted to the ICU under the care of an intensivist during the study period. Further details regarding patient exposures and comorbidities are available in the Supplementary Appendix.
3.2. Model selection
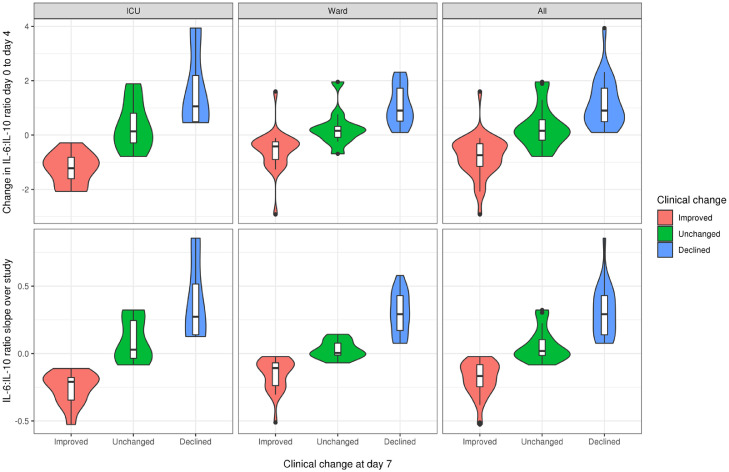
A summary of the performance of each of the models studied in predicting clinical outcome is provided in Table 2. In the unadjusted analysis using nested CV, the best predictor of outcome was the slope of the IL-6:IL-10 ratio across the duration of the study. The second best overall model was the change in ratio between day 0 and day 4, suggesting that it was a close approximation of the slope of IL-6:IL-10 ratio. For both of these models, the association with clinical outcome at day 7 was consistent across the spectrum of COVID-19 patients, with no difference in strength of association observed between those requiring ICU support and those managed at ward level (Fig. 1). We also evaluated the other models mentioned above using independent test sets. The average prediction accuracy remained high, and was close to the average prediction accuracy obtained in the model selection process, reducing our concern for overfitting due to small sample size. No significant improvements were observed for the models studied following adjustment for age and sex (Table S2). While the performance of the slope of IL-6:IL-10 across the duration of the study was slightly stronger than that of the 4-day change in IL-6:IL-10 ratio, the differences were minimal. In association with clinical outcome, the distributions of both the ratio slope and the 4-day change in ratio were similar when stratified according to clinical location (ward vs ICU, Fig. 1). Since calculating slopes in the clinical setting is impractical, the change in IL-6:IL-10 ratio at day 4 was chosen as the preferred model for predicting clinical outcome.
Table 2.
Predictive performance of the models studied.
| Predictor | MSE | MAE | RPS | Improved AUC | Declined AUC | |
|---|---|---|---|---|---|---|
| Biomarker | Timing | |||||
| IL-6:IL-10 | Slope over study | 0.20 | 0.20 | 0.15 | 0.981 | 0.958 |
| IL-6:IL-10 | D4 - D0 | 0.35 | 0.30 | 0.23 | 0.927 | 0.928 |
| IL-6 | Slope over study | 0.45 | 0.35 | 0.26 | 0.877 | 0.888 |
| IL-6 | D4 - D0 | 0.78 | 0.50 | 0.39 | 0.801 | 0.829 |
| IL-6:IL-10 | D2 - D0 | 0.83 | 0.53 | 0.38 | 0.827 | 0.866 |
| IL-6 | D2 - D0 | 1.33 | 0.78 | 0.45 | 0.731 | 0.746 |
| IL-6 | Admission (D0) | 1.53 | 0.88 | 0.46 | 0.724 | 0.702 |
| IL-6:IL-10 | Admission (D0) | 1.53 | 0.88 | 0.46 | 0.727 | 0.706 |
| Dublin-Boston score | 0.39 | 0.34 | 0.22 | 0.921 | 0.897 | |
IL – interleukin.
MSE – mean squared error.
MAE – mean absolute error.
RPS – ranked probability score.
Improved AUC – area under the curve for predicting the binary outcome of whether the patient improved or not.
Declined AUC – area under the curve for predicting the binary outcome of whether the patient declined or not.
D – Hospital day.
Fig. 1.
Association of IL-6:IL-10 ratio models with clinical outcome at day 7 by clinical location. For both the slope of the IL-6:IL-10 ratio across the duration of the study and the change in IL-6:IL-10 ratio between day 0 and day 4, the association with clinical outcome at day 7 was consistent across the spectrum of COVID-19 patients. The distributions of both the ratio slope and the 4-day change in ratio were similar when stratified according to clinical location.
3.3. Association of IL-6 and IL-6:IL-10 ratio with clinical outcome
While the baseline IL-6 and baseline IL-6:IL-10 ratio were both nominally associated with the clinical outcome, the change in IL-6:IL-10 ratio was more significantly associated with the clinical outcome than the change in IL-6. We applied an ordinal regression model to the entire dataset and observed that each 0.1 unit increase in the day 0 to day 4 change in IL-6:IL-10 ratio was associated with a 1.28 times increased odds of having a more severe clinical outcome (OR 1.28, 95% CI 1.17–1.40, P = 9.3 × 10−8, Table 3). Each 10 unit increase in the day 0 to day 4 change in IL-6 was also significantly associated with a more severe clinical outcome (OR 1.14, 95% CI 1.07–1.21, P = 6.2 × 10−5), though with a decreased strength of association and decreased goodness of fit as measured by a higher AIC (Table 3). Therefore, compared to change in IL-6, change in IL-6:IL-10 ratio is a superior predictor of clinical outcome. Adding in sex and age as covariates to these models actually worsened the fit as measured by increased AIC, possibly due to overfitting of the model (Table S3).
Table 3.
Association of IL-6 and IL-6:IL-10 ratio with a more severe clinical outcome.
| Predictors | OR (95% CI)* | AIC | P-value | |
|---|---|---|---|---|
| Biomarker | Timing | |||
| IL-6:IL-10 | Slope over study | 7.44 (3.43–16.13) | 74.1 | 3.7 × 10−7 |
| IL-6:IL-10 | D4 - D0 | 1.28 (1.17–1.40) | 111 | 9.3 × 10−8 |
| IL-6 | Slope over study | 5.04 (2.62–9.70) | 116 | 1.2 × 10−6 |
| IL-6 | D4 - D0 | 1.14 (1.07–1.21) | 150 | 6.2 × 10−5 |
| IL-6:IL-10 | D2 - D0 | 1.19 (1.08–1.32) | 154 | 4.1 × 10−4 |
| IL-6 | D2 - D0 | 1.13 (1.03–1.23) | 163 | 0.0061 |
| IL-6 | Admission (D0) | 0.94 (0.90–0.99) | 168 | 0.020 |
| IL-6:IL-10 | Admission (D0) | 0.94 (0.89–0.99) | 168 | 0.021 |
| Dublin-Boston score | 5.62 (3.22–9.81) | 103 | 1.2 × 10−9 | |
OR – odds ratio.
CI – confidence interval.
AIC - Akaike information criterion.
D – Hospital day.
The cumulative odds ratios (OR) for the proportional odds logistic regression represents the cumulative odds of a more severe clinical outcome (Improved or Unchanged vs. Declined, Improved vs. Unchanged or Declined). Due to differences in scale of IL-6 and IL-6:IL-10 ratio values, the OR is in reference to a 10-unit increase for IL-6 predictors, a 0.1 unit increase for IL-6:IL-10 ratio predictors, and a 1 point increase in the Dublin-Boston score.
3.4. Performance of the Dublin-Boston score
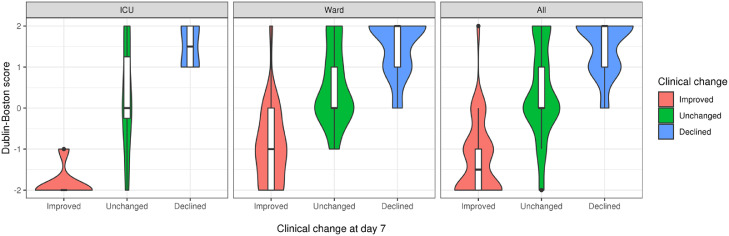
With day 0 to day 4 difference in IL-6:IL-10 ratio being strongly associated with clinical outcome and the most suitable of our tested predictors for use in clinical practice, we developed the 5-point Dublin-Boston score (−2, −1, 0, 1, 2) as a simple proxy for the 4-day change in IL-6:IL-10 ratio. Briefly, the score is obtained by multiplying the day 0 to 4 difference in IL-6:IL-10 ratio by 2 and then rounding to the nearest whole number, setting a minimum value of −2 and a maximum value of 2. The number of individuals with each score and the range of day 0 to 4 differences in IL-6:IL-10 ratio for each score is shown in Table S4. Each 1 point increase in the Dublin-Boston score was associated with a 5.6 times increased odds (OR 5.62, 95% CI −3.22–9.81, P = 1.2 × 10−9) for a more severe outcome (Table 3). As with the 4-day change in IL-6:IL-10 ratio the Dublin-Boston score was clearly stratified across clinical change categories (“Improved”/“Unchanged”/“Declined”), with no difference in the association between score and outcome observed for ICU patients compared to those on the ward (Fig. 2, Table S5).
Fig. 2.
Association of Dublin–Boston score with clinical outcome at day 7 by clinical location. The Dublin-Boston score was designed as a simple proxy for the 4-day change in IL-6:IL-10 ratio. Each 1 point increase in the Dublin-Boston score was associated with a 5.6 times increased odds (OR 5.62, 95% CI −3.22–9.81, P = 1.2 × 10−9) for a more severe outcome at day 7 across the entire cohort. No difference in the association between Dublin-Boston score and clinical outcome was observed for ICU patients compared to those on the ward.
4. Discussion
Here we show that alterations in cytokine balance predict clinical progression, and demonstrate an easily calculated linear score based on the IL-6:IL-10 ratio that can be used to guide clinical decision making. The Dublin-Boston score uses the change between two IL-6:IL-10 ratio measurements taken 4 days apart to guide clinical decision-making by identifying hospitalized patients at risk of impending poor outcome, and is applicable to patients both in the ICU and on the ward. The score, and the change in IL-6:IL-10 from which it is derived, significantly outperform the predictive capabilities of IL-6 alone.
Using inflammatory cytokine balance as a means to project outcome makes mechanistic sense. Both IL-6 and IL-10 are inextricably linked to cell metabolism, which in turn is influenced by factors such as infection, severe inflammation, hypoxia and obesity, all of which are encountered in patients with COVID-19 who require hospitalization. Our findings are consistent with a prior report investigating the use of cytokine ratios in a small cohort of patients with the Systemic Inflammatory Response Syndrome [34]. The link to clinical deterioration is similarly intuitive. Tachypnoea, for example, is a physiological response to the stimulation of irritant, stretch and J receptors within alveolar septae by pulmonary inflammation [35,36]. While tachypnoea alone is unlikely to justify intubation, progressive elevations in the respiratory rate are predictive of impending poor outcome. Early identification of the COVID-19 patient who is more likely to deteriorate allows for increased preparedness and, if it is required, more timely intubation. Indeed, patients with moderate-to-severe ARDS who receive prolonged non-invasive ventilation prior to intubation have increased ICU mortality [37]. Avoiding an extubation that is potentially damaging is also valuable, since those who require re-intubation also display increased mortality.
This study has inherent limitations. While the number of patients is more than three-fold larger than prior similar studies in medically ill patients, the sample size is still small, and lacks a replication cohort. These issues were mitigated somewhat by the statistical approach used, the absence of experimental or biological therapies in the patient population, the analysis of multiple samples taken at regular intervals for each patient, and the clinical heterogeneity of those studied. The criteria for hospitalization and the time from onset of symptoms to presentation and/or diagnosis in patients with COVID-19 remains variable internationally, influenced by health policy factors such as the cost of health care, the availability of testing and the criteria that must be met by a patient to merit a test in some jurisdictions. The patients recruited to the present study were availing of an open-access government-funded public health service, qualified for testing based on symptoms and had a wide range of socioeconomic, ethnic, and demographic backgrounds. The results also do not address asymptomatic individuals with COVID-19 – some individuals experienced resolution of symptoms prior to discharge from hospital, but none were asymptomatic at the time of recruitment to the study.
Comparing our data to the results of previous studies investigating circulating IL-6 and IL-10 as potential disease severity predictors in COVID-19 [5,[38], [39], [40]] is challenging for several reasons.
First, while some of these studies may be larger by number, they are cross-sectional; therefore the total number of samples included in their analyses is substantially less than the number used in the analysis described here. Indeed, our study shows that longitudinal measurements are superior to cross-sectional measurements in predicting clinical decline. Second, in previous studies that state an AUC value for IL-6 and/or IL-10, the AUCs quoted were calculated within a given study population without using nested CV (or any CV) and thus probably represent overfitting; therefore they are unlikely to represent the true predictive abilities of IL-6 and IL-10. To further emphasize this point, our baseline IL-6 measurements had AUCs of 0.7 for predicting decline and 0.72 for predicting clinical improvement. Third, our use of objective, WHO-approved clinical endpoints – rather than symptoms or other subjective measures of clinical improvement – reduces the risk of bias compared to these prior studies. In our study, treating physicians were blinded, insofar as they were unaware of the cytokine levels, IL-6:IL-10 ratios and Dublin-Boston scores for any of the patients under their care.
The models used were adjusted for age and sex only. The rationale for this was to derive a score that was as applicable and as easy to perform as possible. Other clinical variables that are believed to influence outcome, such as body mass index, would potentially improve the predictive ability of the score. However, obtaining accurate weight and height recordings for each patient in an inpatient medical ward or an ICU under the current circumstances is challenging, and may risk overburdening an already stretched hospital staff.
Inclusion of chronic medical comorbidities such as pre-existing lung disease is also challenging, since such a term covers a wide variety of heterogeneous conditions, each with a different prognosis. Furthermore, different degrees of severity exist within each lung disease. A patient with asthma would probably be expected to be less compromised than a patient with cystic fibrosis, for example, while a chronic obstructive pulmonary disease (COPD) patient with a frequent exacerbator phenotype would similarly be expected to do worse than a COPD patient with more stable disease. In this study, we felt that restricting the Dublin-Boston score to a small number of objective variables was preferable, and opted to sacrifice additional precision in favour of ease-of-use. With the performance of the score as is, any improvement in precision would have been uncertain and incremental with a definite increase in model complexity.
Acute thrombotic events have been reported in up to 25% of ICU patients with COVID-19 and 10% of non-ICU patients [41], [42], [43]. While the exact mechanism underlying this phenomenon is incompletely understood, it appears that increased levels of ACE2 expression by endothelial cells following SARS-CoV-2 infection may lead to amplification of endothelial injury and thromboinflammation [44]. Thrombotic events did occur in this population. However, we did not capture a change in the levels of IL-6 or IL-10 based on the development of a thrombotic event. This may be due to the small sample size, but may also be due to the fact that some patients had thrombotic events that went undetected. The present demand for inpatient radiology services coupled with the need to abide by strict sterilization procedures after scanning COVID-19 patients means that ultrasound and/or CT-based imaging for each patient with a diagnosis of COVID-19 is not currently feasible. By the same token, it is recognized that many of the thrombotic events detected in ICU patients are incidental findings that are not clinically significant. Given the complex relationship between cytokines and thromboembolic disease [45], [46], [47], and the lack of standardized screening – in particular for venous thromboembolism – in COVID-19, a separate prospective study of cytokines, thrombotic events, and outcomes in COVID-19 is warranted.
While the Dublin-Boston score and changes in the IL-6:IL-10 ratio both predict clinical outcome, and give an insight into the pathogenesis of COVID-19 inflammation, we emphasize that these data alone do not support attempts to manipulate the ratio directly as a therapeutic target. Although IL-6 may contribute to organ injury and death in sepsis syndromes, it is also required for innate immunity and microbial clearance [7,48]. Imprecise inhibition of the pro-inflammatory effects may therefore represent a double-edged sword.
The IL-6 receptor (IL-6R), is a cell surface receptor expressed by a limited number of cell types, most notably hepatocytes and macrophages. Following binding, the IL-6/IL-6R complex must associate with a gp130 – a protein present on all cell types – for signal transduction to occur [6,49]. This process, known as classic signalling, mediates the anti-inflammatory and anti-microbial effects of IL-6. In certain pathological states, however, cells that do not express the IL-6R are capable of becoming IL-6-responsive. Cleavage of IL-6R is by ADAM-17 produces a soluble IL-6R (sIL-6R), which is capable of binding IL-6 and subsequently the gp130 on cells that were previously unresponsive to IL-6. The signalling that results is termed trans-signalling, and dictates the pro-inflammatory activities of IL-6 [6,49]. An awareness of these distinct paradigms is critically important, since monoclonal antibodies against IL-6R block both types of IL-6R signalling. This is a clinically relevant drawback, as evidenced by increased risk of bacterial infection in patients treated with tocilizumab [48], and further illustrates that direct manipulation of biomarkers such as the IL-6:IL-10 ratio do not assure improved outcomes.
Classical IL-6 signalling also plays an integral role in the endogenous antiprotease response, upregulating the production and release of the acute phase protein alpha-1 antitrypsin (AAT) by the liver [50,51]. The primary role of AAT is to bind neutrophil elastase (NE) [52], an omnivorous protease released by activated or disintegrating neutrophils and a key cause of lung tissue damage and airway inflammation in ARDS [53]. In patients requiring ICU admission, blunting of the AAT response to COVID-19 is associated with poor outcome [4].
Furthermore, the absolute level of IL-6 does not reflect the body's response to IL-6, which largely depends on other factors such as the degree of ADAM-17 activity, and circulating levels of the aforementioned sIL-6R and soluble gp130, which forms a blood buffer against the pro-inflammatory effects of IL-6 [54]. Genetics also play a role – individuals with a single nucleotide polymorphism in the IL-6R gene (rs2228145) display increased IL-6R shedding [55] and a higher inflammatory tolerance [56].
Compensatory changes in these factors vary significantly between different acute and chronic inflammatory conditions, again demonstrating the difficulty in devising anti-IL-6 therapeutic strategies based on data from other diseases, and further highlighting the role of inadequate anti-inflammatory protection in determining outcome. In this regard, treatment aimed at changing the level of a specific cytokine, rather than restoration of cytokine balance by addressing the underlying cause, may prove futile. Indeed, treatment strategies addressing the underlying cause of changes in IL-6 and IL-10 are more likely to be successful, with the Dublin-Boston score also representing a viable means of assessing the response to therapy in the context of properly conducted randomized control trials. Should specific inhibition of IL-6-mediated inflammation be required, selective blockade of trans-signalling would appear more likely to preserve cytokine balance and bacterial clearance than blanket inhibition of IL-6 signalling, and may constitute a safer approach.
Given the scale of the current pandemic, and the wide variety of potential outcomes, tools that help caregivers predict the clinical course of the COVID-19 patient address an area of need. More informed prognosis could help determine when to escalate care, guide clinicians seeking to institute or remove mechanical ventilation, or drive considerations for therapies. The score described here is a first step in this direction.
Contributors
O.J.McE., B.D.H. and N.G.McE. conceptualized the study; O.J.McE., B.D.H., M.M., D.Q., M.H.C., G.F.C. and N.G.McE designed the study; O.J.McE., O.F.McE., N.L.McE., J.C., E.O'C., S.W. and G.F.C. recruited patients and collected and processed samples; O.J.McE., O.F.McE., N.L.McE. and J.C. performed experiments; B.D.H., D.Q., M.M. and M.H.C. performed statistical analyses; O.J.McE., B.D.H., M.M., M.H.C., G.F.C. and N.G.McE. co-wrote the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Competing Interests
M.H.C. has received grant support from Bayer and GSK, and speaking and consulting fees from AstraZeneca and Illumina. N.G.McE. has previously been an investigator in trials for CSL Behring, Galapagos and Vertex. He has sat on advisory boards for CSL Behring, Grifols, Chiesi and Shire. The remaining authors (O.J.McE., B.D.H., D.Q., O.F.McE., M.M., N.L.McE., J.C., E.O'C., S.W. and G.F.C.) have no conflicts to declare.
Acknowledgments
Acknowledgments
The authors thank the patients and their families for engaging with this project, and the staff of Beaumont Hospital for logistical assistance over the course of the study. We note in particular the efforts of Beaumont Hospital Ethics Committee, for ethical guidance during the course of the study.
Funding support
The authors acknowledge the following research funding support: American Thoracic Society International Trainee Scholarship, Elaine Galwey Research Fellowship (O.J.McE.); NIH K08 HL136928, R01 HL089856, and the Parker B Francis Research Opportunity Award (B.D.H.); NIH T32 HL007427 (M.M.); NIH K01 HL129039 (D.Q.); NIH R01 HL135142 and R01 HL089856 (M.H.C.).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.103026.
Contributor Information
Oliver J McElvaney, Email: olivermcelvaney@rcsi.ie.
Noel G McElvaney, Email: gmcelvaney@rcsi.ie.
Appendix. Supplementary materials
References
- 1.Organization WH. Coronavirus disease (COVID-2019) situation reports. Available on:https://wwwWHOInt/docs/default-source/coronaviruse/situation-reports/20200221-sitrep-32-covid-19. 2020.
- 2.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk Factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.McElvaney O.J., McEvoy N., McElvaney O.F., Carroll T.P., Murphy M.P., Dunlea D.M. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garbers C., Heink S., Korn T., Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17(6):395–412. doi: 10.1038/nrd.2018.45. [DOI] [PubMed] [Google Scholar]
- 7.Heinrich P.C., Castell J.V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsusaka T., Fujikawa K., Nishio Y., Mukaida N., Matsushima K., Kishimoto T. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci U S A. 1993;90(21):10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corcoran S.E., O'Neill L.A. HIF1alpha and metabolic reprogramming in inflammation. J Clin Invest. 2016;126(10):3699–3707. doi: 10.1172/JCI84431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palsson-McDermott E.M., Curtis A.M., Goel G., Lauterbach M.A., Sheedy F.J., Gleeson L.E. Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015;21(1):65–80. doi: 10.1016/j.cmet.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palsson-McDermott E.M., O'Neill L.A. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays. 2013;35(11):965–973. doi: 10.1002/bies.201300084. [DOI] [PubMed] [Google Scholar]
- 12.Libermann T.A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu H., Mitomo K., Watanabe T., Okamoto S., Yamamoto K. Involvement of a NF-kappa B-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol Cell Biol. 1990;10(2):561–568. doi: 10.1128/mcb.10.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray A., Tatter S.B., May L.T., Sehgal P.B. Activation of the human "beta 2-interferon/hepatocyte-stimulating factor/interleukin 6" promoter by cytokines, viruses, and second messenger agonists. Proc Natl Acad Sci U S A. 1988;85(18):6701–6705. doi: 10.1073/pnas.85.18.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fong Y., Moldawer L.L., Marano M., Wei H., Tatter S.B., Clarick R.H. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989;142(7):2321–2324. [PubMed] [Google Scholar]
- 16.Kohase M., Henriksen-DeStefano D., May L.T., Vilcek J., Sehgal P.B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- 17.Kohase M., May L.T., Tamm I., Vilcek J., Sehgal P.B. A cytokine network in human diploid fibroblasts: interactions of beta-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol Cell Biol. 1987;7(1):273–280. doi: 10.1128/mcb.7.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sehgal P.B., Helfgott D.C., Santhanam U., Tatter S.B., Clarick R.H., Ghrayeb J. Regulation of the acute phase and immune responses in viral disease. Enhanced expression of the beta 2-interferon/hepatocyte-stimulating factor/interleukin 6 gene in virus-infected human fibroblasts. J Exp Med. 1988;167(6):1951–1956. doi: 10.1084/jem.167.6.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West J.B. Physiological effects of chronic hypoxia. N Engl J Med. 2017;376(20):1965–1971. doi: 10.1056/NEJMra1612008. [DOI] [PubMed] [Google Scholar]
- 20.Multhoff G., Molls M., Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi: 10.3389/fimmu.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McElvaney O.J., Zaslona Z., Becker-Flegler K., Palsson-McDermott E.M., Boland F., Gunaratnam C. Specific inhibition of the NLRP3 inflammasome as an anti-inflammatory strategy in cystic fibrosis. Am J Respir Crit Care Med. 2019 doi: 10.1164/rccm.201905-1013OC. [DOI] [PubMed] [Google Scholar]
- 22.O'Neill L.A., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16(9):553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dang E.V., Barbi J., Yang H.Y., Jinasena D., Yu H., Zheng Y. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton R., Priyadharshini B., Turka L.A. Immunometabolism of regulatory T cells. Nat Immunol. 2016;17(6):618–625. doi: 10.1038/ni.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsonne G., Lekander M., Akerstedt T., Axelsson J., Ingre M. Diurnal Variation of Circulating Interleukin-6 in Humans: a Meta-Analysis. PLoS ONE. 2016;11(11) doi: 10.1371/journal.pone.0165799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molano Franco D., Arevalo-Rodriguez I., Roqué I.F.M., Montero Oleas N.G., Nuvials X., Zamora J. Plasma interleukin-6 concentration for the diagnosis of sepsis in critically ill adults. Cochrane Database Syst Rev. 2019;4(4) doi: 10.1002/14651858.CD011811.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pou K.M., Massaro J.M., Hoffmann U., Vasan R.S., Maurovich-Horvat P., Larson M.G. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham heart study. Circulation. 2007;116(11):1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 28.Roytblat L., Rachinsky M., Fisher A., Greemberg L., Shapira Y., Douvdevani A. Raised interleukin-6 levels in obese patients. Obes Res. 2000;8(9):673–675. doi: 10.1038/oby.2000.86. [DOI] [PubMed] [Google Scholar]
- 29.Trayhurn P., Wood I.S. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33(Pt 5):1078–1081. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- 30.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassionate use of Remdesivir for patients with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calfee C.S., Delucchi K., Parsons P.E., Thompson B.T., Ware L.B., Matthay M.A. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: a language and environment for statistical computing.https://www.R-project.org/ URL. [Google Scholar]
- 33.Varma S., Simon R. Bias in error estimation when using cross-validation for model selection. BMC Bioinformatics. 2006;7:91. doi: 10.1186/1471-2105-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taniguchi T., Koido Y., Aiboshi J., Yamashita T., Suzaki S., Kurokawa A. Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med. 1999;27(7):1262–1264. doi: 10.1097/00003246-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Tobin M.J. Principles and practice of mechanical ventilation. Shock. 2006;26(4):426. [Google Scholar]
- 36.Tobin M.J. McGraw-Hill; 1998. Principles and practice of intensive care monitoring. [Google Scholar]
- 37.Bellani G., Laffey J.G., Pham T., Madotto F., Fan E., Brochard L. Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE study. Am J Respir Crit Care Med. 2017;195(1):67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 38.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou H., Zhang B., Huang H., Luo Y., Wu S., Tang G. Using IL-2R/lymphocytes for predicting the clinical progression of patients with COVID-19. Clin Exp Immunol. 2020;201(1):76–84. doi: 10.1111/cei.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang F., Hou H., Wang T., Luo Y., Tang G., Wu S. Establishing a model for predicting the outcome of COVID-19 based on combination of laboratory tests. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rabinovich A., Cohen J.M., Cushman M., Wells P.S., Rodger M.A., Kovacs M.J. Inflammation markers and their trajectories after deep vein thrombosis in relation to risk of post-thrombotic syndrome. J Thromb Haemost. 2015;13(3):398–408. doi: 10.1111/jth.12814. [DOI] [PubMed] [Google Scholar]
- 46.Sharma A., Singh K., Biswas A., Ranjan R., Kishor K., Pandey H. Impact of interleukin 6 promoter polymorphisms (-174G >C, -572G >C and -597G >A) on plasma IL-6 levels and their influence on the development of DVT: a study from India. Hematology. 2018;23(10):833–838. doi: 10.1080/10245332.2018.1483546. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Zhang Z., Wei R., Miao X., Sun S., Liang G. IL (interleukin)-6 contributes to deep vein thrombosis and is negatively regulated by miR-338-5p. Arterioscler Thromb Vasc Biol. 2020;40(2):323–334. doi: 10.1161/ATVBAHA.119.313137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smolen J.S., Beaulieu A., Rubbert-Roth A., Ramos-Remus C., Rovensky J., Alecock E. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371(9617):987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 49.Jones S.A., Jenkins B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18(12):773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 50.Castell J.V., Gomez-Lechon M.J., David M., Andus T., Geiger T., Trullenque R. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242(2):237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- 51.Perlmutter D.H., May L.T., Sehgal P.B. Interferon beta 2/interleukin 6 modulates synthesis of alpha 1-antitrypsin in human mononuclear phagocytes and in human hepatoma cells. J Clin Invest. 1989;84(1):138–144. doi: 10.1172/JCI114133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carrell R.W., Jeppsson J.O., Laurell C.B., Brennan S.O., Owen M.C., Vaughan L. Structure and variation of human alpha 1-antitrypsin. Nature. 1982;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- 53.Lee C.T., Fein A.M., Lippmann M., Holtzman H., Kimbel P., Weinbaum G. Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory-distress syndrome. N Engl J Med. 1981;304(4):192–196. doi: 10.1056/NEJM198101223040402. [DOI] [PubMed] [Google Scholar]
- 54.Jostock T., Mullberg J., Ozbek S., Atreya R., Blinn G., Voltz N. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268(1):160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 55.Garbers C., Monhasery N., Aparicio-Siegmund S., Lokau J., Baran P., Nowell M.A. The interleukin-6 receptor Asp358Ala single nucleotide polymorphism rs2228145 confers increased proteolytic conversion rates by ADAM proteases. Biochim Biophys Acta. 2014;1842(9):1485–1494. doi: 10.1016/j.bbadis.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 56.Ferreira R.C., Freitag D.F., Cutler A.J., Howson J.M., Rainbow D.B., Smyth D.J. Functional IL6R 358Ala allele impairs classical IL-6 receptor signaling and influences risk of diverse inflammatory diseases. PLoS Genet. 2013;9(4) doi: 10.1371/journal.pgen.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.